Abstract
Polymeric alkylpyridinium salts (poly-APS) isolated from the Mediterranean marine sponge, Haliclona (Rhizoniera) sarai, effectively inhibit barnacle larva settlement and natural marine biofilm formation through a non-toxic and reversible mechanism. Potential use of poly-APS-like compounds as antifouling agents led to the chemical synthesis of monomeric and oligomeric 3-alkylpyridinium analogues. However, these are less efficient in settlement assays and have greater toxicity than the natural polymers. Recently, a new chemical synthesis method enabled the production of poly-APS analogues with antibacterial, antifungal and anti-acetylcholinesterase activities. The present study examines the antifouling properties and toxicity of six of these synthetic poly-APS using the barnacle (Amphibalanus amphitrite) as a model (cyprids and II stage nauplii larvae) in settlement, acute and sub-acute toxicity assays. Two compounds, APS8 and APS12-3, show antifouling effects very similar to natural poly-APS, with an anti-settlement effective concentration that inhibits 50% of the cyprid population settlement (EC50) after 24 h of 0.32 mg/L and 0.89 mg/L, respectively. The toxicity of APS8 is negligible, while APS12-3 is three-fold more toxic (24-h LC50: nauplii, 11.60 mg/L; cyprids, 61.13 mg/L) than natural poly-APS. This toxicity of APS12-3 towards nauplii is, however, 60-fold and 1200-fold lower than that of the common co-biocides, Zn- and Cu-pyrithione, respectively. Additionally, exposure to APS12-3 for 24 and 48 h inhibits the naupliar swimming ability with respective IC50 of 4.83 and 1.86 mg/L.
1. Introduction
Marine biofouling is a dynamic natural process that occurs on ocean-submerged surfaces and leads to the undesired accumulation of organic polymers and of microbial, plant and animal communities and their by-products [1]. Although there is a wide diversity of fouling organisms and a variety of contributing environmental factors, a general sequence of fouling events is frequently observed [2]. The initial conditioning of the submerged surfaces by the adsorption of organic macromolecules is followed by the attachment of marine bacteria, which form a complex multi-species biofilm [3,4]. The complexity of “microfoulers” is further increased when fungi, diatoms and protozoa colonize this microbial slime layer [5]. Within hours to days, “soft macrofouling” is observed, as algal spores and various invertebrate larvae begin to attach and develop [6,7]. Shelled invertebrates, like barnacles, mussels and tubeworms, represent the “hard macrofouling” phase, which results in the formation of a mature fouling community [8].
The settlement and accumulation of marine organisms is a severe problem on engineered structures that are submerged in the sea, and it incurs substantial economic costs in the shipping [9,10], desalination [11] and offshore oil and gas [12] industries and in marine aquaculture [13]. Traditionally, the most effective strategy for controlling biofouling has been achieved using paints and antifouling coatings that contain toxic constituents (e.g., Cu, Zn) or biocides (e.g., tributyltin, bis(tributyltin) oxide) [14,15]. However, the accumulation of these compounds in harbors and ports led to massive pollution problems [14] and had detrimental effects on non-target marine organisms [16]. Consequently, the use of organotin-based antifouling coatings was prohibited by the Antifouling System Convention of the International Maritime Organization (effective from 17 September 2008).
Modern antifouling approaches are investigating behavioral, chemical and physical defense mechanisms that have evolved in living organisms, to translate these into novel antifouling applications [17]. The creation of self-polishing and foul-release coatings has been inspired by the skin of marine mammals and fish, which respond to environmental stimuli, like temperature and pH [1]. Bio-inspired physical antifouling strategies have exploited the physical properties for fouling prevention, such as the surface energy and microtopography [1,15,18,19,20]. Antifoulants based on natural products have been proposed as one of the best ecologically relevant antifouling solutions [21,22], due to their lower toxicity, reversible effects at lower effective concentrations and biodegradability, compared with conventional biocides [23]. Natural antifouling compounds are produced as secondary metabolites by a wide range of organisms, and they include terpenoids, steroids, carotenoids, phenolics, furanones, alkaloids, peptides and lactones [24,25,26].
To date, over 80 different bioactive 3-alkylpyridinium and 3-alkylpyridine compounds have been identified in marine sponges of the order Haplosclerida [27,28]. One of the most studied of these compounds are water-soluble polymeric 3-alkylpyridinium salts (poly-APS), secondary metabolites produced by the Mediterranean marine sponge, Haliclona (Rhizoniera) sarai [29]. Poly-APS (5.52 kDa; [30]) have been chemically defined as polymers that are composed of 29 monomeric N-butyl-3-butyl pyridinium units, with 3-octyl chains linked to the nitrogen of the adjacent unit in a head-to-tail organization (see Figure 1). In aqueous solutions, poly-APS behave as cationic detergents at concentrations >0.23 g/L and form large supramolecular structures of a 23 nm mean hydrodynamic radius [31]. At concentrations >1 g/L, poly-APS have toxic and lethal effects in rodents upon intravenous administration [23].
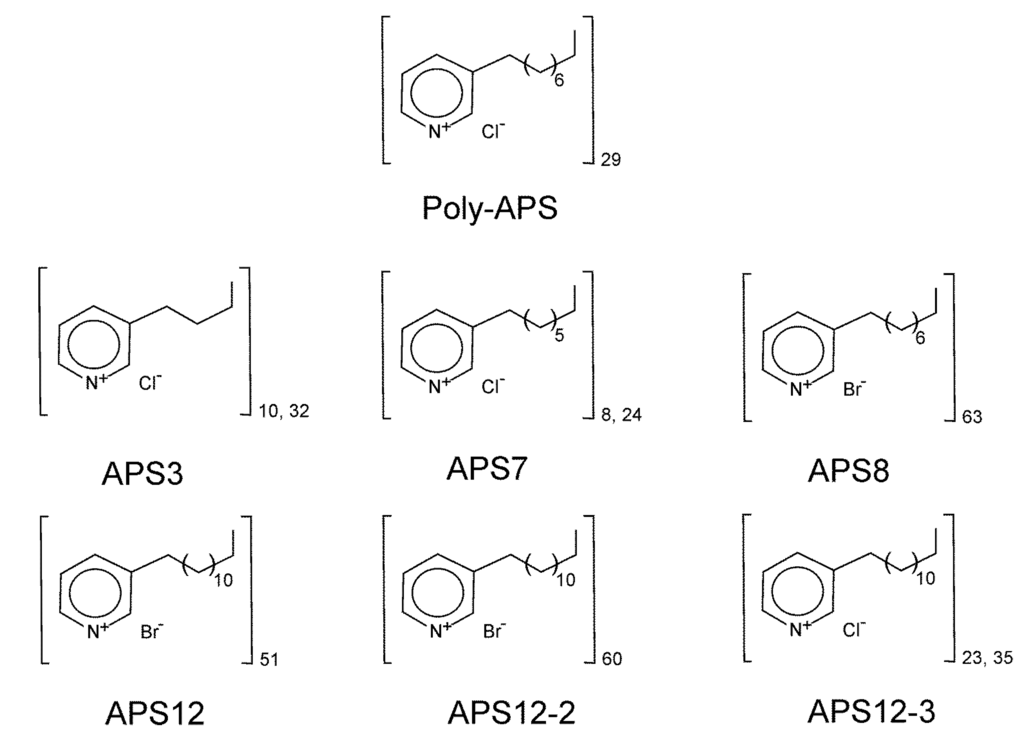
Figure 1.
Chemical structures of natural polymeric 3-alkylpyridinium salts (poly-APS) and the synthetic poly-APS investigated. Molecular weights: natural poly-APS, 5.52 kDa; APS3, 1.46 kDa; APS7, 2.33 kDa; APS8, 11.9 kDa; APS12, 12.5 kDa; APS12-2, 14.7 kDa; APS12-3, 6.08 kDa.
Among the numerous biological activities of poly-APS that were detailed by [27], their antifouling activity is of particular interest for the current study. In a static laboratory bioassay [2] using the most common hard macrofouler species, the barnacle, Amphibalanus amphitrite, poly-APS effectively deterred the settlement of cyprids (effective concentration that inhibits 50% of the cyprid population settlement (EC50), 0.27 mg/L), with low toxicity towards nauplii (lethal concentration that kills 50% of the cyprid population (LC50), 30.01 mg/L) and the non-target organisms, the alga, Tetraselmis suecica, and the mussel, Mytilus galloprovincialis [32]. Additionally, in a laboratory anti-microfouling activity assay and in the range of 0.1 mg/L to 1.0 mg/L, poly-APS prevented the formation of the natural marine biofilm, through inhibition of the growth of certain marine bacteria [33]. The molecular mechanisms behind poly-APS antifouling activity presumably involve neurotransmission blockade/modulation [34] through acetylcholinesterase inhibition, combined with their surfactant-like properties that decrease the surface tension [28].
Despite extensive studies of such natural antifoulants over the past 20 years, their incorporation into antifouling paints has been hampered by their limited supply [24]. Sufficient amounts of target poly-APS for broad biological screening and of poly-APS analogues for structure-activity relationship studies can be supplied through synthetic organic chemistry [35]. Using such an approach, dimers and tetramers of linear 3-alkylpyridinium salts have been produced that have high antibacterial and moderate anti-acetylcholinesterase activities [36]. In the barnacle anti-settlement assay, these oligomeric compounds did not reach the antifouling potential of natural poly-APS and were considerably more toxic [37]. In contrast, screening for novel antimicrobial agents has revealed that some of these APS analogues have considerable antibacterial activity towards biofilm-forming marine bacteria and has indicated that their charged pyridinium moiety and bromine atom, and the length of their alkyl chain, are decisive factors in this bioactivity [38]. Recently, new microwave-assisted polymerization has allowed the production of high-molecular-weight analogues of poly-APS [39,40]. Certain poly-APS analogues have been seen to behave similarly to natural poly-APS when their antifungal, antibacterial, anti-acetylcholinesterase, antitumoral and membrane-damaging activities have been assessed [39,40,41].
In the present study, we investigated the antifouling activity of six synthetic poly-APS analogues using A. amphitrite cyprids as the macrofouling model in a static laboratory settlement assay. In parallel, we examined the toxicity towards A. amphitrite cyprids after 24 h, 48 h and 72 h of exposure to these synthetic poly-APS and calculated their therapeutic ratios. We monitored the acute and sub-acute toxicity towards II stage A. amphitrite nauplii after 24 h and 48 h exposure to these poly-APS analogues. Finally, the behavior of II stage nauplii was studied for one of the most promising analogues, APS12-3, using swimming speed alteration assay as a measure of sub-lethal toxicity.
2. Results and Discussion
Marine biofouling is a complex and dynamic natural process that is very difficult to reproduce under laboratory conditions. As direct evaluation of antifouling coatings in situ is expensive and time-consuming, several bioassays have been developed to estimate the antifouling potential of novel natural products [2]. Due to the large diversity of organisms implicated in the marine biofouling process, it has been recommended that as many target species are used as possible, taken from both the microfouling and macrofouling communities [25]. Generally, bacteria, diatoms and fungi isolated from marine biofilms are studied in microfouling bioassays, while sessile hard-foulers (e.g., barnacles, tube worms, mussels) and soft-foulers (e.g., the bryozoan, Bugula neritina, the polychaete, Hydroides elegans) or seaweed (e.g., Ulva) are frequently used as representative macrofouling organisms. In our previous studies [32,33,42], we demonstrated that natural poly-APS can effectively inhibit the settlement and/or growth of different target fouling organisms. Using synthetic monomeric and oligomeric analogues of natural poly-APS, we also demonstrated their considerable antimicrobial activity [38]. However, in the anti-settlement assay against A. amphitrite cyprids, we identified only one synthetic poly-APS analogue (1,8-di(3-pyridyl)octane) that showed similar efficacy to natural poly-APS, although with a noticeably different toxic mechanism of action [37].
In the present study, we focused on the antifouling activities of synthetic poly-APS using A. amphitrite as the primary invertebrate model for biofouling [43]. The structures of the synthetic poly-APS evaluated in this study are illustrated in Figure 1.
2.1. Anti-Settlement Assay
We investigated the settlement of laboratory-reared cyprids of A. amphitrite after 24 h, 48 h and 72 h of exposure to these synthetic poly-APS in 24-well plates (see the Experimental Section). The results of the 72 h anti-settlement assays are shown in Figure 2a.
APS8 was the most effective of the synthetic poly-APS for the inhibition of the settlement of cyprids (p < 0.01; Figure 2a). With an EC50 of 0.32 mg/L after 24 h (Table 1), APS8 matches the anti-settlement activity of natural poly-APS (EC50, 0.27 mg/L). Moreover, when these are expressed as molar concentrations, APS8 is almost two-fold more effective than natural poly-APS (Table 1). The biphasic dose response seen for APS8 (Figure 2a) is characterized by a significant inhibition of settlement at 0.5 and 1 mg/L, followed by the loss of anti-settlement activity at concentrations from 5 to 10 mg/L and then by the return of inhibition at 50 and 100 mg/L, thus suggesting the phenomenon of hormesis [44]. The underlying mechanism of such adaptive responses might be mediated via specific receptor and/or cell-signaling pathways [45,46]. For natural poly-APS, one of the possible molecular mechanisms of this anti-settlement activity is interference with the cyprid cholinergic system [28]. A recent novel hypothesis proposed that the cellular quality control systems that are involved in the recognition, repair and prevention of cell stress represent the underlying molecular mechanisms that account for the benefits of hormesis [47].
We observed a hormetic-like response also for APS7, even though this is not supported by a posteriori comparison of the means and less clearly for APS12. However, for both analogues, 50% inhibition of the cyprid settlement occurred at concentrations that are an order of magnitude higher than APS8 (Table 1). For all of these tested synthetic poly-APS, the settlement inhibition decreased with the time of exposure. This effect is particularly evident for polymer APS3, which shows no significant effect on the cyprid settlement (p = 0.45; F = 1.08) and is thus less interesting for further antifouling research.
The synthetic polymers, APS12-2 and APS12-3, inhibit the settlement of cyprids in a concentration-dependent manner, with the respective EC50 of 8.78 and 0.89 mg/L at 24 h (Table 1). As reported by Zovko et al. [40], APS12-3 has the highest antibacterial and antifungal activity among all of these synthetic poly-APS. By comparing the data for the inhibition of cyprid settlement with those for the toxicity of APS12-3 (Figure 2b), for the longer incubation times of 48 h and 72 h, it can be concluded that the inhibitory effects probably occur due to the toxic action of APS12-3. Interestingly, however, the potent inhibition of cyprid settlement by APS12-3 is reversible at concentrations up to 1.6 mg/L (Figure 3). The other synthetic poly-APS tested here did not show any significant toxic effects against these barnacle cyprids at concentrations of up to 100 mg/L (Figure 2b).
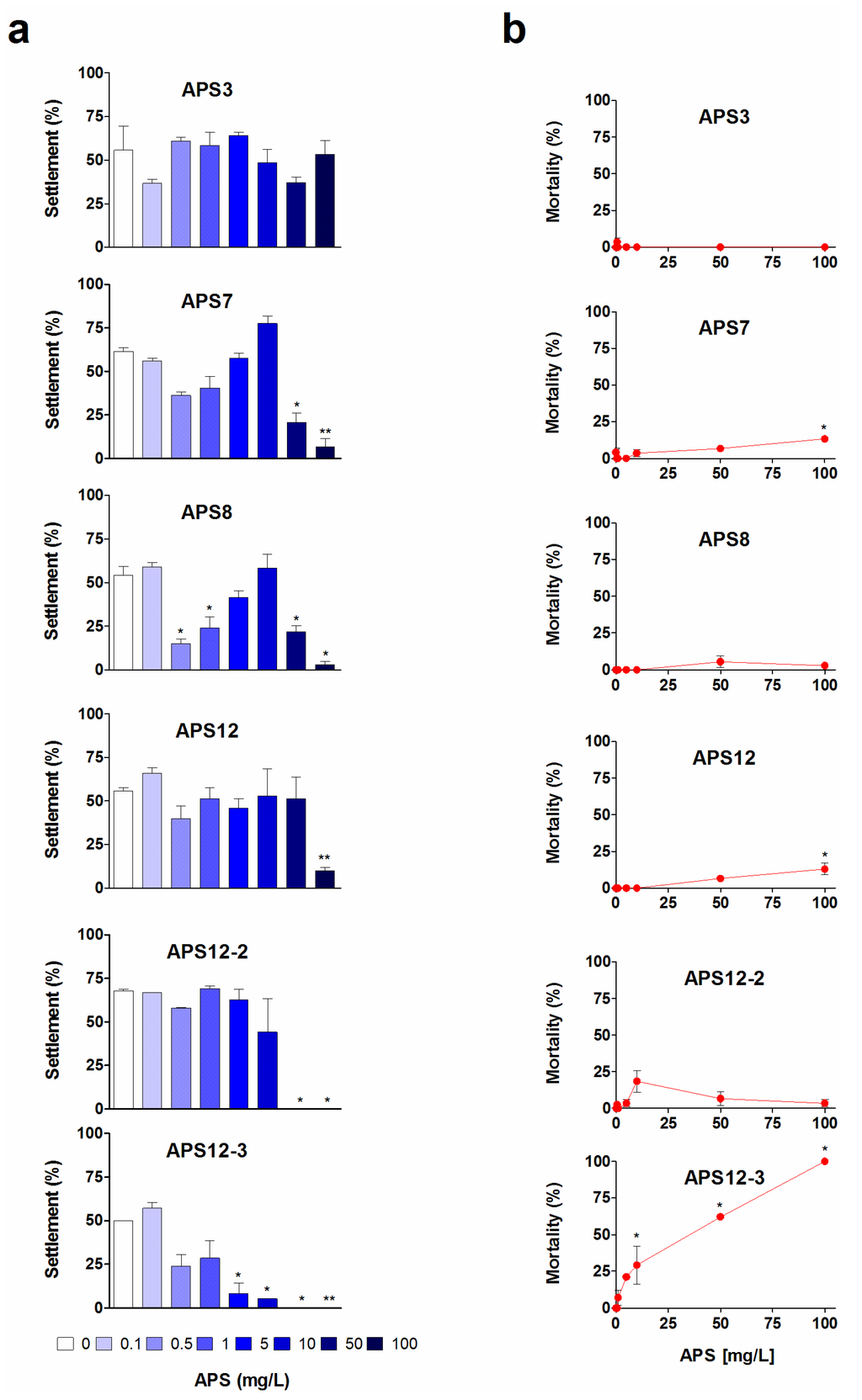
Figure 2.
Anti-settlement activity (a) and toxicity (b) of synthetic poly-APS on A. amphitrite cyprids after 72-h exposure to the different poly-APS concentrations (as indicated). Data are expressed as the means ± standard error. * p < 0.05; ** p < 0.001.

Table 1.
Antifouling activities of natural poly-APS, Zn and Cu pyrithiones, and the synthetic poly-APS, assessed as the settlement (EC50) and mortality (LC50) of A. amphitrite cyprids.
| Compound | Treatment (h) | EC50 (mg/L) | LC50 (mg/L) | EC50 (μM) | LC50 (μM) |
|---|---|---|---|---|---|
| Poly-APS 1 | 24 | 0.27 (0.15–0.47) | 0.049 | ||
| Zn pyrithione 1 | 24 | 0.02 | 0.063 | ||
| Cu pyrithione 1 | 24 | <0.01 | <0.032 | ||
| APS3 | 24 | 5.72 (4.24–7.72) | >100 | 3.9 | |
| 48 | >100 | >100 | |||
| 72 | >100 | >100 | |||
| APS7 | 24 | 10.50 (8.47–13.01) | >100 | 4.5 | |
| 48 | 25.86 (23.29–28.71) | >100 | 11.1 | ||
| 72 | 29.38 (26.17–32.99) | >100 | 12.6 | ||
| APS8 2 | 24 | 0.32 (0.26–0.39) | >100 | 0.026 | |
| 48 | 0.50 (0.36–0.70) | >100 | 0.042 | ||
| 72 | 2.33 (1.78–3.04) | >100 | 0.195 | ||
| APS12 | 24 | nc | >100 | ||
| 48 | nc | >100 | |||
| 72 | 49.82 (37.18–66.76) | >100 | 4.0 | ||
| APS12-2 | 24 | 8.78 (8.37-9.20) | >100 | 0.597 | |
| 48 | 9.38 (8.76–10.05) | >100 | 0.638 | ||
| 72 | 11.13 (10.38–11.94) | >100 | 0.757 | ||
| APS12-3 2 | 24 | 0.89 (0.48–1.65) | 61.13 (51.65–72.36) | 0.146 | 10.0 |
| 48 | 4.03 (3.49–4.65) | 24.24 (20.09–29.24) | 0.661 | 4.0 | |
| 72 | 4.76 (4.44–5.11) | 17.97 (14.88–21.70) | 0.781 | 2.9 |
Data are expressed as EC50 or LC50 (95% confidence interval); EC50, effective concentration that inhibits 50% of the cyprid population settlement; LC50, lethal concentration that kills 50% of the cyprid population; nc, not calculable; 1 data measured after a 24 h-treatment with poly-APS or commercial co-biocide [32]; 2 EC50 calculated from an additional experiment (see the Experimental Section).
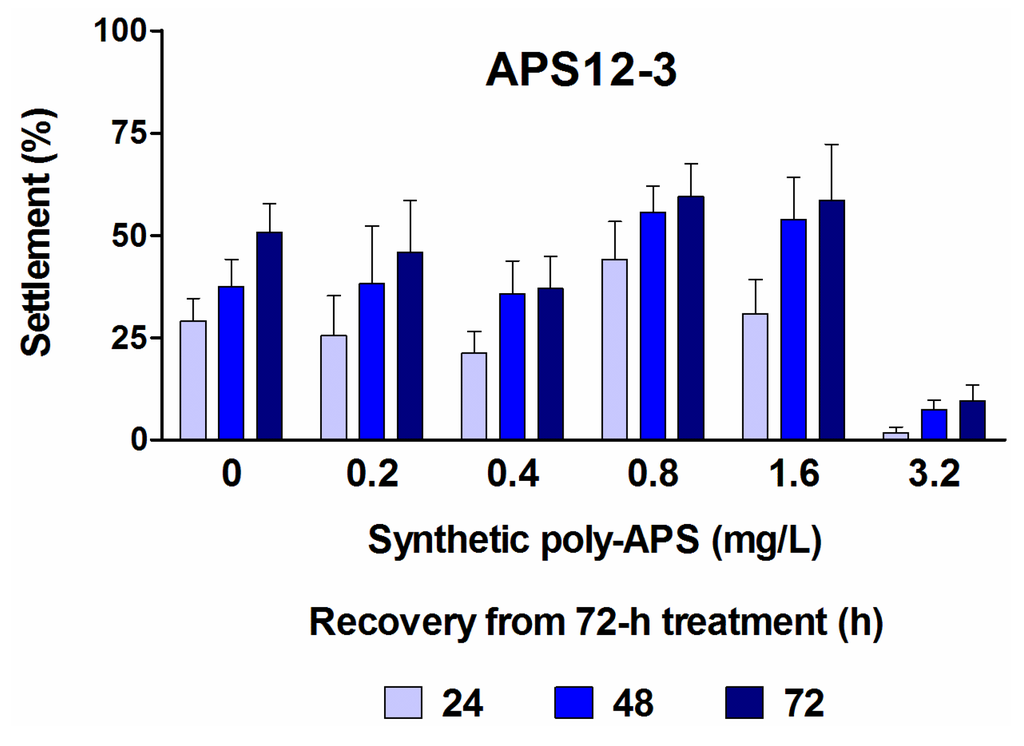
Figure 3.
Recovery of A. amphitrite cyprid settlement in fresh filtered natural seawater after 72-h treatment with different concentrations of APS12-3 monitored for three consecutive days. Data are the means ± standard error.
2.2. Naupliar Mortality, Immobility and Swimming Speed Alteration Assays
Amphibalanus amphitrite nauplii normally swim continuously, and their inability to move is evidence of toxicity [48]. On this basis, we measured the acute (mortality) and sub-acute (immobility) toxicity end-points of II stage nauplii exposed to the different solutions of the synthetic poly-APS for 24 h and 48 h. These immobility and naupliar mortality results are shown in Figure 4.
In ecotoxicological studies, the behavioral changes are more sensitive, short-term indicators of chemical toxicity than the assessment of lethal effects. Therefore, we investigated the sub-lethal toxicity of one of the synthetic analogues with the highest anti-settlement activity, APS12-3, using a swimming speed alteration test (Figure 5). Due to a lack of compound, the same evaluation has not been performed also for APS8. The synthetic poly-APS APS12-3 inhibited the mobility of nauplii in a concentration-dependent manner. The EC50 for immobility was 9.43 mg/L (after 24 h), and the ability of the nauplii to swim was completely lost at 50 mg/L APS12-3, and above. Thus, APS12-3 is moderately toxic to these nauplii, and indeed, the mortality (LC50 of 11.60 mg/L after 24 h) is three-fold greater compared to that for natural poly-APS (24-h LC50 of 30.01 mg/L; Table 2). The greater toxicity of APS12-3 towards this model organism, compared to natural poly-APS, is evidenced also by their 24-h swimming speed inhibition IC50 of 4.83 and >10 mg/L, respectively.
Increasing concentrations of APS12-2 also progressively inhibited the naupliar mobility and resulted in increased mortality. Prolonging the time of exposure to APS12-2 increased this immobility of nauplii (EC50, 36.92 mg/L at 24 h; 2.28 mg/L at 48 h). The toxicity of APS12-2 towards these nauplii is comparable to that of APS12-3.

Table 2.
Lethal (mortality) and sub-lethal (immobility) toxicity of natural poly-APS, Zn and Cu pyrithiones and the synthetic poly-APS, as assessed with A. amphitrite II stage nauplii.
| Compound | Treatment (h) | EC50 (mg/L) | LC50 (mg/L) | EC50 (μM) | LC50 (μM) |
|---|---|---|---|---|---|
| Poly-APS 1 | 24 | >10 | 30.01 (21.71–41.49) | >1.81 | 5.43 |
| Zn pyrithione 1 | 24 | 0.23 (0.16–0.33) | 0.19 (0.13–0.30) | 0.725 | 0.6 |
| Cu pyrithione 1 | 24 | 0.03 (0.03–0.04) | <0.01 | 0.095 | <0.032 |
| APS3 | 24 | >100 | >100 | ||
| 48 | >100 | >100 | |||
| APS7 | 24 | >100 | >100 | ||
| 48 | 30.64 (25.43–36.9) | 94.02 (81.73–108.17) | 13.1 | 40.3 | |
| APS8 | 24 | >100 | >100 | ||
| 48 | >100 | 79.37 (68.29–92.25) | 6.7 | ||
| APS12 | 24 | >100 | >100 | ||
| 48 | >100 | >100 | |||
| APS12-2 | 24 | 36.92 (29.33–46.46) | >100 | 2.5 | |
| 48 | 2.28 (1.95–2.67) | 4.80 (4.21–5.46) | 0.15 | 0.32 | |
| APS12-3 | 24 | 9.43 (8.10–10.97) | 11.60 (10.06–13.38) | 1.5 | 1.9 |
| 48 | 3.61 (3.14–4.16) | 5.44 (4.64–6.37) | 0.59 | 0.89 |
Data are expressed as EC50 or LC50 (95% confidence interval); EC50, effective concentration that inhibits mobility of 50% naupliar population; LC50, lethal concentration that kills 50% of the naupliar population; 1 data measured after a 24 h-treatment with poly-APS or commercial co-biocide [32].
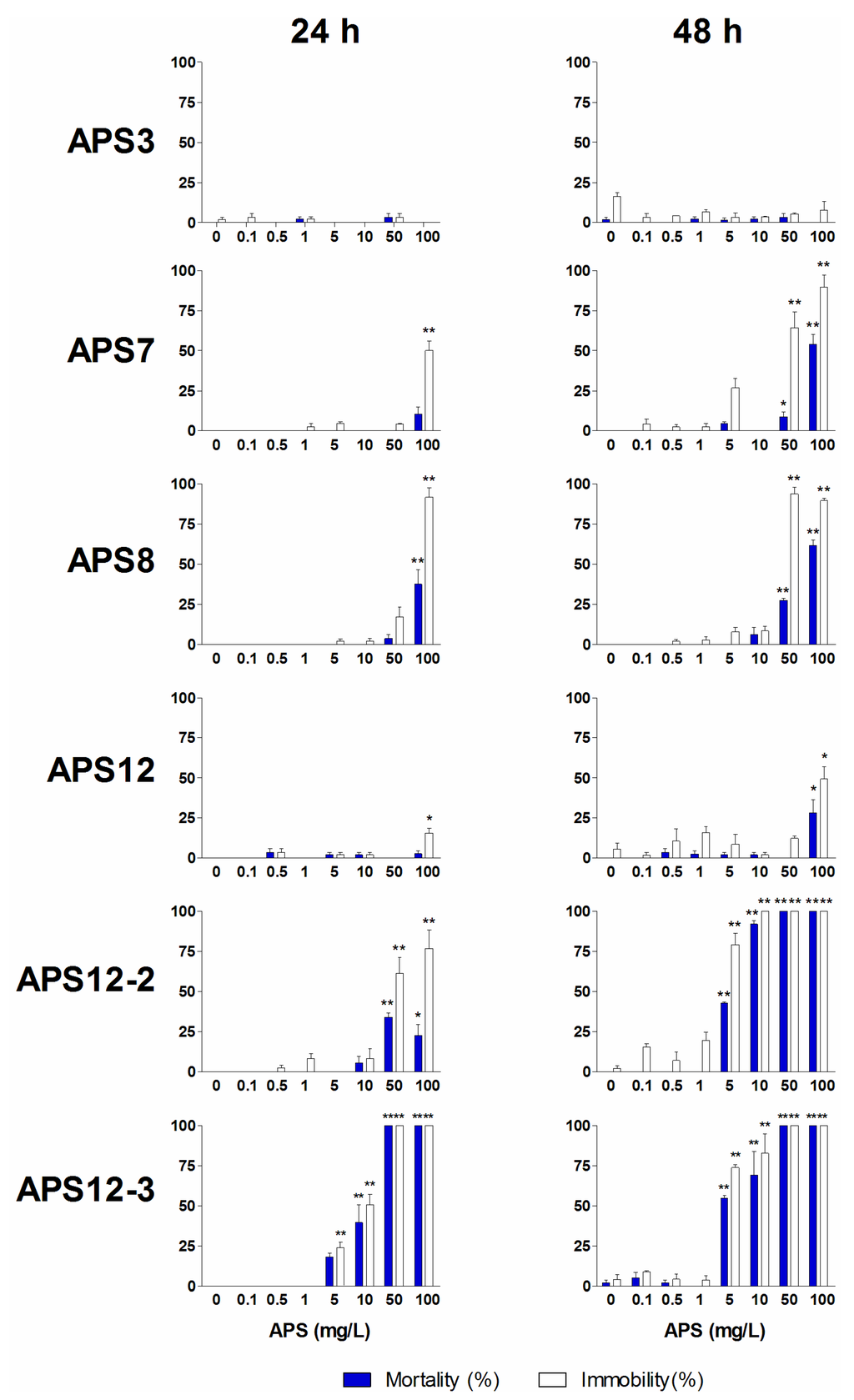
Figure 4.
Immobility (white bars) and mortality (blue bars) of the synthetic poly-APS for A. amphitrite II stage nauplii after 24 h and 48 h exposure. Data are the means ± standard error. * p < 0.05; ** p < 0.001.
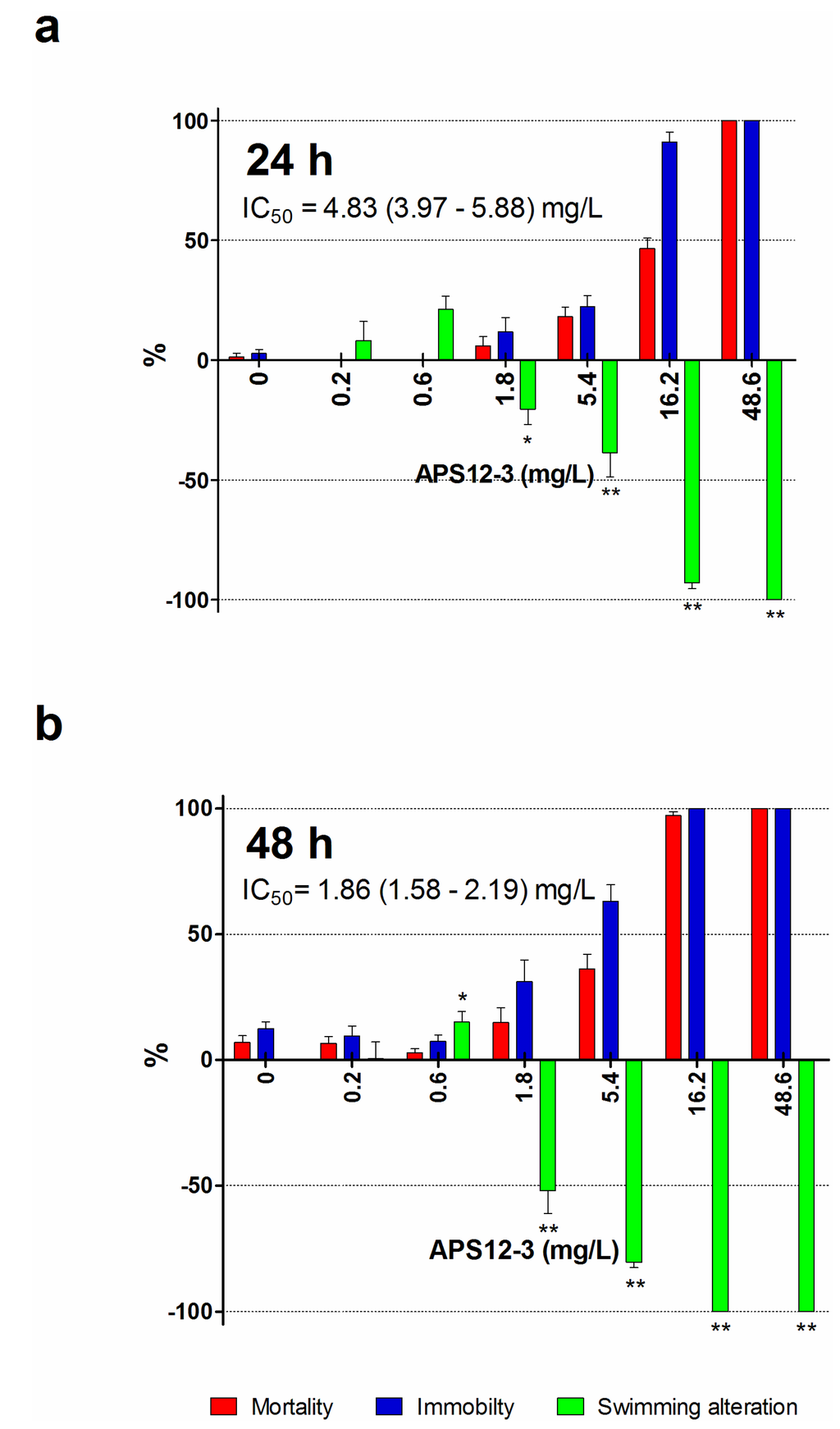
Figure 5.
Swimming speed alteration (green bars), immobility (blue bars) and mortality (red bars) of the APS12-3 for A. amphitrite II stage nauplii after 24-h (a) and 48-h (b) incubations. Data are the means ± standard error. * p < 0.05; ** p < 0.001.
At concentrations above 10 mg/L, APS7 and APS8 inhibited the naupliar mobility. This effect was more prominent after 48-h exposure at the higher doses of 50 mg/L and 100 mg/L APS7 and APS8. Both APS7 and APS8 showed a low toxicity towards these nauplii, with 48-h LC50 values of 94.02 mg/L and 79.37 mg/L, respectively.
The naupliar immobility was only minor upon exposure to APS3 and APS12.
A therapeutic ratio (TR; lethal concentration for 50% mortality (LC50) divided by the effective concentration for 50% inhibition of settlement (EC50)) is commonly used to evaluate compounds’ potential [2,26]. Generally, compounds with a TR > 50 and EC50 < 5 mg/L are considered to be promising non-toxic antifouling candidates [25]. We calculated TR values both for naupliar (TRN) and cyprids (TRC) toxicity (Table 3). TRC denotes an antifouling mechanism (toxic or non-toxic), whereas TRN indicates an environmental impact of a compound, since it is calculated by using LC50 towards non-target organisms (e.g., nauplii as representatives of the plankton) [49].
After a 24-h treatment of cyprids with synthetic analogue APS12-3, this analogue has a very promising antifouling activity (EC50 = 0.89 mg/L), and it seems to act through a non-toxic mechanism with a TRC of 68.68. However, it is potentially hazardous for the environment (TRN = 13.3). After 72 h, the antifouling activity of APS12-3 is less significant, and the compound displays a high toxicity towards target and non-target organisms (TRC = 3.77 and TRN = 1.35, respectively).
Another synthetic analogue, APS8, shows an anti-settlement activity (EC50, 0.32 mg/L) similar to natural poly-APS (EC50, 0.27 mg/L). Its TRN is higher than that of the natural compound (158.74 and 111.15, respectively) suggesting an even lower environmental risk. Based on this, we propose its use as one of the pharmacophores in the prospective studies of non-toxic antifouling compounds.

Table 3.
Therapeutic ratio values calculated using both data of naupliar (TRN) and cyprids (TRC) toxicity.
| Compounds | Treatment (h) | EC50 (mg/L) | LC50(N) (mg/L) | LC50(C) (mg/L) | TRC | TRN |
|---|---|---|---|---|---|---|
| Poly-APS 1 | 24 | 0.27 | 30.01 | / | / | 111.15 |
| Zn pyrithione 1 | 24 | 0.02 | 0.19 | / | / | 9.50 |
| Cu pyrithione 1 | 24 | <0.01 | <0.01 | / | / | / |
| APS3 | 24 | 5.72 | >100 | >100 | nc | nc |
| 48 | nc | >100 | >100 | nc | nc | |
| 72 | nc | / | >100 | nc | / | |
| APS7 | 24 | 10.50 | >100 | >100 | nc | nc |
| 48 | 25.86 | 94.02 | >100 | nc | 3.64 | |
| 72 | 29.38 | / | >100 | nc | / | |
| APS8 | 24 | 0.32 | >100 | >100 | nc | |
| 48 | 0.50 | 79.37 | >100 | nc | 158.74 | |
| 72 | 2.33 | / | >100 | nc | / | |
| APS12 | 24 | nc | >100 | >100 | nc | nc |
| 48 | nc | >100 | >100 | nc | nc | |
| 72 | 49.82 | / | >100 | nc | / | |
| APS12-2 | 24 | 8.78 | >100 | >100 | nc | 11.39 |
| 48 | 9.38 | 4.8 | >100 | nc | 0.51 | |
| 72 | 11.13 | / | >100 | nc | / | |
| APS12-3 | 24 | 0.89 | 11.6 | 61.13 | 68.68 | 13.3 |
| 48 | 4.03 | 5.44 | 24.24 | 6.01 | 1.35 | |
| 72 | 4.76 | / | 17.97 | 3.77 | / |
1 Data measured after a 24 h-treatment with poly-APS or commercial co-biocide [32]; nc, not calculable.
3. Experimental Section
3.1. Chemicals
Stock solutions (100 mg/L) of each synthetic poly-APS were prepared by dissolving them in filtered (0.22 μm) natural seawater. The information on the synthesis and NMR analyses of APS8, APS12 and APS12-2 is available in [39] and of APS3, APS7 and APS12-3 in [40]. The chemical structures of the synthetic poly-APS are illustrated in Figure 1. The Zn pyrithione (Zinc Omadine®) and Cu pyrithione (Copper Omadine®) were from Arch Chemicals Incorporated, Atlanta, USA.
3.2. Rearing of Amphibalanus amphitrite Larvae
Adult barnacles were maintained in aerated, filtered (0.45 μm) natural seawater at 20 °C, on a 16-h:8-h light-dark cycle. They were fed every two days with Artemia salina (50 to 100 mL; 200 larvae/mL) and Tetraselmis suecica (100 to 200 mL; 2 × 106 cells/mL). The seawater was changed three times per week, and the barnacles were periodically rinsed with fresh water to remove epibionts or debris.
To obtain the nauplii for cyprid cultures, the adults were left to dry for 30 min to 40 min and then immersed in fresh seawater. The hatched nauplii were attracted to a light source and collected using a Pasteur pipette. They were reared to the cyprid stage as described in [50], by keeping them at 28 °C in natural filtered (0.22 μm) seawater and feeding them three times a week with Tetraselmis suecica (2 × 106 cells/mL). In these conditions nauplii reach the cyprids stage in 5–6 days. The cyprids were harvested by filtration and aged for 4 days prior to use, in filtered (0.45 μm) natural seawater at 4 °C in the dark [48].
3.3. Settlement Assay
The effects of poly-APS on the barnacle cyprids settlement were tested using these A. amphitrite cyprids. In the preliminary settlement assays, all poly-APS-like compounds were tested within a wide range of concentrations (from 0 to 100 mg/L). The settlement assays were conducted in 24-well microplates (Greiner Bio-One International AG, Austria) by adding 20 to 25 cyprids per well, with each well containing 2 mL of the relevant poly-APS solution (i.e., 0.1, 0.5, 1, 5, 10, 50, 100 mg/L), or control seawater (i.e., 0 mg/L poly-APS). For each compound, the experiment was performed in duplicate (two wells per concentration).
The test plates were sealed to prevent evaporation and incubated at 28 °C in the dark. The settlement was evaluated after 24 h, 48 h and 72 h of incubation. The larvae were examined under a dissecting microscope, to record the number of dead and permanently attached and metamorphosed individuals. The experiments were terminated by the addition of three droplets of 40% formaldehyde into each test well and the counting of the settled and non-settled larvae. The results were expressed as the percentages (±standard error) of the settlement of the total number of larvae incubated (20–25). The EC50 was determined as the concentration of poly-APS causing 50% inhibition of the cyprids’ population settlement.
The settlement assay was repeated only for those synthetic poly-APS-like compounds that showed the best antifouling activities: APS8 and APS12-3. To better define the EC50, the assays were slightly modified in terms of the range of concentrations of these poly-APS solutions (0, 0.3, 0.6, 1.2, 2.4, 4.8, 9.6, 19.2 mg/L for APS8; 0, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8 mg/L for APS12-3), and the experiments were performed with three replicates (three wells per concentration). The data for the inhibition of cyprid settlement are not illustrated, but EC50 values are reported in Table 1.
As APS12-3 caused significant mortality of these cyprids at the higher concentrations, another experiment was designed to study the recovery of the settlement ability of these cyprid larvae. Briefly, after the 72 h treatment with APS12-3, the unsettled cyprids were collected from the wells, rinsed with filtered seawater and transferred into new microplates with clean fresh filtered (0.45 μm) natural seawater. The percentages of settled cyprids were then determined after 24 h, 48 h and 72 h at 28 °C.
3.4. Toxicity Assay
Acute (mortality) and sub-acute (immobility) toxicities of the synthetic poly-APS were assessed using II stage nauplii or cyprids of A. amphitrite. All of these tests were performed in duplicate (two wells per concentration) in 24-well microplates (Greiner Bio-One International AG, Kremsmünster, Austria), each well containing 2 mL of the relevant poly-APS solution (i.e., 0.1, 0.5, 1, 5, 10, 50 and 100 mg/L), or filtered seawater as control (i.e., 0 mg/L poly-APS), with 20 to 25 larvae per well.
The naupliar mortality and immobility were evaluated after 24 h and 48 h of incubation at 20 °C in the dark, while the cyprids’ mortality was evaluated after 24 h, 48 h and 72 h of incubation at 28 °C. After the exposure to the relevant poly-APS or control filtered seawater, the larvae were examined under a dissecting microscope, and the number of dead larvae was recorded. The data are presented as the percentage of mortality ± standard error, and the LC50 is expressed as the concentration of the poly-APS that induced death in 50% of the tested organisms. When assessing naupliar immobility, the number of immobile larvae is expressed as the sum of dead larvae (not swimming and moving appendages for 10 s of observation) and non-swimming larvae (not shifting their barycenter, but moving their appendages). The EC50 was calculated as the concentration of the toxicant that caused 50% immobility of the exposed organisms after 24 and 48 h.
3.5. Naupliar Swimming Speed Test
A swimming speed assay [51,52] was used to measure the behavioral effects of the synthetic analogue APS12-3 on the A. amphitrite larvae. Briefly, II stage nauplii (15–20 per well) were exposed to the APS12-3 test solutions in 24-well microplates for 48 h, at 20 °C in the dark, without aeration and feeding. All of these experiments were performed in duplicate (two wells per concentration).
A Swimming Behavioral Recorder System (e-magine IT, Genova, Italy) was used to track the paths of the swimming nauplii. Prior to the video recording under infrared light, the nauplii were adapted to the dark for 2 min, to gain their steady swimming speeds and to reach a uniform spatial distribution. The swimming behavior was monitored in the dark at 20 °C, for about three seconds, at 25 frames/s. The resulting digital images were analyzed using advanced image processing software to reconstruct the individual naupliar swimming paths and to measure the average swimming speed (mm/s) for each of the nauplii (15–20 nauplii). Finally, the data are expressed as percentages in terms of the swimming alteration, after normalization to the mean swimming speed (v) of the control (filtered natural seawater), as follows (Equation 1):
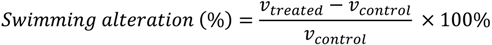
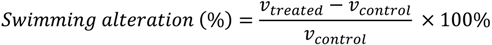
The IC50 value was determined as the concentration of APS12-3 that caused changes in swimming behavior in 50% of the test nauplii.
3.6. Statistical Analyses
The EC50 for the cyprid settlement inhibition after 24, 48 and 72 h, the EC50 for the naupliar immobility after 24 and 48 h, the LC50 for the larval mortality after 24, 48 and 72 h and the IC50 for naupliar swimming speed alteration were calculated using a trimmed Spearman–Karber analysis [53]. One-way analysis of variance (ANOVA) was performed, with the level of significance set at p < 0.05 or p < 0.001, followed by Student–Newman–Keuls (SNK) tests to compare the treatment means [54].
4. Conclusions
The present study was designed to determine the antifouling potential of six synthetic poly-APS that differ in the lengths of their alkyl chains (3–12 carbon atoms), their degree of polymerization (eight to 63 monomeric subunits) and the nature of their counter ions (chloride or bromide). Although several studies have reported that these structural features influence the biological activities of amphiphilic compounds, particularly in terms of their membrane-damaging potential and antimicrobial activities [55,56,57,58,59], we were not able to observe such a relationship.
The antifouling activity of natural poly-APS is believed to derive from its surfactant-like properties, or is potentially due to the inhibition of the cholinergic system, which is involved in cyprid settlement [28]. The underlying antifouling mechanism of these synthetic poly-APS remains unsolved; however, the hormetic responses observed imply the involvement of receptor system(s). Recently, a strong interaction between the synthetic poly-APS APS8 and human α7 nicotinic acetylcholine receptors was reported [60]. Acetylcholine serves as a neurotransmitter/neuromodulator during the settlement of A. amphitrite larvae [50], and therefore, the antifouling activity of APS8 and possibly also of these other synthetic poly-APS might derive from the binding of these synthetic poly-APS to these receptors and the subsequent competition with acetylcholine.
Although numerous natural products with anti-settlement activities have been reported to date, only a few have shown potential for commercialization [24]. Among these, natural poly-APS has been suggested as a non-toxic natural antifoulant [22,26]. With the recent chemical synthesis of various poly-APS [39,40] now ensuring an adequate supply of these compounds, we focused our current research on the finding of synthetic poly-APS with comparable or improved antifouling activities to natural poly-APS. Among the polymers tested, APS8 prevented the settlement of A. amphitrite cyprids via a non-toxic mechanism and with similar potency to natural poly-APS. On the other hand, APS12-3 showed an anti-settlement efficacy similar to the natural poly-APS, but with higher toxicity.
Acknowledgments
Financial support from the Slovenian Research Agency through grant J1-4044 (Tom Turk), from the Italian National Research Council (CNR)—Institute of Marine Science and from the Erasmus Programme (Ivanka Dragić) is gratefully acknowledged. We sincerely thank Christopher Berrie for critical reading and editing of the manuscript.
Author Contributions
Veronica Piazza, Francesca Garaventa, Marco Faimali, Kristina Sepčić, Tom Turk and Sabina Berne contributed to conception and design of the study. Veronica Piazza and Ivanka Dragić performed the experiments. Veronica Piazza, Francesca Garaventa, Sabina Berne and Kristina Sepčić analyzed and interpreted the data. All authors participated in drafting the article and critically revising it.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirschner, C.M.; Brennan, A.B. Bio-Inspired Antifouling Strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- Briand, J.-F. Marine antifouling laboratory bioassays: An overview of their diversity. Biofouling 2009, 25, 297–311. [Google Scholar]
- Jain, A.; Bhosle, N.B. Biochemical composition of the marine conditioning film: Implications for bacterial adhesion. Biofouling 2009, 25, 13–19. [Google Scholar] [CrossRef]
- Dobretsov, S. Marine Biofilms. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Wiley-Blackwell: Oxford, UK, 2009; pp. 123–136. [Google Scholar]
- Railkin, A.I.; Ganf, T.A.; Manylov, O.G. Biofouling as a Process. In Marine Biofouling: Colonization Processes and Defenses; CRC Press: Boca Raton, FL, USA, 2003; pp. 25–39. [Google Scholar]
- Joint, I.; Tait, K.; Callow, M.E.; Callow, J.A.; Milton, D.; Williams, P.; Cámara, M. Cell-to-cell communication across the prokaryote-eukaryote boundary. Science 2002, 298, 1207. [Google Scholar] [CrossRef]
- Hadfield, M.G.; Paul, V.J. Natural chemical cues for settlement and metamorphosis of marine-invertebrate larvae. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 431–461. [Google Scholar]
- Hadfield, M.G. Biofilms and marine invertebrate larvae: What bacteria produce that larvae use to choose settlement sites. Ann. Rev. Mar. Sci. 2011, 3, 453–470. [Google Scholar] [CrossRef]
- Schultz, M.P. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 2007, 23, 331–341. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Page, H.M.; Dugan, J.E.; Piltz, F. Fouling and antifouling in oil and other offshore industries. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Wiley-Blackwell: Oxford, UK, 2009; pp. 252–266. [Google Scholar]
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Rosenhahn, A.; Schilp, S.; Kreuzer, H.J.; Grunze, M. The role of “inert” surface chemistry in marine biofouling prevention. Phys. Chem. Chem. Phys. 2010, 12, 4275–4286. [Google Scholar] [CrossRef]
- Sonak, S.; Pangam, P.; Giriyan, A.; Hawaldar, K. Implications of the ban on organotins for protection of global coastal and marine ecology. J. Environ. Manag. 2009, 90, S96–S108. [Google Scholar] [CrossRef]
- Ralston, E.; Swain, G. Bioinspiration—The solution for biofouling control? Bioinspir. Biomim. 2009, 4, 015007. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Stoodley, P.; Dennington, S.P.; Goodes, L.R.; Werwinski, S.; Mart, U.; Wood, R.J.K.; Stokes, K.R. Designing biomimetic antifouling surfaces. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 4729–4754. [Google Scholar] [CrossRef]
- Scardino, A.J.; de Nys, R. Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 2011, 27, 73–86. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Rittschof, D. Natural product antifoulants: One perspective on the challenges related to coatings development. Biofouling 2000, 15, 119–127. [Google Scholar] [CrossRef]
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep. 2004, 21, 94–104. [Google Scholar] [CrossRef]
- Turk, T.; Frangež, R.; Sepčić, K. Mechanisms of toxicity of 3-alkylpyridinium polymers from marine sponge Reneira sarai. Mar. Drugs 2007, 5, 157–167. [Google Scholar] [CrossRef]
- Raveendran, T.V.; Limna Mol, V.P. Natural product antifoulants. Curr. Sci. 2009, 97, 508–520. [Google Scholar]
- Qian, P.-Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar] [CrossRef]
- Fusetani, N. Antifouling marine natural products. Nat. Prod. Rep. 2011, 28, 400–410. [Google Scholar] [CrossRef]
- Turk, T.; Sepčić, K.; Mancini, I.; Guella, G. 3-Akylpyridinium and 3-alkylpyridine compounds from marine sponges, their synthesis, biological activities and potential use. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; Volume 35, pp. 355–397. [Google Scholar]
- Sepčić, K.; Turk, T. 3-Alkylpyridinium compounds as potential non-toxic antifouling agents. In Antifouling Compounds SE-4; Fusetani, N., Clare, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 42, pp. 105–124. [Google Scholar]
- Sepčić, K.; Guella, G.; Mancini, I.; Pietra, F.; Serra, M.D.; Menestrina, G.; Tubbs, K.; Maček, P.; Turk, T. Characterization of anticholinesterase-active 3-alkylpyridinium polymers from the marine sponge Reniera sarai in aqueous solutions. J. Nat. Prod. 1997, 60, 991–996. [Google Scholar] [CrossRef]
- Grandič, M.; Sepčić, K.; Turk, T.; Juntes, P.; Frangež, R. In vivo toxic and lethal cardiovascular effects of a synthetic polymeric 1,3-dodecylpyridinium salt in rodents. Toxicol. Appl. Pharmacol. 2011, 255, 86–93. [Google Scholar] [CrossRef]
- Malovrh, P.; Sepčić, K.; Turk, T.; Maček, P. Characterization of hemolytic activity of 3-alkylpyridinium polymers from the marine sponge Reniera sarai. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 124, 221–226. [Google Scholar] [CrossRef]
- Faimali, M.; Sepčić, K.; Turk, T.; Geraci, S. Non-toxic antifouling activity of polymeric 3-alkylpyridinium salts from the Mediterranean sponge Reniera sarai (Pulitzer-Finali). Biofouling 2003, 19, 47–56. [Google Scholar] [CrossRef]
- Garaventa, F.; Faimali, M.; Sepčić, K.; Geraci, S. Laboratory analysis of antimicrofouling activity of Poly-APS extracted from Reniera sarai (Porifera: Demospongiae). Biol. Mar. Mediterr. 2003, 10, 565–567. [Google Scholar]
- Qian, P.-Y.; Chen, L.; Xu, Y. Mini-review: Molecular mechanisms of antifouling compounds. Biofouling 2013, 29, 381–400. [Google Scholar] [CrossRef]
- Mancini, I.; Defant, A.; Guella, G. Recent synthesis of marine natural products with antibacterial activities. Anti-Infect. Agents Med. Chem. 2007, 6, 17–48. [Google Scholar] [CrossRef]
- Mancini, I.; Sicurelli, A.; Guella, G.; Turk, T.; Maček, P.; Sepčić, K. Synthesis and bioactivity of linear oligomers related to polymeric alkylpyridinium metabolites from the Mediterranean sponge Reniera sarai. Org. Biomol. Chem. 2004, 2, 1368–1375. [Google Scholar] [CrossRef]
- Faimali, M.; Garaventa, F.; Mancini, I.; Sicurelli, A.; Guella, G.; Piazza, V.; Greco, G. Antisettlement activity of synthetic analogues of polymeric 3-alkylpyridinium salts isolated from the sponge Reniera sarai. Biofouling 2005, 21, 49–57. [Google Scholar] [CrossRef]
- Chelossi, E.; Mancini, I.; Sepčić, K.; Turk, T.; Faimali, M. Comparative antibacterial activity of polymeric 3-alkylpyridinium salts isolated from the Mediterranean sponge Reniera sarai and their synthetic analogues. Biomol. Eng. 2006, 23, 317–323. [Google Scholar] [CrossRef]
- Houssen, W.E.; Lu, Z.; Edrada-Ebel, R.; Chatzi, C.; Tucker, S.J.; Sepčić, K.; Turk, T.; Zovko, A.; Shen, S.; Mancini, I.; et al. Chemical synthesis and biological activities of 3-alkyl pyridinium polymeric analogues of marine toxins. J. Chem. Biol. 2010, 3, 113–125. [Google Scholar] [CrossRef]
- Zovko, A.; Vaukner Gabrič, M.; Sepčić, K.; Pohleven, F.; Jaklič, D.; Gunde-Cimerman, N.; Lu, Z.; Edrada-Ebel, R.; Houssen, W.E.; Mancini, I.; et al. Antifungal and antibacterial activity of 3-alkylpyridinium polymeric analogs of marine toxins. Int. Biodeterior. Biodegrad. 2012, 68, 71–77. [Google Scholar] [CrossRef]
- Grandič, M.; Aráoz, R.; Molgó, J.; Turk, T.; Sepčić, K.; Benoit, E.; Frangež, R. Toxicity of the synthetic polymeric 3-alkylpyridinium salt (APS3) is due to specific block of nicotinic acetylcholine receptors. Toxicology 2013, 303, 25–33. [Google Scholar] [CrossRef]
- Eleršek, T.; Kosi, G.; Turk, T.; Pohleven, F.; Sepčić, K. Influence of polymeric 3-alkylpyridinium salts from the marine sponge Reniera sarai on the growth of algae and wood decay fungi. Biofouling 2008, 24, 137–143. [Google Scholar] [CrossRef]
- Holm, E.R. Barnacles and biofouling. Integr. Comp. Biol. 2012, 52, 348–355. [Google Scholar] [CrossRef]
- Calabrese, E.J. Biphasic dose responses in biology, toxicology and medicine: Accounting for their generalizability and quantitative features. Environ. Pollut. 2013, 182, 452–460. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. Hormesis provides a generalized quantitative estimate of biological plasticity. J. Cell Commun. Signal. 2011, 5, 25–38. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Wiegant, F.A.C.; de Poot, S.A.H.; Boers-Trilles, V.E.; Schreij, A.M.A. Hormesis and cellular quality control: A possible explanation for the molecular mechanisms that underlie the benefits of mild stress. Dose-Response 2012, 11, 413–430. [Google Scholar]
- Rittschof, D.; Clare, A.S.; Gerhart, D.J.; Mary, S.A.; Bonaventura, J. Barnacle in vitro assays for biologically active substances: Toxicity and settlement inhibition assays using mass cultured Balanus amphitrite amphitrite Darwin. Biofouling 1992, 6, 115–122. [Google Scholar] [CrossRef]
- Piazza, V.; Roussis, V.; Garaventa, F.; Greco, G.; Smyrniotopoulos, V.; Vagias, C.; Faimali, M. Terpenes from the red alga Sphaerococcus coronopifolius inhibit the settlement of barnacles. Mar. Biotechnol. 2011, 13, 764–772. [Google Scholar] [CrossRef]
- Faimali, M.; Falugi, C.; Gallus, L.; Piazza, V.; Tagliafierro, G. Involvement of acetyl choline in settlement of Balanus amphitrite. Biofouling 2003, S19, 213–220. [Google Scholar]
- Faimali, M.; Garaventa, F.; Piazza, V.; Greco, G.; Corrà, C.; Magillo, F.; Pittore, M.; Giacco, E.; Gallus, L.; Falugi, C.; et al. Swimming speed alteration of larvae of Balanus amphitrite as a behavioural end-point for laboratory toxicological bioassays. Mar. Biol. 2006, 149, 87–96. [Google Scholar] [CrossRef]
- Garaventa, F.; Gambardella, C.; di Fino, A.; Pittore, M.; Faimali, M. Swimming speed alteration of Artemia sp. and Brachionus plicatilis as a sub-lethal behavioural end-point for ecotoxicological surveys. Ecotoxicology 2010, 19, 512–519. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Russo, R.C.; Thurston, R.V. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977, 11, 714–719. [Google Scholar] [CrossRef]
- De Muth, J.E. Basic Statistics and Pharmaceutical Statistical Applications, 2nd ed; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006; p. 744. [Google Scholar]
- Kleszczyńska, H.; Bielecki, K.; Sarapuk, J.; Bonarska-Kujawa, D.; Pruchnik, H.; Trela, Z.; Łuczyński, J. Biological activity of new N-oxides of tertiary amines. Z. Naturforsch. C J. Biosci. 2009, 61, 715–720. [Google Scholar]
- Zarif, L.; Riess, J.G.; Pucci, B.; Pavia, A.A. Biocompatibility of alkyl and perfluoroalkyl telomeric surfactants derived from THAM. Biomater. Artif. Cells Immobil. Biotechnol. 1993, 21, 597–608. [Google Scholar]
- Kuroda, K.; DeGrado, W.F. Amphiphilic polymethacrylate derivatives as antimicrobial agents. J. Am. Chem. Soc. 2005, 127, 4128–4129. [Google Scholar] [CrossRef]
- Sarapuk, J.; Kleszczyńska, H.; Pernak, J.; Kalewska, J.; Rózycka-Roszak, B. Influence of counterions on the interaction of pyridinium salts with model membranes. Z. Naturforsch. C 1999, 54, 952–955. [Google Scholar]
- Kleszczynska, H.; Sarapuk, J.; Rozycka-Roszak, B. The role of counterions in the interaction of some cationic surfactants with model membranes. Pol. J. Environ. Stud. 1998, 7, 327–329. [Google Scholar]
- Zovko, A.; Viktorsson, K.; Lewensohn, R.; Kološa, K.; Filipič, M.; Xing, H.; Kem, W.R.; Paleari, L.; Turk, T. APS8, a polymeric alkylpyridinium salt blocks α7 nAChR and induces apoptosis in non-small cell lung carcinoma. Mar. Drugs 2013, 11, 2574–2594. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).