Abstract
In our search for bioactive metabolites from marine organisms, we have investigated the polar fraction of the organic extract of the Red Sea sponge Theonella swinhoei. Successive chromatographic separations and final HPLC purification of the potent antifungal fraction afforded a new bicyclic glycopeptide, theonellamide G (1). The structure of the peptide was determined using extensive 1D and 2D NMR and high-resolution mass spectral determinations. The absolute configuration of theonellamide G was determined by chemical degradation and 2D NMR spectroscopy. Theonellamide G showed potent antifungal activity towards wild and amphotericin B-resistant strains of Candida albicans with IC50 of 4.49 and 2.0 μM, respectively. Additionally, it displayed cytotoxic activity against the human colon adenocarcinoma cell line (HCT-16) with IC50 of 6.0 μM. These findings provide further insight into the chemical diversity and biological activities of this class of compounds.
1. Introduction
The order Lithistida include the genera Theonella, Discodermia, Aciculites, Microscleroderma, and Callipelta. Lithistid sponges have been shown to yield a wide variety of bioactive marine natural products that include unique cyclic peptides and depsipeptides [1,2,3]. The genus Theonella is known to be a rich source of structurally diverse, biologically active peptides [1] including polytheonamides [4], cyclotheonamides [5], theonellapeptolides [6], theonellamides [7], theonegramides [8], keramamides [9], mozamides [3], mutoporins [10], microsclerodermins [11], cupolamide [12], oriamide [13], and cyclolithistide A [14]. Many Theonella derived peptides demonstrate potent cytotoxicity [4,7,12,13], thrombin inhibition [5], phosphatase inhibition [10], protease inhibition [5], antifungal [7,8,11,14,15],and anti-HIV properties [16]. Our previous investigation on the Red Sea Theonella swinhoei led to the isolation of several macrolides including swinholide A, I and hurghadolide A [17]. As a continuation of this work, we have investigated the polar active fraction of the organic extract of the sponge. Here, we describe the isolation, structure elucidation, and biological activity of a new bicyclic glycopeptide, theonellamide G (1). Theonellamides A–F were previously isolated from Theonella sp. [7,18]. Theonellamides A–E have been found to possess cytotoxic activity, while a potent antifungal activity was reported for theonellamide F [7,18]. Theonellamides represent a new class of sterol-binding molecules that induce glucan overproduction, damage cellular membranes, and activate Rho1-mediated 1,3-β-d-glucan synthesis [19,20,21]. The absolute stereochemistry of the amino acid residues of theonellamides was determined using chemical methods, chiral GC, and Marfey’s analyses. Interestingly, the Red Sea sample did not contain any of the previously reported theonellamides.
2. Results and Discussion
2.1. Purification of Compound 1
The frozen sponge was extracted with a mixture of MeOH/CH2Cl2 (1:1). The combined extracts were suspended in MeOH/H2O (9:1) and partitioned between n-hexane and 90% MeOH followed by fractionation between CH2Cl2 and 60% MeOH. The CH2Cl2 fraction was subjected to size exclusion chromatography on Sephadex LH-20 (Merck, Darmstadt, Germany) followed by ODS flash column chromatography (Yamazen Corporation, Osaka, Japan) of the active antifungal fraction. Final HPLC purification of the polar and potent antifungal fraction on a preparative C30 HPLC column afforded theonellamide G (1) (Figure 1).
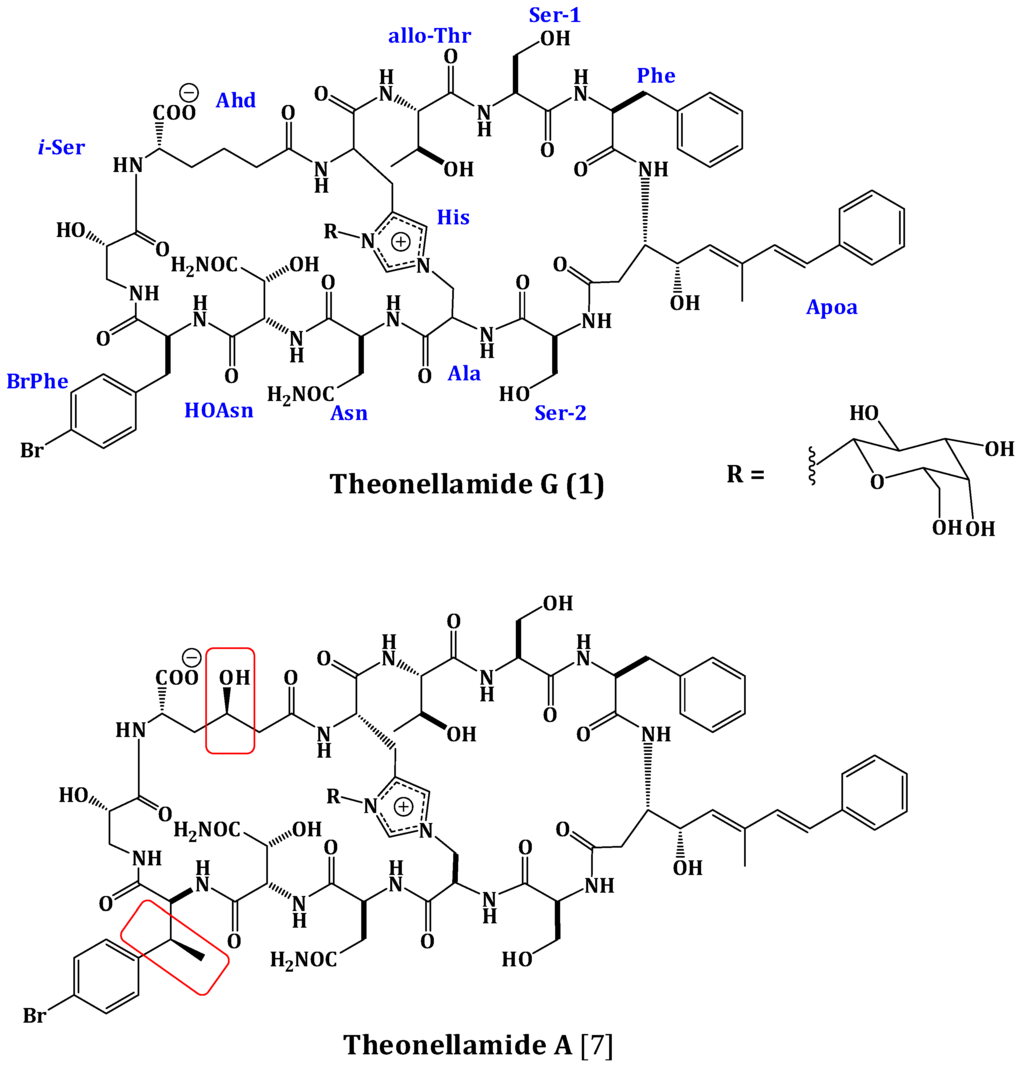
Figure 1.
Structures of Theonellamide G (1) and Theonellamide A.
Figure 1.
Structures of Theonellamide G (1) and Theonellamide A.
2.2. Structure Elucidation of Compound 1
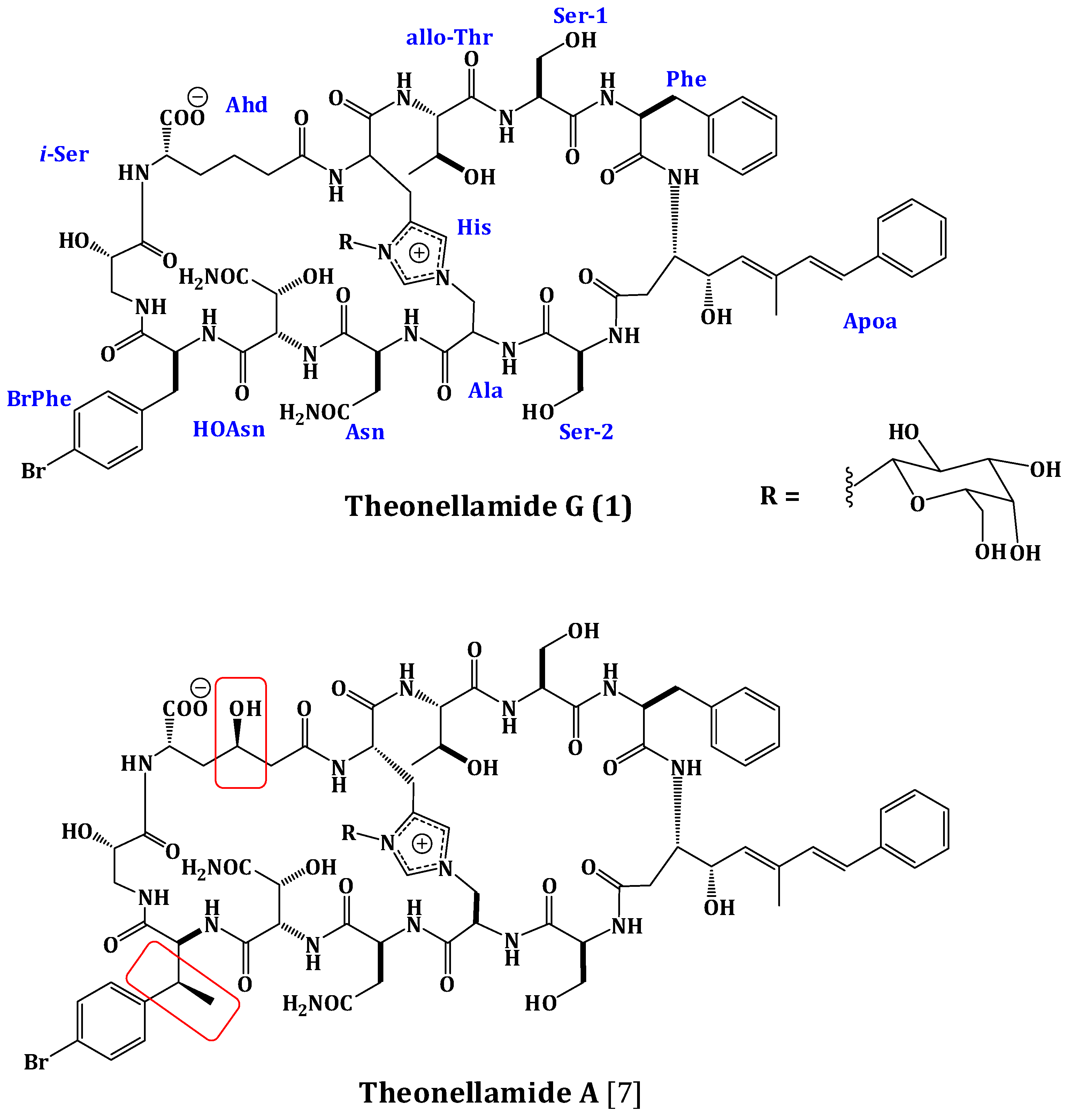
Compound 1 was obtained as an optically active powder. The molecular formula of 1 was C75H97BrN16O27 on the basis of HRFABMS and NMR data, requiring 36 degrees of unsaturation. Compound 1 is 30 mass units less than theonellamide A (Figure 1) [7], indicating loss of methyl and hydroxyl functionalities. The UV absorption bands at 289 and 306 nm suggested the presence of (5E,7E)-3-amino-4-hydroxy-6-methyl-8-phenyl-5,7-octadienoic acid (Apoa) moiety in 1 [7,15,18].The IR spectrum showed absorption bands at 3324 and 1655 cm−1, corresponding to amino and carbonyl groups, respectively. The NMR data of 1 were similar to those of theonellamide A but new signals for p-bromophenylalanine residue in 1, replacing the signals of the β-methyl-p-bromophenylalanine (β-MeBrPhe) residue in theonellamide A, were observed [7,15,18] (Supplementary Figures S1–S6). The signals at δH 4.34 (1H, m, 9-αH)/55.8, 3.01 (1H, brd, J = 14.4 Hz, 9-βHa) and 2.65 (1H, m, 9-βHb)/δC 37.2, 7.21 (2H, d, J = 6.6 Hz, H-2‴, 6‴)/δC 129.2, 7.28 (2H, d, J = 6.6 Hz, H-3‴, 5‴)/δC 131.8, 120.6 (C-4‴), and 172.6 (9-CO) (Table 1) were consistent with the p-bromophenylalanine residue [7,15,18]. The 1H-1H COSY correlations from 9-αH to 9-βHa and 9-βHb, H-2‴ to H-3‴, and H-5‴ to H-6‴, as well as, the HMBC cross peaks of 9-αH to 9-CO, 9-βH to 9-CO, C-1‴, and C-2‴, H-2‴ and H-6‴ to C-1‴, C-4‴, and H-3‴ and H-5‴ to C-1‴, C-2‴, and C-6‴, supported the assignment of the p-bromophenylalanine (BrPhe) residue. Furthermore, the chemical shift of C-1‴ in 1 (δC 137.7) compared to 141.6 ppm in theonellamide A [7] supported the absence of the β-methyl group in p-bromophenylalanine moiety in 1. In addition, a new spin system consisting of three coupled methylenes at δH 2.22 and 2.01 (11-αH), 1.37 and 1.02 (11-βH), and 1.78 and 1.53 (11-γH) together with a methine at δH 4.57 (11-δH) and NH group at δH 7.63 (11-NH) was observed in 1H-1H COSY spectrum,suggesting the presence of2-aminohexanedioic acid (Ahd) residue (Figure 2). The HMBC cross peaks from 11-αH to 11-CO and 11-βC, 11-γH to 11-αC, and 11-δH to 11-βC and 11-COO− corroborated this spin system.
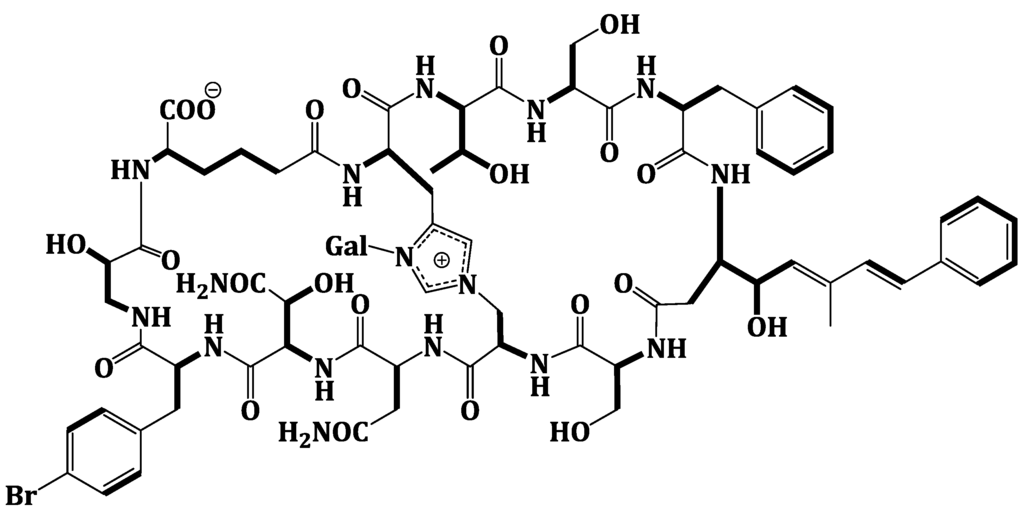
Figure 2.
Observed 1H-1H COSY Correlations of Theonellamide G (1).
Figure 2.
Observed 1H-1H COSY Correlations of Theonellamide G (1).
Extensive analysis of the 1D and 2D NMR data (Supplementary Figures S1–S6). of 1 revealed the presence of 12 spin systems which could be assigned to 12 amino acid residues [Thr, two Ser residues, Ala, Asn, OHAsn, Phe, BrPhe, His, i-Ser, Apoa, and Ahd] (Figure 2 and Figure 3). They were confirmed by the presence of 12 carbonyls between 170.0 and 175.0 ppm and 12 α-carbons in the region of 36.9–70.2. The carbonyl carbon atδC 175.0 was assigned to the α-free carboxylic acid group of Ahd [7,15,18]. In addition, the 1H and 13C NMR spectra showed signals at δH/δC 5.03/89.0, 3.83/69.5, 3.45/73.7, 3.66/69.8, 3.67/79.0, and 3.76 and 3.36/62.0, indicating the presence of a hexose moiety. It was confirmed by the observed 1H-1H COSY and HMBC correlations (Table 1). It was identified as d-galactose based on the 1H and 13C NMR data in addition to co-TLC with authentic sample upon the acid hydrolysis of 1 using solvent system S2 (Rf = 0.33) [7,15,18]. The 1H and 13C NMR chemical shift values of the galactose moiety were similar to those reported in theonellamides A and E [7] (Supplementary Figures S1 and S2). Thecoupling constant value (JH-1, H-2 = 9.0 Hz) indicated an axial configuration of the anomeric proton [7,15,18]. Thus, 34 of the 36 double bond equivalent required by the molecular formula were encountered by the amino acid residues and galactose moiety, indicating 1 was a bicyclic peptide. The sequence of amino acids and the bicyclic nature of 1 were established by a detailed examination of the NOESY and HMBC spectra (Figure 3; Supplementary Figures S5 and S6). In the NOESY spectrum, the cross peaks observed between the α-H and NH group of adjacent amino acids and between the NH and NH of adjacent residue established the presence of two substructures; alloThr-Ser-Phe-Apoa-Ser-Ala (substructure A) and Asn-OHAsn-BrPhe-iSer-Ahd-His (substructure B). In particular, the two substructures were corroborated by HMBC (2JCH and 3JCH) correlations of NH and α-H of each amino acid to the amide carbonyl carbons. The sequence alloThr-Ser-Phe-Apoa-Ser-Ala was confirmed by the key HMBC cross peaks of 1-NH/2-CO, 3-NH/2-CO, 4-βH/3-CO, 5-NH/4-CO, and 6-NH/5-CO. The HMBC correlations of 8-NH/7-CO, 9-NH/8-CO, 10-NH/9-CO, 11NH/10-CO, and 12NH/11-CO proved the substructure B. Substructures A and B were connected on the basis of NOESY correlations from 1-αH to 12-NH, 1-NH to 12-αH, 6-αH and 6-βH to 7-αH and 7-NH, 6-NH to 7-NH, H-2″″ to 6-βH and 6-NH, H-5″″ to 6-αH and 6-βH, and further confirmed by the HMBC correlations of 1-αH to 12-CO, 6-βH to C-2″″, and 7-αH and 7-NH to 6-CO.
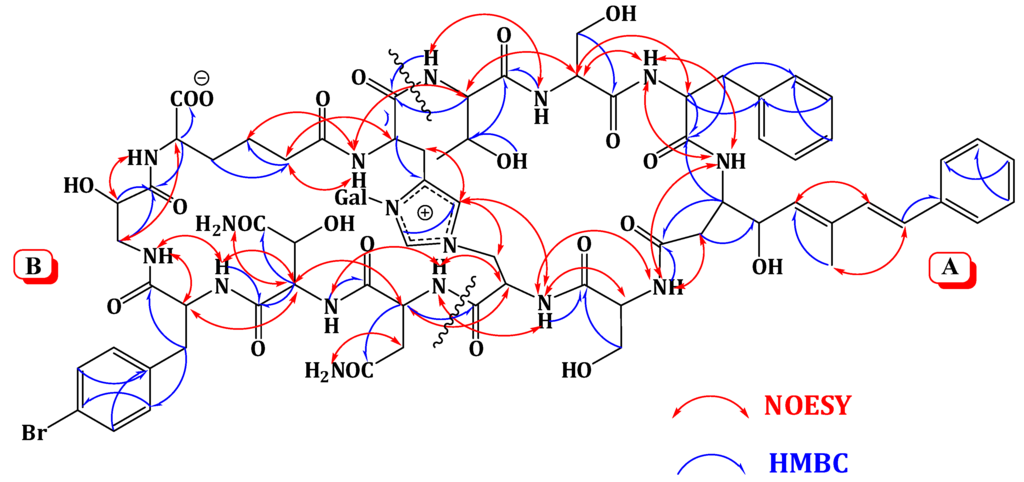
Figure 3.
Significant Observed NOESY and HMBC Correlations of Theonellamide G (1).
Figure 3.
Significant Observed NOESY and HMBC Correlations of Theonellamide G (1).
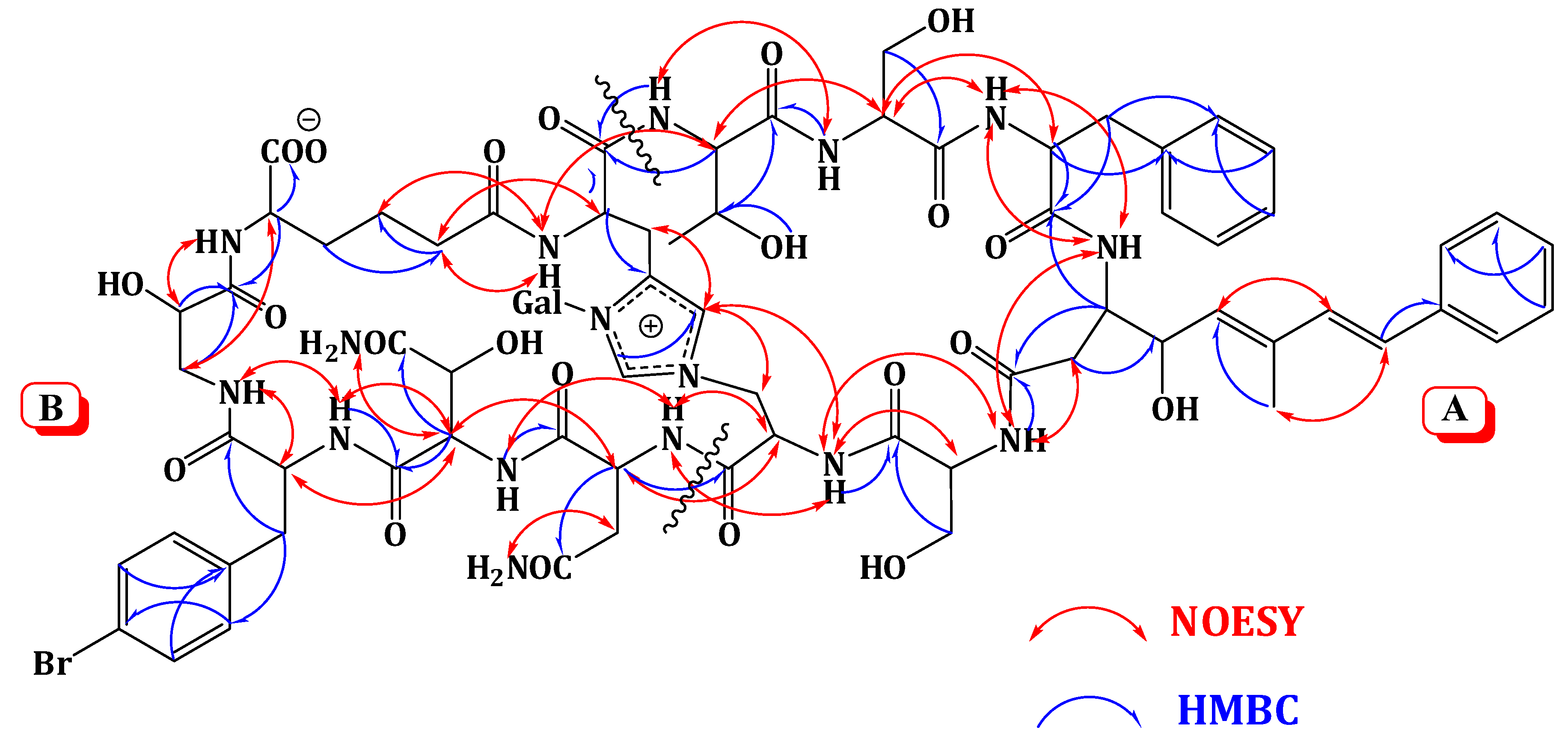
The attachment of the galactose moiety at postion-3 of His moiety was apparent from the NOESY correlation of the anomeric proton at δH 5.03 to H-2″″ and secured by its HMBC cross peaks to C-2″″ and C-4″″. The absolute configuration of 1 was determined by acid hydrolysis followed by chiral GC-MS and Marfey’s analyses. Chiral GC-MS of the acid hydrolysate and LC-MS of the hydrolysate product of 1 derivatized with N-α-(5-fluoro-2,4-dinitrophenyl)-l-leucinamide (Marfey’s reagent) indicated the presence of l-Asn, l-alloThr, l-Ser, 2S-i-Ser, l-Phe, l-BrPhe, 2S,3R-HOAsn, and d-galactose. However, the absolute stereochemistry of Apoa, Hisala, and Ahd residues could not be determined. This stereochemical assignment was confirmed by comparison of NMR coupling constant values and chemical shifts with literature [7,15,18]. The E,E geometry of the olefinic double bonds Δ4δ and Δ4ζ of Apoa was assigned on the basis of the intense NOESY cross peaks between δH 5.12 (1H, m, 4-δH) and 6.47 (1H, d, J = 16.2 Hz, 4-ζH) and between δH 6.56 (1H, d, J = 16.2 Hz, 4-ηH) and 1.63 (3H, s, 4-εCH3) and confirmed by the coupling constant values and the 13C chemical shift of 4-εCH3 (δC 13.4). In conclusion, comparison of the spectral data of 1 with those of theonellamide A suggested the replacement of the β-MeBrPhe and α-amino-γ-hydroxyadipic acid in theonellamide A with BrPhe and Ahd in 1. Thus, the structure of 1 was unambiguously elucidated as depicted and the trivial name theonellamide G was given to it.
Theonellamide G (1) showed potent antifungal activity towards wild and amphotericin B-resistant strains of Candida albicans with IC50 of 4.49 and 2.0 μM, respectively, compared to 1.48 μM for the positive antifungal control amphotericin-B against the wild type (Table 2). Additionally, compound 1 displayed cytotoxic activity against the human colon adenocarcinoma cell line (HCT-16) with IC50 of 6.0 μM, compared to 2.0 μM for etoposide (positive anticancer control) (Table 2).

Table 1.
NMR Spectroscopic Data of Theonellamide G (1) (DMSO-d6:H2O, 4:1).
| Amino acid | C | δH m (J in Hz) | δC m | HMBC | NOESY |
|---|---|---|---|---|---|
| allo-Thr1 | CO | − | 173.1 C | − | − |
| α | 4.18 d (9.8) | 58.9 CH | 1 β, 12CO | 1γ, 2α, 12NH | |
| β | 3.55 m | 69.0 CH | 1CO | 12α, 2NH | |
| γ | 0.84 brs | 21.4 CH3 | 1α, 1β | 1α, 2NH, 12α, 12β | |
| NH | 7.65 d (7.8) | − | 12CO | 2α, 2NH, 12α, 5″″ | |
| OH | 5.12 m | − | 1β | − | |
| Ser-12 | CO | − | 170.0 C | − | − |
| α | 4.45 m | 56.6 CH | − | 3α, 3NH | |
| β | 3.64 m | 61.1 CH2 | 2α, 2CO | 2α, 3NH | |
| NH | 7.73 d (3.6) | − | 1CO | 1NH, 3α, 3NH | |
| Phe3 | CO | − | 171.6 C | − | − |
| α | 4.55 t (8.3) | 54.9 CH | 3CO, 1′ | 2α, 2NH | |
| β | 2.81 dd (13.3, 6.8) 2.67 m | 39.3 CH2 | 3α, 3CO, 1′, 2′, 3′ | 2NH | |
| 1′ | − | 137.2 C | − | ||
| 2′, 6′ | 7.12 d (6.6) | 129.9 CH | 1′, 3′, 5′ | ||
| 3′, 5′ | 7.01 t (6.6) | 131.8 CH | 2′, 6′ | ||
| 4″ | 7.28 d (6.6) | 129.7 CH | 2′, 6′ | ||
| NH | 7.93 d (7.8) | − | 2CO | 2α, 2NH, 4NH | |
| Apoa4 | CO | − | 172.6 C | − | |
| α | 2.55 q (10.3) 2.30 brd (13.8) | 36.9 CH2 | 4γ, 4CO | 5NH | |
| β | 4.46 m | 52.2 CH | 3CO, 4CO | 4α, 4γ, 5NH | |
| γ | 4.42 t (8.4) | 68.8 CH | 5NH, 4β, ε-CH3 | ||
| δ | 5.12 m | 132.4 CH | 4ζ, 4ε | 4β, 4ζ, 4γ | |
| ε | − | 137.9 C | − | ||
| ζ | 6.47 d (16.2) | 133.9 CH | 4δ, 4ε, 1″ | 4δ | |
| η | 6.56 d (16.2) | 128.7 CH | 4δ, 4ε, 3ζ, 4ε-CH3, 1″ | ε-CH3 | |
| 1″ | − | 137.2 C | − | − | |
| 2″, 6″ | 7.12 d (6.6) | 129.9 CH | 1″, 3″, 5″ | ||
| 3″, 5″ | 7.01 t (6.6) | 131.8 CH | 2″, 6″ | ||
| 4″ | 7.28 d (6.6) | 129.7 CH | 2″, 6″ | ||
| ε-CH3 | 1.63 s | 13.4 CH3 | 4δ, 4ε, 4ζ | 4η, 4γ | |
| NH | 8.45 brs | − | 4CO | 3NH, 3α, 5NH | |
| Ser-25 | CO | − | 172.8 C | − | − |
| α | 3.74 m | 56.8 CH | 4α, 4β, 4NH, 6α, 6NH | ||
| β | 3.76 m 3.63 m | 62.0 CH2 | 5CO | 4α, 4β, 4NH, 6α, 6NH | |
| NH | 7.78 brs | − | 4CO | 4α, 4NH, 6NH | |
| Ala6 | CO | − | 170.0 C | − | − |
| α | 5.08 m | 51.4 CH | 6CO | 7α, 7NH, 2″″ | |
| β | 4.90 brd (12.6) | 50.6 CH2 | 2″″ | 7α, 7NH, 2″″, 5″″ | |
| NH | 8.27 d (9.6) | − | 5CO | 5α, 7NH, 5″″ | |
| Asn7 | CO | − | 171.4 C | − | − |
| α | 4.11 t (7.2) | 52.9 CH | 6CO, 7-CONH2 | 6α, 8α, 6NH | |
| β | 2.36 dt (13.3, 7.2) 2.12 brd (13.3) | 37.3 CH2 | 7α, 7-CONH2 | 6NH | |
| CONH2 | − | 172.7 C | − | − | |
| NH | 7.67 d (11.2) | − | 6CO | 6α, 6NH | |
| NH2 | 7.69 brs | − | − | 7α, 7β | |
| HOAsn8 | CO | − | 170.9 C | − | − |
| α | 5.34 t (8.4) | 54.9 CH | 8CO, 8-CONH2 | 7α, 9α, 9β, 9NH | |
| β | 4.22 d (11.7) | 72.9 CH | − | − | |
| CONH2 | − | 174.8 C | − | − | |
| NH | 8.32 brs | − | 7CO | 7NH | |
| NH2 | 7.78 s | − | − | 8α, 8NH | |
| OH | 6.78 brs | − | − | − | |
| BrPhe9 | CO | − | 172.6 C | − | − |
| α | 4.34 m | 55.8 CH | 9CO | 10NH | |
| β | 3.01 brd (14.4) 2.65 m | 37.2 CH2 | 9CO, 9α, 1‴, 2‴ | 10NH | |
| 1‴ | − | 137.7 C | − | − | |
| 2‴, 6‴ | 7.21 d (6.6) | 129.2 CH | 1‴, 4‴ | − | |
| 3‴, 5‴ | 7.28 d (6. 6) | 131.8 CH | 1‴, 2‴, 6‴ | − | |
| 4‴ | − | 120.6 C | − | − | |
| NH | 8.71 brs | − | 8CO | 8α, 10NH | |
| i-Ser10 | CO | − | 171.9 C | − | − |
| α | 4.17 d (11.2) | 70.2 CH | 10CO | 11NH | |
| β | 3.95 m 2.96 brd (7.2) | 43.8 CH2 | 10CO | 11δ, 11NH | |
| NH | 7.47 d (7.2) | − | 9CO | 9α, 9β, 9NH, 11γ, 11NH | |
| Ahd−11 | CO | − | 173.1 C | − | − |
| α | 2.22 m 2.01 m | 35.9 CH2 | 11CO, 11β | 12α, 12NH | |
| β | 1.37 m 1.02 m | 22.7 CH2 | 11γ | 12NH | |
| γ | 1.78 m 1.53 m | 32.5 CH2 | 11α | 12NH | |
| δ | 4.57 t (7.3) | 54.9 CH | 11β, 11γ, 10CO, 11-COO− | 10NH, 12NH | |
| COO− | − | 175.0 C * | − | ||
| NH | 7.63 d (6.6) | − | 10CO | 10NH, 12NH | |
| His12 | CO | − | 171.1 C | − | − |
| α | 4.82 m | 54.5 CH | 12CO, 4″″ | 1γ, 1NH, 11α | |
| β | 3.24 t (13.5) 3.01 brd (14.4) | 26.3 CH2 | 12α, 4″″ | 1NH, 1CO, 11α, 2″″, 5″″ | |
| 2″″ | 8.84 s | 137.4 CH | 4″″, 5″″ | 1-Gal, 6β, 6NH, 11β | |
| 4″″ | − | 131.8 C | − | − | |
| 5″″ | 7.26 brs | 124.4 CH | 4″″ | 6α, 6β, 11β, 12β | |
| NH | 8.40 brs | − | 11CO | 1NH, 11α, 11β, 5″″ | |
| Gal13 | 1 | 5.03 d (9.0) | 89.0 CH | 2, 3, 2″″, 4″″ | 12NH, 12β, 2″″ |
| 2 | 3.83 m | 69.5 CH | 4, 3 | ||
| 3 | 3.45 m | 73.7 CH | 4, 5 | ||
| 4 | 3.66 m | 69.8 CH | 3, 6 | ||
| 5 | 3.67 m | 79.0 CH | 3, 4 | ||
| 6 | 3.76 m 3.63 m | 62.0 CH2 | 4, 5 |
* δ value was abstracted from HMBC spectrum.

Table 2.
Antifungal and Cytotoxic Activities of Theonellamide G (1) a.
| Compound | C. albicans (W.T.) b | C. albicans (AmBR) c | HCT-116 |
|---|---|---|---|
| MIC (μM) | MIC (μM) | IC50 (μM) | |
| Theonellamide G (1) | 4.49 | 2.0 | 6.0 |
| Amphotericin B d | 1.48 | − | |
| Etoposide e | − | − | 2.0 |
a Upper limit on the antifungal assay is 500 μg/mL; b Wild type (ATCC 32354); c Amphotericin B-resistant type (ATCC 90873); d Positive antifungal control; e Positive cytotoxic control.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotation was measured on a JASCO DIP-370 digital polarimeter (Jasco Co., Tokyo, Japan) at 25 °C at the sodium D line (589 nm). UV spectrum was recorded on a Hitachi 300 spectrometer (Hitachi High-Technologies Corporation, Kyoto, Japan). The IR spectrum was measured on a Shimadzu Infrared-400 spectrophotometer (Shimadzu, Kyoto, Japan). NMR spectra were determined on BRUKER Unity INOVA 600 instruments (600 MHz for 1H and 150 MHz for 13C NMR) (Bruker BioSpin, Billerica, MA, USA). Positive HRFABMS spectrum was determined on a Finnigan MAT-312 spectrometer (ThermoFinnigan GmbH, Tokyo, Japan). HPLC purification was performed on a preparative RP C30 column (Develocil, C30-UG-5, 250 × 20 mm, Phenomenex) (Nomura Chemical, Setouchi-shi, Japan) using 25% n-propanol in water. Column chromatographic separation was carried out on Sephadex LH-20 (0.25–0.1 mm, Merck, Darmstadt, Germanyand ODS flash column chromatography (Yamazen Corporation, Osaka, Japan), while reversed phase chromatography was performed on YMC*Gel (ODS-AQ-HG, YMC Europe GmbH, Dinslaken, Germany). TLC analyses were conducted on pre-coated silica gel F254 aluminum sheets (layer thickness 0.2 mm, Merck, Darmstadt, Germany). Standard amino acids were purchased from Sigma-Aldrich Chemical Co. (Taufkirchen, Germany) and Trademax Pharmaceuticals & Chemicals Co., Ltd. (Shanghai, China). The solvent systems were used for TLC analyses; CHCl3:MeOH (9:1, S1) and CHCl3:EtOAc:MeOH:H2O (2.8:3.2:3.5:0.5, S2).
3.2. Animal Materials
The marine sponge was collected by scuba diving at a depth of 4–5 m of Hurghada in the Red Sea coast. The sponge is cylindrical in shape and dark red-brown in color. The cut-off fragment measures 7.5 cm high and 4.5 cm in diameter. It has a central canal of 1.5 cm diameter leading to a narrow vent with sphincter-like membrane at the top. The in-situ photo shows the vent to be similar in diameter as the central canal. The surface is slightly bumpy, generally smooth, but furrowed lengthwise. The ectosomal skeleton consists of a dense mass of curved acanthomicrorhabds of 15–24 × 2–3 μm, overlying a lose reticulation of reduced phyllotriaenes with cladome spanning 120–180 μm and thin undivided cladi 55–120 × 4–7 μm in size. A subectosomal region measuring about 1 mm in thickness bridges an area devoid of desmas, the skeleton of which consists of bundles of strongylotes, measuring 25–70 μm in diameter, enclosing 4–20 strongylotes. The latter are slightly anisotylote with either end more or less swollen, 405–620 × 3–6 μm in size. The choanosomal skeleton consists of a loose reticulation of tetraclone desmas strengthened by bundles of strongylotes. Desmas cladomes measure 400–550 μm, rhabds smooth, 120–230 × 15–20 μm, and cladi smooth with simple zygoses, 150–250 × 12–16 μm. Compared with the type specimen there are some differences (lighter skeletal, smooth instead of tuberculated desmas, shorter strongylotes) which are judged to be infraspecific variation. The voucher fragment is registered in the collection of the Zoological Museum of Amsterdam under registration number POR 16637 and in the Red Sea Invertebrates Collection at Faculty of Pharmacy, Suez Canal University, Ismailia, Egypt, under registration number DY-RS-59.
3.3. Extraction and Purifications of Compound 1
The frozen sponge materials (1.5 kg, wet weight) were extracted with a mixture of MeOH/CH2Cl2 (1:1) (3 × 1000 mL) at room temperature. The combined extracts were concentrated under reduced pressure and suspended in MeOH/H2O (9:1) (1000 mL). The resulting mixture was extracted with n-hexane (3 × 400 mL) to give 7.2 g of n-hexane residue. The remaining methanolic layer was diluted with H2O to (3:2) MeOH/H2O and then extracted with CH2Cl2 (3 × 400 mL) to give 2.4 g of CH2Cl2 residue. The CH2Cl2 residue was subjected to a Sephadex LH-20 column (Merck, Darmstadt, Germany) using MeOH as an eluent to afford nine fractions. Fraction 4 (730 mg) was subjected to ODS flash chromatography starting with 30% aqueous MeOH through pure MeOH to afford 10 subfractions. The potent antifungal subfraction eluted with 40% H2O in MeOH (subfraction 4) (86 mg) was subjected to final HPLC purification on a preparative C30 column (Develocil, C30-UG-5, 250 × 20 mm, Nomura Chemical, Setouchi-shi, Japan) using 25% n-propanol in water at a flow rate of 5.5 mL/min to afford compound 1 (11.5 mg).
Theonellamide G (1): White amorphous powder; 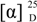 +15.85° (C 0.42, MeOH:H2O, 4:1); UV (λmax, MeOH) (log ε) 289 (4.82), 306 (3.57) nm; IR νmax (KBr) 3324, 2965, 1655, 1062 cm−1; NMR data, see Table 1; HRFABMS m/z 1733.5983 (calcd for C75H9879BrN16O27, 1733.5970, [M + H]+).
+15.85° (C 0.42, MeOH:H2O, 4:1); UV (λmax, MeOH) (log ε) 289 (4.82), 306 (3.57) nm; IR νmax (KBr) 3324, 2965, 1655, 1062 cm−1; NMR data, see Table 1; HRFABMS m/z 1733.5983 (calcd for C75H9879BrN16O27, 1733.5970, [M + H]+).
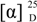 +15.85° (C 0.42, MeOH:H2O, 4:1); UV (λmax, MeOH) (log ε) 289 (4.82), 306 (3.57) nm; IR νmax (KBr) 3324, 2965, 1655, 1062 cm−1; NMR data, see Table 1; HRFABMS m/z 1733.5983 (calcd for C75H9879BrN16O27, 1733.5970, [M + H]+).
+15.85° (C 0.42, MeOH:H2O, 4:1); UV (λmax, MeOH) (log ε) 289 (4.82), 306 (3.57) nm; IR νmax (KBr) 3324, 2965, 1655, 1062 cm−1; NMR data, see Table 1; HRFABMS m/z 1733.5983 (calcd for C75H9879BrN16O27, 1733.5970, [M + H]+). 3.4. Acid Hydrolysis and Absolute Configuration of Amino Acids Using LC-MS Analysis of the Marfey Derivatives of 1
Compound 1 (1.0 mg) was treated with 2 mL 6 N HCl (pa) and heated in sealed ampoule at 110 °C for 24 h under N2 gas. The resulting solution was concentrated, with consecutive addition of H2O (5 mL) to ensure complete elimination of HCl. To 50 μL of acid hydrolysate (or authentic amino acid standard at comparable concentration), 100 μL FDNPL (1% N-(5-flouro-2,4-dinitrophenyl)-l-leucinamide in acetone) and 20 μL 1 M NaHCO3 were added. The mixture was heated at 40 °C for 1 h over a hot plate with frequent mixing. After cooling, 10 μL of 2 M HCl was added and then concentrated to dryness before dissolving in 1000 μL MeOH. Standards of l and d amino acids were treated separately with FDNPL in the same manner. The FDNPL derivatives were analyzed using LC-MS by comparison of the retention time and molecular weight with those of standard amino acids FDNPL derivatives [22,23].
3.5. Chiral GC-MS Analysis of 1
About 0.2 mg of 1 was placed in sealed ampoule containing 6 N HCl (0.5 mL) and heated at 110 °C for 12 h. After evaporation of the solvent under a stream of N2 gas, the residue was dissolved in 10% HC1/MeOH and heated at 100 °C for 30 min. The product was evaporated, dissolved in trifluoroacetic anhydride (50 μL) and CH2C12 (50 μL), reacted at 100 °C for 10 min, and evaporated in a stream of N2 gas. The residue was dissolved in EtOAc (100 μL). Aliquot (30 μL) was injected into a Hewlett-Packard 5890 GC-MS (Hewlett-Packard, Cary, NC, USA) fitted with an Alltech Chirasil-l-Val capillary column (Varian, Palo Alto, CA, USA). The temperature was ramped form 60 °C to 210 °C over a period of 45 min. The retention time (tR, min) of the residues in the hydrolysate of 1 matched standards for l-alloThr (14.02; d-alloThr, 12.785), l-Ser (13.42; d-Ser, 12.365), l-Asn (15.78; d-Asn, 15.34), l-Phe (22.967; d-Phe, 22.124), l-BrPhe (32.104; d-BrPhe, 31.68), (2S)-iSer (16.344; (2R)-iSer, 16.152), and d-Gal (18.582; l-Gal, 18.982).
3.6. Evaluation of Cytotoxic Activity
Cytotoxicity was tested against human colon adenocarcinoma (HCT-116) cancer cell line by using the MTT [17,24]. The cells were incubated overnight at 37 °C in 5% CO2/air in microtiter plates. Tested compound, etoposide (positive control), and DMSO (negative control) were added to the top row of a 96-well microtiter plate and serially diluted (1:4) downward. After a 72 h incubation, cell viability was determined colorimetrically using a Molecular Devices Emax microplate reader (490 nm), recording the amount of MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium) reduced to formazan using the Cell Titer 96 AQueous non radioactive cell proliferation protocol (Promega,Madison, WI, USA). Minimum inhibitory concentration (IC50, μM) values were calculated using the program SOFTmax PRO (Molecular Devices, Sunnyvale, CA, USA). The results were shown in Table 2.
3.7. Antifungal Assay with C. albicans
The minimal inhibitory concentration (MIC; lowest concentration of the compound able to inhibit microorganism growth) of compound 1 was evaluated against two strains of Candida albicans ATCC 32354 (wild type) and ATCC 90873 (amphotericin B-resistant). These strains were purchased from the American Type Culture Collection (ATCC). Inhibitory activity was determined by a standard microdilution liquid antifungal assay [25]. Candida albicans was incubated overnight at 37 °C in RPMI 1640 media (GibcoBRL, Invitrogen Corp, Carlsbad, CA, USA) and aliquots transferred to 96-well plates the next day. The indicator Alamar Blue was added to the C. albicans culture before they were transferred to the plates. Samples were added along with amphotericin B (Sigma, St. Louis, MO, USA) and DMSO (solvent) as positive and negative controls, respectively, and serially diluted. The plates were then incubated overnight for 14–16 h. Minimum inhibitory concentration (MIC) values were determined by the change in color from blue to pink of the media according to the indicator Alamar Blue. The results of the activity were shown in Table 2.
4. Conclusions
In conclusion, the investigation of the Red Sea sponge Theonella swinhoei led to isolation of a new bicyclic glycopeptide, theonellamide G (1). The structure was determined using extensive spectroscopic studies. Theonellamide G showed potent antifungal activity towards wild and amphotericin B-resistant strains of Candida albicans with IC50 of 4.49 and 2.0 μM, respectively, compared to 1.48 μM for the positive antifungal control amphotericin-B against the wild type. Additionally, it displayed cytotoxic activity against the human colon adenocarcinoma cell line (HCT-16) with IC50 of 6.0 μM, compared to 2.0 μM for etoposide (positive anticancer control).
Acknowledgements
This project was supported by the NSTIP strategic technologies program in the Kingdom of Saudi Arabia—Project No. (11-BIO1556-03). The authors also, acknowledge with thanks Science and Technology Unit, King Abdulaziz University for technical support.
Author Contributions
Conceived and designed the experiments: DTAY. Performed the experiments: DTAY LAS GAM JMB FHB. Analyzed the data: DTAY LAS GAM JMB FHB SRMI. Wrote the paper: DTAY SRMI.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Winder, P.L.; Pomponi, S.A.; Wright, A.E. Natural Products from the Lithistida: A Review of the Literature since 2000. Mar. Drugs 2011, 9, 2643–2682. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.G.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Harper, M.K.; Faulkner, D.J. Mozamides A and B, cyclic peptides from a Theonellid sponge from Mozambique. J. Nat. Prod. 1997, 60, 779–782. [Google Scholar] [CrossRef]
- Hamada, T.; Suyawara, T.; Matsunaga, S.; Fusetani, N. Polytheonamides, unprecedented highly cytotoxic polypeptides from the marine sponge Theonella swinhoei 2. Structure elucidation. Tetrahedron Lett. 1994, 35, 609–612. [Google Scholar] [CrossRef]
- Nakao, Y.; Oku, N.; Matsunaga, S.; Fusetani, N. Cyclotheonamides E2 and E3, new potent serine protease inhibitors from the marine sponge of the genus Theonella. J. Nat. Prod. 1998, 61, 667–670. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kanzaki, K.; Katayama, S.; Ohashi, K.; Okada, H.; Ikegami, S.; Kitagawa, I. Marine natural products. XXXIII. Theonellapeptolide IId, a new tridecapeptide lactone from the Okinawan marine sponge Theonella swinhoei. Chem. Pharm. Bull. 1994, 42, 1410–1415. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N. Theonellamides A–E, cytotoxic bicyclic peptides, from a marine sponge Theonella sp. J. Org. Chem. 1995, 60, 1177–1181. [Google Scholar] [CrossRef]
- Bewley, C.A.; Faulkner, D.J. Theonegramide, an antifungal glycopeptides from the Philippine Lithistid Sponge Theonella swinhoei. J. Org. Chem. 1994, 59, 4849–4852. [Google Scholar] [CrossRef]
- Kobayashi, J.; Itagaki, F.; Shigemori, H.; Takao, T.; Shimonishi, Y. Keramamides E, G, H, and J, new cyclic peptides containing an oxazole or a thiazole ring from a Theonella sponge. Tetrahedron 1995, 51, 2525–2532. [Google Scholar] [CrossRef]
- de Silva, E.D.; Williams, D.E.; Andersen, R.J.; Klix, H.; Holmes, C.F.B.; Allen, T.M. A potent protein phosphatase inhibitor isolated from the Papau New Guinea sponge Theonella swinhoei Gray. Tetrahedron Lett. 1992, 33, 1561–1564. [Google Scholar]
- Schmidt, E.W.; Faulkner, D.J. Microsclerodermins C–E, antifungal cyclic peptides from the lithistid sponges Theonella sp. and Microscleroderma sp. Tetrahedron 1998, 54, 3043–3056. [Google Scholar] [CrossRef]
- Bonnington, L.S.; Tanaka, J.; Higa, T.; Kimura, J.; Yoshimura, Y.; Nakao, Y.; Yoshida, W.Y.; Scheuer, P.J. Cupolamide A: A cytotoxic cyclic heptapeptide from two samples of the sponge Theonella cupola. J. Org. Chem. 1997, 62, 7765–7767. [Google Scholar] [CrossRef]
- Chill, L.; Kashman, Y.; Schleyer, M. Oriamide, a new cytotoxic cyclic peptide containing a novel amino acid from the marine sponge Theonella sp. Tetrahedron 1997, 53, 16147–16152. [Google Scholar] [CrossRef]
- Clark, D.P.; Carroll, J.; Naylor, S.; Crews, P. An antifungal cyclodepsipeptide, cyclolithistide A from the sponge Theonella swinhoei. J. Org. Chem. 1998, 63, 8757–8764. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Bewley, C.A.; Faulkner, D.J. Theopalauamide, a bicyclic glycopeptide from filamentous bacterial symbionts of the Lithistid sponge Theonella swinhoei from Palau and Mozambique. J. Org. Chem. 1998, 63, 1254–1258. [Google Scholar] [CrossRef]
- Ford, P.W.; Gustafson, K.R.; McKee, T.C.; Shigematsu, N.; Maurizi, L.K.; Pannell, L.K.; Williams, D.E.; de Silva, E.D.; Lassota, P.; Allen, T.M.; et al. Papuamides A–D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Mooberry, S.L. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the Red Sea sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 154–157. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Hashimoto, K.; Wӓlchlit, M. A novel antifungal bicyclic peptide from a marine sponge Theonella sp. J. Am. Chem. Soc. 1989, 111, 2582–2588. [Google Scholar] [CrossRef]
- Nishimura, S.; Arita, Y.; Honda, M.; Iwamoto, K.; Matsuyama, A.; Shirai, A.; Kawasaki, H.; Kakeya, H.; Kobayashi, T.; Matsunaga, S.; et al. Marine antifungal theonellamides target 3β-hydroxysterol to activate Rho1 signaling. Nat. Chem. Biol. 2010, 6, 519–526. [Google Scholar] [CrossRef]
- Espiritu, R.A.; Matsumori, N.; Murata, M.; Nishimura, S.; Kakeya, H.; Matsunaga, S.; Yoshida, M. Interaction between the marine sponge cyclic peptide theonellamide A and sterols in lipid bilayers as viewed by surface plasmon resonance and solid-state (2)H nuclear magnetic resonance. Biochemistry 2013, 52, 2410–2418. [Google Scholar] [CrossRef]
- Nishimura, S.; Ishii, K.; Iwamoto, K.; Arita, Y.; Matsunaga, S.; Ohno-Iwashita, Y.; Sato, S.B.; Kakeya, H.; Kobayashi, T.; Yoshida, M. Visualization of sterol-rich membrane domains with fluorescently-labeled theonellamides. PLoS One 2013, 8, e83716. [Google Scholar]
- Ibrahim, S.R.M.; Min, C.C.; Teuscher, F.; Ebel, R.; Kakoschke, C.; Lin, W.; Wray, V.; Edrada-Ebel, R.; Proksch, P. Callyaerins A–F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg. Med. Chem. 2010, 18, 4947–4956. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Edrada-Ebel, R.; Mohamed, G.A.; Youssef, D.T.A.; Wray, V.; Proksch, P. Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. ARKIVOC 2008, 2008, 164–171. [Google Scholar] [CrossRef]
- Soria-Mercado, I.E.; Prieto-Davo, A.; Jensen, P.R.; Fenical, W. Antibiotic terpenoid chlorodihydroquinones from a new marine actinomycete. J. Nat. Prod. 2005, 68, 904–910. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 8th ed.; CLSI Document M7-A8; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
