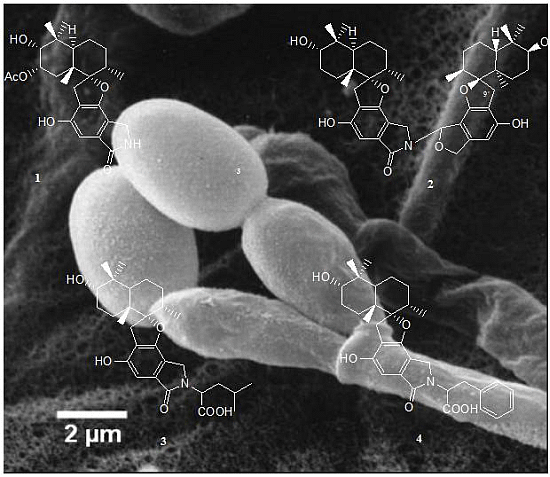
Spirocyclic Drimanes from the Marine Fungus Stachybotrys sp. Strain MF347
Abstract
:1. Introduction
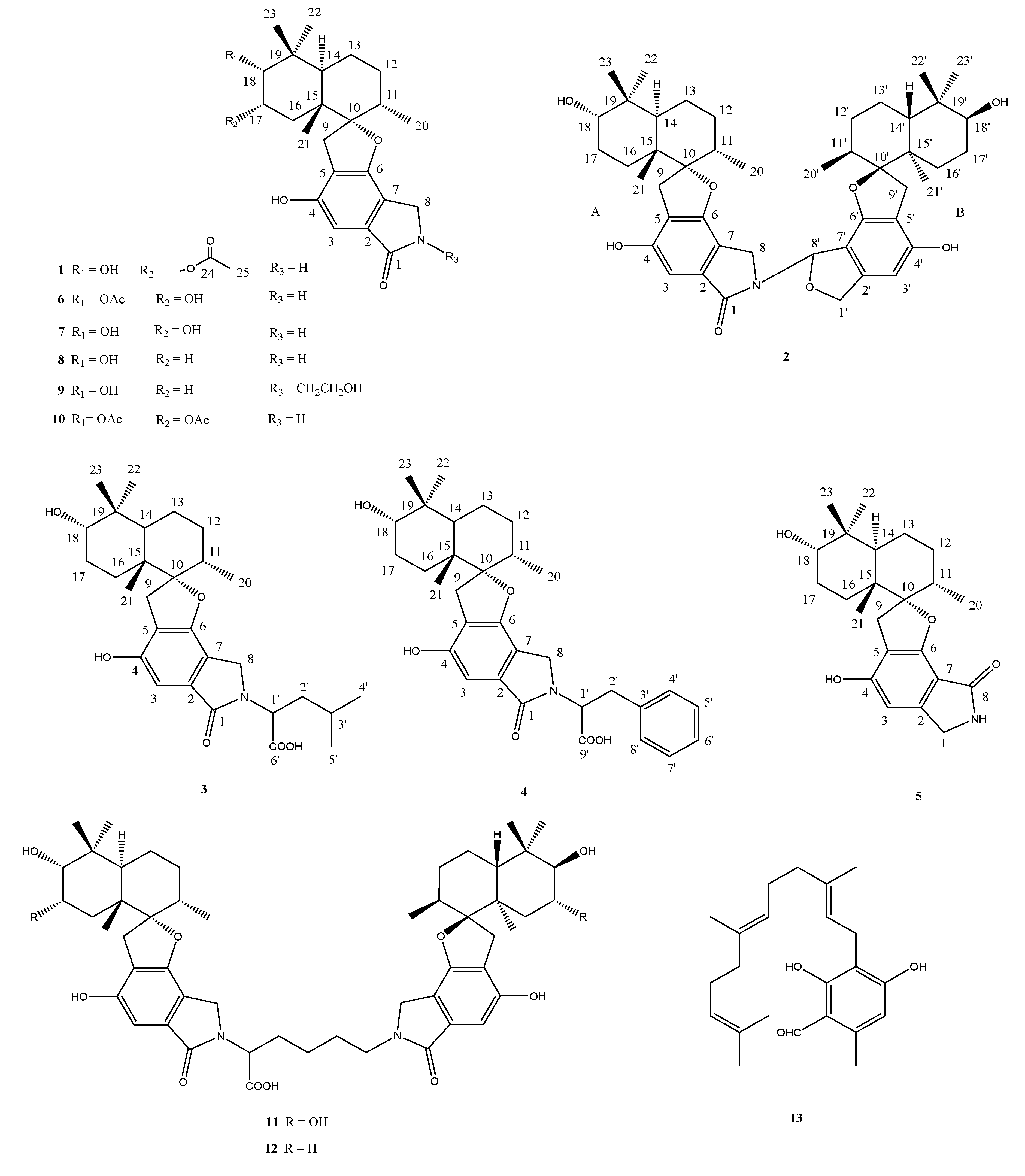
2. Results and Discussion
2.1. Identification of Strain MF347

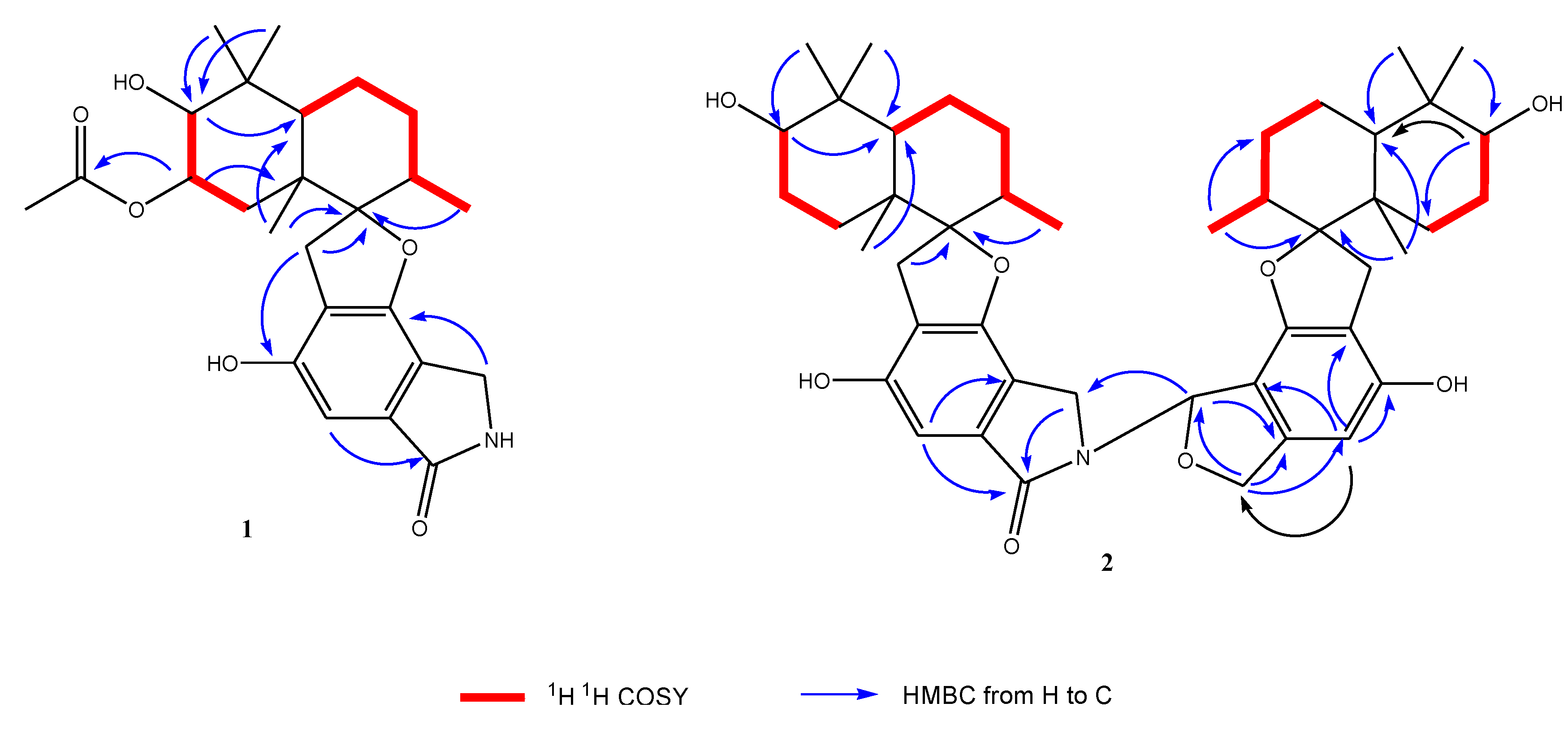
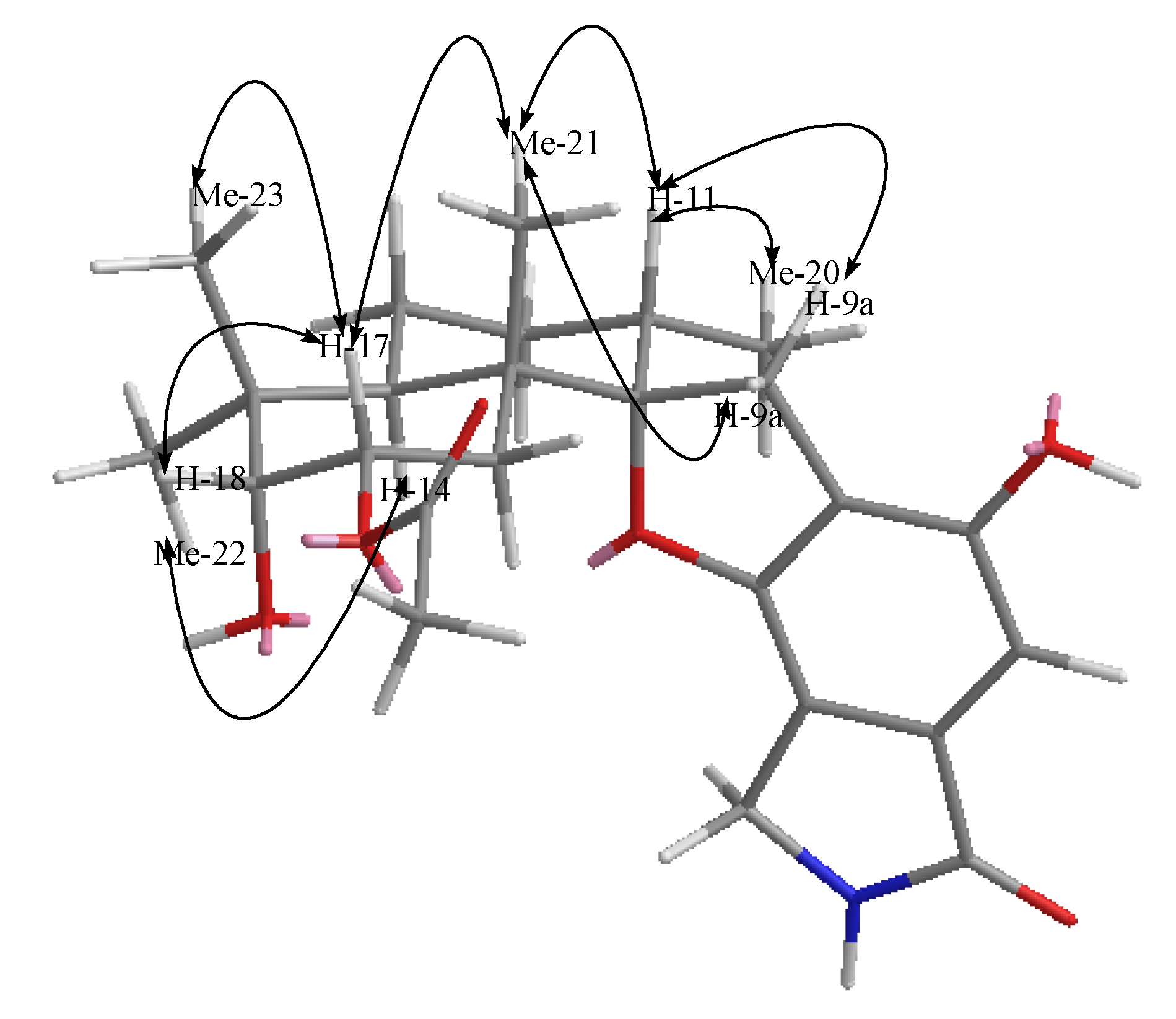
2.2. Structural Elucidation
| Position | 1 | |
|---|---|---|
| δC a,b, mult. | δH c, mult. (J in Hz) | |
| 1 | 174.0, C | - |
| 2 | 134.9, C | - |
| 3 | 102.3, CH | 6.74, s |
| 4 | 155.2, C | - |
| 5 | 118.6, C | - |
| 6 | 157.5, C | - |
| 7 | 116.8, C | - |
| 8 | 43.9, CH2 | 4.47, d (17.4), 4.31, d (17.4) |
| 9 | 33.1, CH2 | 3.25, d (17.0), 2.93, d (17.0) |
| 10 | 99.2, C | - |
| 11 | 38.0, CH | 1.91, m |
| 12 | 32.1, CH2 | 1.66, 1.56, 2 m |
| 13 | 21.7, CH2 | 1.54, 1.62, 2 m |
| 14 | 40.8, CH | 2.15, m |
| 15 | 44.8, C | - |
| 16 | 30.8, CH2 | 1.96, t (12.3), 1.36, dd (12.3, 4.4) |
| 17 | 72.0, CH | 5.21, ddd (12.3, 4.4, 2.1) |
| 18 | 77.0, CH | 3.48, d (2.1) |
| 19 | 39.9, C | - |
| 20 | 15.8, CH3 | 0.78, d (6.6) |
| 21 | 17.3, CH3 | 1.17, s |
| 22 | 29.1, CH3 | 0.99, s |
| 23 | 22.5, CH3 | 1.07, s |
| 24 | 172.6, C | - |
| 25 | 21.2, C | 1.99, s |
| Position | Unit A | Position | Unit B | ||
|---|---|---|---|---|---|
| δC a,b, mult. | δH c, mult. (J in Hz) | δC a,b, mult. | δH c, mult. (J in Hz) | ||
| 1 | 171.1, C | - | 1′ | 74.0, CH2 | 5.21 dd (12.2, 2.1), 5.00, d (12.2) |
| 2 | 134.8, C | - | 2′ | 143.2, C | - |
| 3 | 102.4, CH | 6.77, s | 3′ | 100.2, CH | 6.29, s |
| 4 | 155.3, C | - | 4′ | 156.6, C | - |
| 5 | 119.3, C | - | 5′ | 113.8, C | - |
| 6 | 157.6 d, C | - | 6′ | 157.7 d, C | - |
| 7 | 114.4, C | - | 7′ | 109.1, C | - |
| 8 | 44.3, CH2 | 4.30, d (16.8), 3.86, d (16.8) | 8′ | 85.8, CH | 7.24, br d (2.1) |
| 9 | 33.0, CH2 | 3.23, d (17.0), 2.85, d (17.0) | 9′ | 32.4, CH2 | 3.15 d (16.2), 2.76 d (16.2) |
| 10 | 99.6, C | - | 10′ | 99.6, C | - |
| 11 | 38.5, CH | 1.84, m | 11′ | 38.0, CH | 1.72, m |
| 12 | 32.2, CH2 | 1.54, 1.44, overlap | 12′ | 32.0, CH2 | 1.23, 0.80, 2m |
| 13 | 22.0, CH2 | 1.54, 1.39, overlap | 13′ | 22.0, CH2 | 1.54, 1.39, overlap |
| 14 | 41.4, CH | 2.05, m | 14′ | 41.2, CH | 1.99, m |
| 15 | 43.4, C | - | 15′ | 43.5, C | - |
| 16 | 25.3, CH2 | 1.80, 1.15, overlap | 16′ | 25.3, CH2 | 1.80, 1.15, overlap |
| 17 | 26.1 e, CH2 | 1.96, 1.55, overlap | 17′ | 26.0 e, CH2 | 1.96, 1.55, overlap |
| 18 | 76.5, CH | 3.34, overlap | 18′ | 76.7, CH | 3.34, overlap |
| 19 | 38.0, C | - | 19′ | 38.0, C | - |
| 20 | 16.0, CH3 | 0.67, d (6.5) | 20′ | 16.1, CH3 | 0.49, d (6.5) |
| 21 | 16.5, CH3 | 1.10, s | 21′ | 16.5, CH3 | 1.02, s |
| 22 | 29.0 f, CH3 | 0.98, s | 22′ | 28.8 f, CH3 | 0.96, s |
| 23 | 23.1 g, CH3 | 0.89, s | 23′ | 23.0 g, CH3 | 0.87, s |
| Position | 3 | 4 | ||
|---|---|---|---|---|
| δC a,b, mult. | δH c, mult. (J in Hz) | δC a,b, mult. | δH c, mult. (J in Hz) | |
| 1 | 171.8, C | - | 171.7, C | - |
| 2 | 134.4, C | - | 134.4, C | - |
| 3 | 102.2, CH | 6.72, s | 102.1, CH | 6.63, s |
| 4 | 155.2, C | - | 155.1, C | - |
| 5 | 119.2, C | - | 119.0, C | - |
| 6 | 157.6, C | - | 157.5, C | - |
| 7 | 114.6, C | - | 114.6, C | - |
| 8 | 45.7, CH2 | 4.64, d (16.8), 4.30, d (16.8) | 46.2, CH2 | 4.58, d (16.8), 4.29, d (16.8) |
| 9 | 32.2, CH2 | 3.27, d (16.9), 2.88, d (16.9) | 32.2, CH2 | 3.24, d (16.9), 2.83, d (16.9) |
| 10 | 99.8, C | - | 99.7, C | - |
| 11 | 38.5, CH | 1.88, m | 38.5, CH | 1.88, m |
| 12 | 33.0, CH2 | 1.62, 1.57, 2 m | 33.0, CH2 | 1.62, 1.55, 2 m |
| 13 | 22.1, CH2 | 1.51, 1.59, 2 m | 22.1, CH2 | 1.53, 1.59, overlap |
| 14 | 41.4, CH | 2.16, dd (11.7, 2.5) | 41.4, CH | 2.13, m |
| 15 | 43.5, C | - | 43.4, C | - |
| 16 | 25.4, CH2 | 1.86, 1.10, 2 m | 25.4, CH2 | 1.88, 1.12, 2 m |
| 17 | 26.0, CH2 | 2.00, 1.56, 2 m | 26.0, CH2 | 2.00, 1.55, 2 m |
| 18 | 76.5, CH | 3.37, t (2.5) | 76.5, CH | 3.36, t (2.5) |
| 19 | 38.6, C | - | 38.6, C | - |
| 20 | 16.0, CH3 | 0.76, d (6.6) | 15.9, CH3 | 0.70, d (6.6) |
| 21 | 16.6, CH3 | 1.08, s | 16.6, CH3 | 1.07, s |
| 22 | 29.0, CH3 | 1.01, overlap | 29.0, CH3 | 1.01, s |
| 23 | 23.0, CH3 | 0.91, s | 23.0, CH3 | 0.92, s |
| 1′ | 53.0, CH | 5.02, dd (11.4, 4.4) | 57.0, CH | 5.27, dd (11.1, 5.1) |
| 2′ | 39.4, CH2 | 1.98 overlap | 36.6, CH2 | 3.55, dd (14.8, 5.1), 3.26 (11.1, 5.0) |
| 3′ | 26.3, CH | 1.51 overlap | 138.7, C | - |
| 4′ | 21.4, CH3 | 1.01, d (6.9) | 129.6, CH | 7.28, dd (8.0, 2.1) |
| 5′ | 23.5, CH3 | 1.02, d (6.9) | 129.6, CH | 7.25, td (8.0, 2.1) |
| 6′ | 172.0 d, C | - | 127.7, CH | 7.17, tt (8.0, 2.1) |
| 7′ | - | - | 129.6, CH | 7.25, td (8.0, 2.1) |
| 8′ | - | - | 129.6, CH | 7.28, dd (8.0, 2.1) |
| 9′ | - | - | 174.0 d, C | - |
2.3. Biological Activities
| No. | Name | Antibiotic Activity | Cytotoxic Activity | |||
|---|---|---|---|---|---|---|
| B. subtilis | S. epidermidis | S. aureus MRSA | NIH-3T3 | HepG2 | ||
| 2 | stachyin B | 1.42 (±0.07) | 1.02 (±0.09) | 1.75 (±0.09) | 13.01 (±0.46) | 14.27 (±1.54) |
| 11 | stachybocin B | 1.77 (±0.32) | 4.44 (±0.28) | 3.94 (±0.53) | >50 | >50 |
| 12 | stachybocin A | 2.03 (±0.23) | 2.84 (±0.35) | 3.71 (±0.22) | >50 | >50 |
| 13 | ilicicolin B | 1.06 (±0.11) | 3.18 µM (±0.33) | 0.74 (±0.12) | 30.00 (±1.20) | >50 |
3. Experimental Section
3.1. General Experimental Procedures
| Compound | Purification Step | Column | Gradient | Flow (mL/min) | UV Detection at (nm) | tR (min) | Yield (mg) |
|---|---|---|---|---|---|---|---|
| 1 | - | NP | 0 min 20% C, 20 min 70% C | 5 | 224 | 8.8 | 7.6 |
| 2 | 1st | RP | 0 min 62% B, 20 min 63% B | 15 | 215 | 6.0 | 4.5 |
| - | 2nd | NP | 0 min 20% C, 20 min 30% C | 5 | - | - | - |
| 3 | 1st | RP | 0 min 50% B, 20 min 60% B | 15 | 219 | 9.5 | 14.6 |
| 4 | 1st | NP | 0 min 5% C, 20 min 15% C | 5 | 215 | 14.8 | 4.3 |
| - | 2nd | NP | 0 min 15% C, 20 min 35% C | 5→8 | - | - | - |
| 5 | - | NP | 0 min 20% C, 20 min 70% C | 5 (8 min) | 224 | 10.7 | 8.2 |
| - | - | - | - | 8 (12 min) | - | - | - |
| 6 | - | NP | 0 min 20% C, 20 min 70% C | 5 | 224 | 7.2 | 62.9 |
| 7 | - | NP | 0 min 30% C, 20 min 60% C | 5 | 218 | 6.4 | 19 |
| 8 | - | NP | 0 min 15% C, 20 min 20% C | 5 | 215 | 12 | 71.0 |
| 9 | - | NP | 0 min 20% C, 20 min 70% C | 5 | 215 | 13.6 | 10.5 |
| 10 | 1st | RP | isocratic: 40% B, 20 min | 15 | 215 | 10.5 | 6.7 |
| - | 2nd | NP | 0 min 10% C, 20 min 25% C | 5→6 | - | - | - |
| 11 | - | NP | 0 min 20% C, 20 min 70% C | 5→8 | 215 | 12.0 | 7.9 |
| 12 | 1st | RP | 0 min 62% B, 20 min 63% B | 15 | 215 | 8.0 | 14.6 |
| - | 2nd | RP | isocratic: 61% B, 20 min | 15 | - | - | - |
| 13 | 1st | NP | 0 min 0% C, 20 min 50% C | 5 | 215 | 9.4 | 4.9 |
3.2. I Fungal Material and Cultivation Conditions
3.3. Fermentation, Extraction and Isolation of the Compounds
3.4. Biological Activities Assays
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Crous, P.W.; Gams, W.; Stalpers, J.A.; Robert, V.; Stegehuis, G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004, 50, 19–22. [Google Scholar]
- Domsch, K.-H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi; Academic Press Ltd.: London, UK, 1980; Volume 1. [Google Scholar]
- Jarvis, B.B.; Salemme, J.; Morais, A. Stachybotrys toxins. 1. Nat. Toxins 1995, 3, 10–16. [Google Scholar] [CrossRef]
- Li, D.-W.; Yang, C.S. Taxonomic history and current status of Stachybotrys chartarum and related species. Indoor Air 2005, 15, 5–10. [Google Scholar]
- Gupta, N.; Das, S.; Sabat, J.; Basak, U.C. Isolation, identification and growth of Stachybotrys sp. obtained from mangrove ecosystem of Bhitarkanika, Orissa. Int. J. Plant Sci. 2007, 2, 64–68. [Google Scholar]
- Abdullah, S.K.; Al-Saadoon, A.H.; Al-Salihy, M.H. Fungi from the tidal zone of Khawr AL-Zubair Canal Southern Iraq. Marsh Bull. 2007, 2, 18–31. [Google Scholar]
- Boland, G.J.; Grund, D.W. Fungi from the salt marshes of Minas Basin, Nova Scotia. Proc. N. S. Inst. Sci. 1979, 29, 393–402. [Google Scholar]
- Xu, X.; de Guzman, F.S.; Gloer, J.B.; Shearer, C.A. Stachybotrins A and B: Novel bioactive metabolites from a brackish water isolate of the fungus Stachybotrys sp. J. Org. Chem. 1992, 57, 6700–6703. [Google Scholar] [CrossRef]
- Sponga, F.; Cavaletti, L.; Lazzarini, A.; Borghi, A.; Ciciliato, A.; Losi, D.; Marinelli, F. Biodiversity and potentials of marine-derived microorganisms. J. Biotechnol. 1999, 70, 65–69. [Google Scholar] [CrossRef]
- Osterhage, C. Isolation, Structure Determination and Biological Activity Assessment of Secondary Metabolites from Marine-Derived Fungi. Ph.D. Thesis, Carol Wilhelmina Technical University, Braunschweig, Germany, 2001; p. 186. [Google Scholar]
- Toledo-Hernández, C.; Bones-González, A.; Ortiz-Vázquez, O.E.; Sabat, A.M.; Bayman, P. Fungi in the sea fan Gorgonia ventalina: Diversity and sampling strategies. Coral Reef. 2007, 26, 725–730. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.-H.; Sun, L.-C.; Chen, Z.-H.; Zhang, J.; Bao, B. Isolation of fibrinolytic active compound from marine fungi and initial identification of the strain. Nat. Prod. Res. Dev. 2012, 24, 57–61. [Google Scholar]
- Kaise, H.; Shinohara, M.; Miyazaki, W.; Izawa, T.; Nakano, Y.; Sugawara, M.; Sugiura, K. Structure of K-76, a complement inhibitor produced by Stachybotrys complementi nov. sp. K-76. J. Chem. Soc. Chem. Commun. 1979, 16, 726–727. [Google Scholar]
- Ayer, W.A.; Miao, S. Secondary metabolites of the aspen fungus Stachybotrys cylindrospora. Can. J. Chem. 1992, 71, 487–493. [Google Scholar] [CrossRef]
- Nakamura, M.; Ito, Y.; Ogawa, K.; Michisuji, Y.; Sato, S.; Takada, M.; Hayashi, M.; Yaginuma, S.; Yamamoto, S. Stachybocins, novel endothelin receptor antagonists, produced by Stachybotrys sp. M6222. I. Taxonomy, fermentation, isolation and characterization. J. Antibiot. 1995, 48, 1389–1395. [Google Scholar] [CrossRef]
- Ogawa, K.; Nakamura, M.; Hayashi, M.; Yaginuma, S.; Yamamoto, S.; Furihara, K.; Shiin-Ya, K.; Seto, H. Stachybocins, novel endothelin receptor antagonists, produced by Stachybotrys sp. M6222. II. Structure determination of stachybocins A, B and C. J. Antibiot. 1995, 48, 1396–1400. [Google Scholar] [CrossRef]
- Vázqueza, M.J.; Vegad, A.; Rivera-Sagredoe, A.; Jiménez-Alfarob, M.D.; Dı́ezb, E.; Hueso-Rodrı́guezc, J.A. Novel sesquiterpenoids as tyrosine kinase inhibitors produced by Stachybotrys chartarum. Tetrahedron 2004, 60, 2379–2385. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef]
- Saleema, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef]
- Sakai, K.; Watanabe, K.; Masuda, K.; Tsuji, M.; Hasumi, K.; Endo, A. Isolation, characterization and biological activities of novel triprenyl phenols as pancreatic cholesterol esterase inhibitors produced by Stachybotrys sp. F-1839. J. Antibiot. 1995, 48, 447–456. [Google Scholar] [CrossRef]
- Eder, C.; Kurz, M.; Toti, L. Spirobenzofuranlactam Derivatives, Methods for Their Preparation, and Use Thereof. European Patent EP1572697, 21 February 2007. [Google Scholar]
- Li, Y.; Wu, C.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Chartarlactams A–P, phenylspirodrimanes from the sponge-associated fungus Stachybotrys chartarum with antihyperlipidemic activities. J. Nat. Prod. 2014, 77, 138–147. [Google Scholar] [CrossRef]
- Zhang, P.; Bao, B.; Dang, H.T.; Hong, J.; Lee, H.J.; Yoo, E.S.; Bae, K.S.; Jung, J.H. Anti-inflammatory sesquiterpenoids from a sponge-derived fungus Acremonium sp. J. Nat. Prod. 2009, 72, 270–275. [Google Scholar] [CrossRef]
- Wickerham, L.J. Taxonomy of yeasts. U.S. Dept. Agric. Tech. Bull. 1951, 1029, 1–56. [Google Scholar]
- Ohlendorf, B.; Schulz, D.; Erhard, A.; Nagel, K.; Imhoff, J.F. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J. Nat. Prod. 2012, 75, 1400–1404. [Google Scholar] [CrossRef]
- Silber, J.; Ohlendorf, B.; Labes, A.; Erhard, A.; Imhoff, J.F. Calcarides A–E, antibacterial macrocyclic and linear polyesters from a Calcarisporium strain. Mar. Drugs 2013, 11, 3309–3323. [Google Scholar] [CrossRef]
- Jansen, N.; Ohlendorf, B.; Erhard, A.; Imhoff, J.F. Helicusin E, Isochromophilone X and isochromophilone XI: New chloroazaphilones produced by the fungus Bartalinia robillardoides strain LF550. Mar. Drugs 2013, 11, 800–816. [Google Scholar] [CrossRef]
- Schulz, D.; Beese, P.; Ohlendorf, B.; Erhard, A.; Zinecker, H; Dorador, C.; Imhoff, J.F. Abenquines A–D: Aminoquinone derivatives produced by Streptomyces sp. strain DB634. J. Antibiot. 2011, 64, 763–768. [Google Scholar] [CrossRef]
- Baki, A.; Bielik, A.; Molnar, L.; Szendrei, G.; Keseru, G.M.A. A high throughput luminescent assay for glycogen synthase kinase-3β inhibitors. Assay Drug Develop. Technol. 2007, 5, 75–83. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, B.; Oesker, V.; Wiese, J.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Spirocyclic Drimanes from the Marine Fungus Stachybotrys sp. Strain MF347. Mar. Drugs 2014, 12, 1924-1938. https://doi.org/10.3390/md12041924
Wu B, Oesker V, Wiese J, Malien S, Schmaljohann R, Imhoff JF. Spirocyclic Drimanes from the Marine Fungus Stachybotrys sp. Strain MF347. Marine Drugs. 2014; 12(4):1924-1938. https://doi.org/10.3390/md12041924
Chicago/Turabian StyleWu, Bin, Vanessa Oesker, Jutta Wiese, Susann Malien, Rolf Schmaljohann, and Johannes F. Imhoff. 2014. "Spirocyclic Drimanes from the Marine Fungus Stachybotrys sp. Strain MF347" Marine Drugs 12, no. 4: 1924-1938. https://doi.org/10.3390/md12041924
APA StyleWu, B., Oesker, V., Wiese, J., Malien, S., Schmaljohann, R., & Imhoff, J. F. (2014). Spirocyclic Drimanes from the Marine Fungus Stachybotrys sp. Strain MF347. Marine Drugs, 12(4), 1924-1938. https://doi.org/10.3390/md12041924