Abstract
A novel spirocyclic drimane coupled by two drimane fragment building blocks 2 and a new drimane 1 were identified in mycelia and culture broth of Stachybotrys sp. MF347. Their structures were established by spectroscopic means. This is the first example of spirocyclic drimane coupled by a spirodihydrobenzofuranlactam unit and a spirodihydroisobenzofuran unit; and the connecting position being N-C instead of an N and N connecting unit. Strain MF347 produced also the known spirocyclic drimanes stachybocin A (12) and stachybocin B (11) featured by two sesquiterpene-spirobenzofuran structural units connected by a lysine residue; the known spirocyclic drimanes chartarlactam O (5); chartarlactam K (6); F1839A (7); stachybotrylactam (8); stachybotramide (9); and 2α-acetoxystachybotrylactam acetate (10); as well as ilicicolin B (13), a known sesquiterpene. The relative configuration of two known spirobenzofuranlactams (3 and 4) was determined. All compounds were subjected to biological activity tests. The spirocyclic drimane 2, 11, and 12, as well as the sesquiterpene 13, exhibited antibacterial activity against the clinically relevant methicillin-resistant Staphylococcus aureus (MRSA).
1. Introduction
The fungal genus Stachybotrys (class: Sordariomycetes, order: Hypocreales) comprises approximately 100 species [1]. Members of Stachybotrys spp. are distributed worldwide and are commonly isolated from soil and various decaying plant substrates. Most species are able to decompose cellulose efficiently. Dangerous toxinogenic isolates of the Stachybotrys chartarum complex have gained importance, when colonizing cellulosic substrates in moist indoor environments [2]. S. chartarum is reported to be involved in animal and human toxicoses, which are associated with “sick building syndrome” in wet buildings [3,4].
Marine isolates of Stachybotrys spp. have been gained from various marine environments as the rhizosphere of mangroves, soil and mud of the intertidal zone, intertidal pools, brackish waters, marine sediments and sponges, marine algae, and sea fans [5,6,7,8,9,10,11,12].
During the course of a study of isolates of S. chartarum obtained from various areas around the world, spirocyclic drimanes were discovered as the major class of secondary metabolites produced by this fungus [3]. This type of compound has been reported to be produced by other species of Stachybotrys and members of this class are potent immunosuppressants, particularly the dialdehyde derivatives [13]. However, dialdehyde derivatives were rarely isolated from the genus Stachybotrys, since dialdehydes are relatively unstable and often undergo conversion to stachybotrylactones [14]. Although many spirocyclic drimanes were reported to be toxins, they showed various biological effects, such as immunosuppressive activity [13], endothelin receptor antagonistic activity [15,16], and tyrosine kinase inhibitory activity [17].
Fungi from marine habitats, living in a stressful habitat, are of great interest as new promising sources of biologically active products. As marine organisms live in a biologically competitive environment with unique conditions of pH, temperature, pressure, oxygen, light, nutrients, and salinity, the chemical diversity of the secondary metabolites from marine fungi is considerably high [18,19,20]. As far as is known, only two studies focused on biologically active compounds produced by Stachybotrys spp. strains originated from marine habitats and identified stachybotrin A and stachybotrin B, as well as a fibrinolytic active compound of unknown structure [8,12]. In order to find new natural products from marine microorganisms exhibiting, e.g., antibacterial and cytototoxic activities, Stachybotrys sp. strain MF347 was cultured and the secondary metabolites in the mycelia and the culture broth were investigated. A novel spirocyclic drimane coupled by two drimane fragment building blocks and a new drimane were identified. This is the first example of spirocyclic drimane coupled by a spirodihydrobenzofuranlactam unit and a spirodihydroisobenzofuran unit, and the connecting position being N–C instead of an N and N connecting unit (Chart 1).
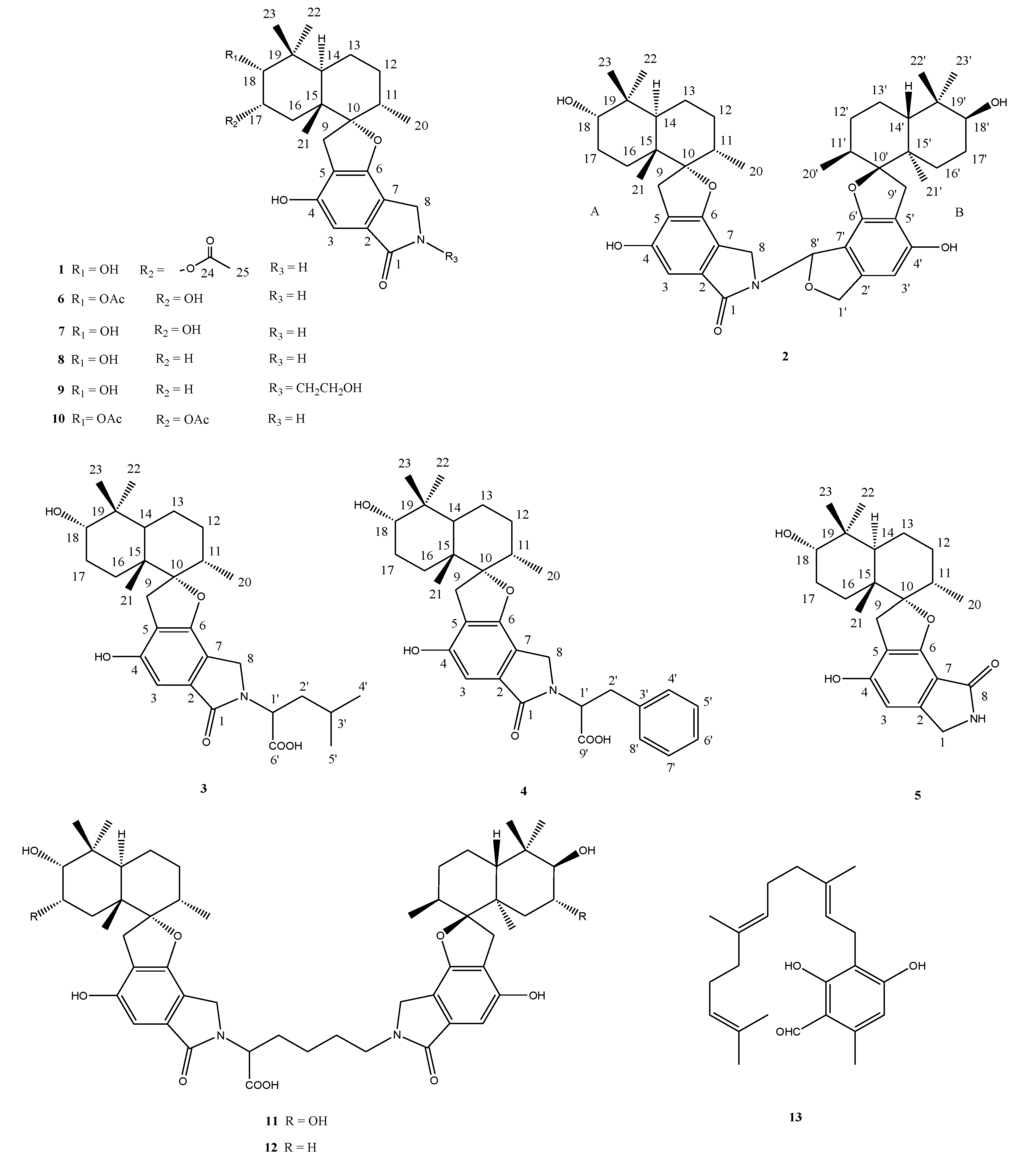
Chart 1.
Structures of compounds 1–13.
2. Results and Discussion
2.1. Identification of Strain MF347
The fungus was isolated by Dr. Karsten Schaumann from a marine driftwood sample and could be taxonomically classified as a Stachybotrys species. Colonies growing on WSP30 agar attaining a diameter of 40 mm within 14 days of incubation at 26 °C. Colonies were greyish brown with a brown back side (Figure 1). Ellipsoidal conidia were produced by clusters of phialides in slimy masses at the top of conidiophores (Figure 2). These morphological features are characteristic for the genus Stachybotrys, including the well-known species Stachybotrys chartarum.
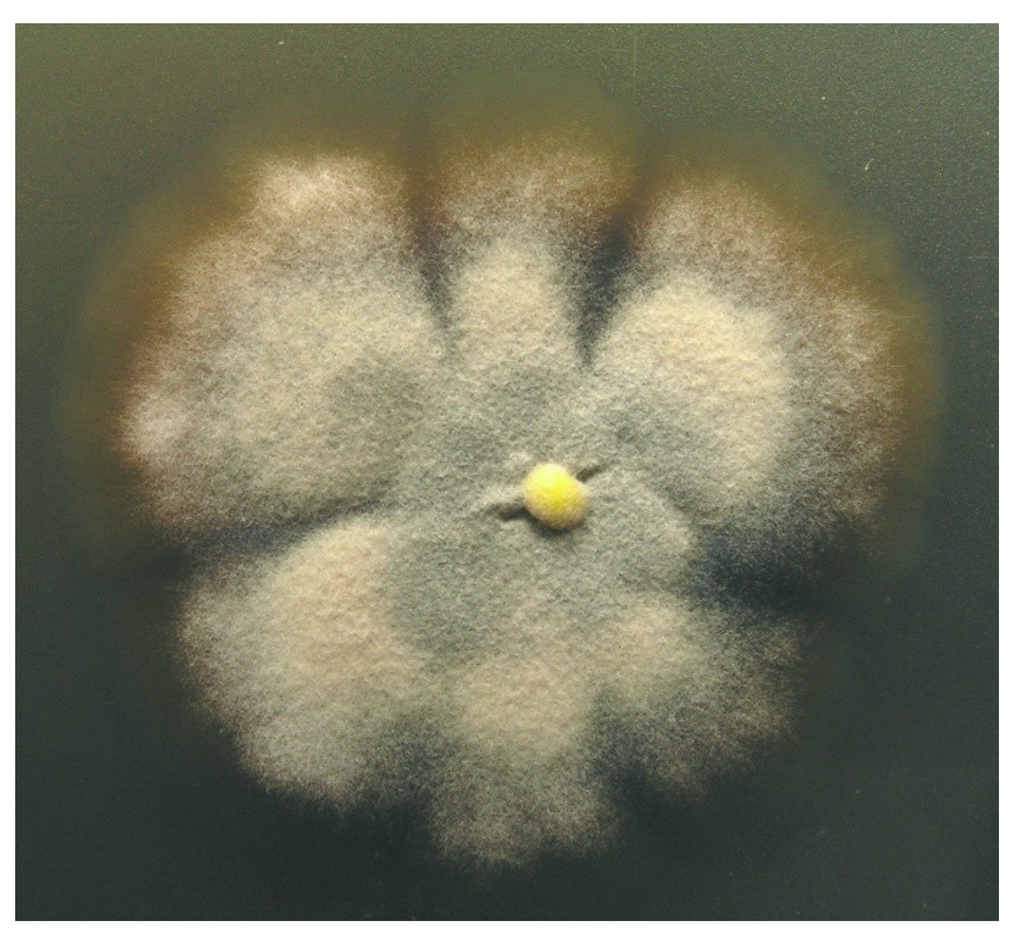
Figure 1.
Colony of Stachybotrys sp. MF347, grown for 14 days at WSP30 agar.
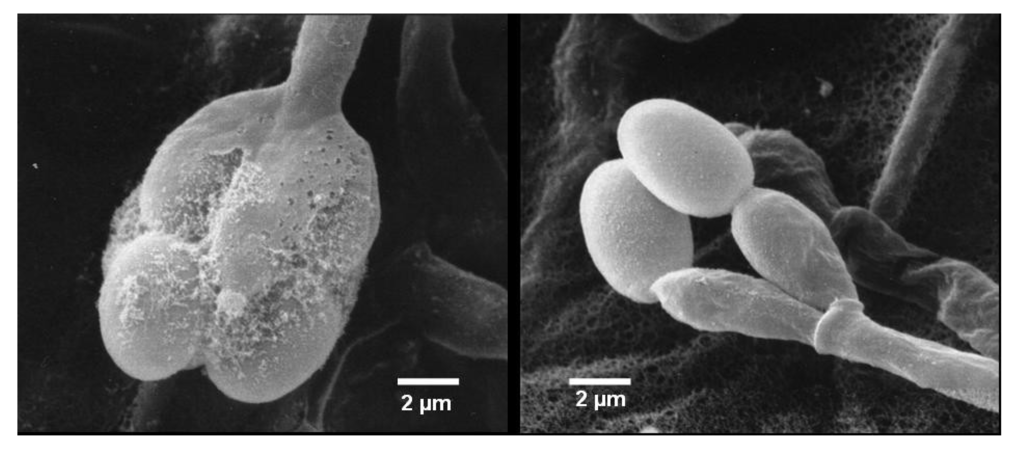
Figure 2.
Stachybotrys sp. MF347, scanning electron micrograph showing conidiophore with phialides and conidia.
2.2. Structural Elucidation
Compound 1 was isolated as a yellow powder. The molecular formula was determined to be C25H33NO6 by analysis of the HR-TOF-MS ion peak at m/z 466.2181 [M + Na]+ (calcd. 466.2200). The IR spectrum suggested the presence of an α,β-unsaturated γ-lactam (1673 cm−1) group. 1H and 13C NMR spectrum (Table 1) showed signals in close agreement with those of the known spirodihydrobenzofuranlactam, F 1839 A (7) [21], isolated from the same fungus, except that the acetoxyl group was added in the molecule of 1. Analysis of the 1D and 2D NMR data (Figure 3) and comparison with those of F 1839 A led to the identification of the planar structure of 1 as drawn [21]. The acetoxyl group was positioned at C-17 from the observation of the HMBC cross peak from the oxygenated proton signal at δH 5.21 (ddd, J = 12.6, 4.4, 2.6 Hz, H-17) to the acetoxyl carbon signal at δC 172.6 (s, C-24). The 1,3-diaxial NOESY cross peaks of H-17β/Me-23, H-17β/Me-21β, Me-21β/H-11β revealed a 9.14-trans ring fusion between two cylcohexyl chair rings of the bicyclic decalin with a β-oriented methyl group at C-15 and α-oriented methyl group at C-11 (Figure 4). The NOESY correlations from the axial methyl proton signals at δH 1.17 (s, Me-21) to the one of the methylene proton signal at δH 3.25 (d, J = 17.0 Hz, H-9a), and from axial proton signal at δH 1.91 (m, H-11) to one of the methylene proton signal at δH 2.93 (d, J = 17.0 Hz, H-9b) indicated a β-oriented CH2 and an α-oriented oxygen atom bridge at the spirocyclic carbon center of C-10. The diagnostic NOESY correlation from the proton signal at δH 5.21 (ddd, J = 12.6, 4.4, 2.6 Hz, H-17β) to the proton signal at δH 3.48, (d, J = 2.1 Hz, H-18β) revealed that the both oxygen at C-17 and C-18 were α-oriented. The relatively small coupling constant between H-17 and H-18 of 2.1 Hz and large coupling constant between H-17 and H-16 of 12.6 Hz confirmed an equatorial β-oriented H-18 and an axial β-oriented H-17 in 1. Thus, compound 1 was identified as a new spirodihydrobenzofuranlactam, and given the trivial name stachyin A.

Table 1.
NMR data (500 MHz) for compound 1 in CD3OD.
| Position | 1 | |
|---|---|---|
| δC a,b, mult. | δH c, mult. (J in Hz) | |
| 1 | 174.0, C | - |
| 2 | 134.9, C | - |
| 3 | 102.3, CH | 6.74, s |
| 4 | 155.2, C | - |
| 5 | 118.6, C | - |
| 6 | 157.5, C | - |
| 7 | 116.8, C | - |
| 8 | 43.9, CH2 | 4.47, d (17.4), 4.31, d (17.4) |
| 9 | 33.1, CH2 | 3.25, d (17.0), 2.93, d (17.0) |
| 10 | 99.2, C | - |
| 11 | 38.0, CH | 1.91, m |
| 12 | 32.1, CH2 | 1.66, 1.56, 2 m |
| 13 | 21.7, CH2 | 1.54, 1.62, 2 m |
| 14 | 40.8, CH | 2.15, m |
| 15 | 44.8, C | - |
| 16 | 30.8, CH2 | 1.96, t (12.3), 1.36, dd (12.3, 4.4) |
| 17 | 72.0, CH | 5.21, ddd (12.3, 4.4, 2.1) |
| 18 | 77.0, CH | 3.48, d (2.1) |
| 19 | 39.9, C | - |
| 20 | 15.8, CH3 | 0.78, d (6.6) |
| 21 | 17.3, CH3 | 1.17, s |
| 22 | 29.1, CH3 | 0.99, s |
| 23 | 22.5, CH3 | 1.07, s |
| 24 | 172.6, C | - |
| 25 | 21.2, C | 1.99, s |
a Recorded at 125 MHz; b multiplicities inferred from DEPT and HMQC experiments; c recorded at 500 MHz.
Figure 3.
Key 1H 1H COSY and HMBC correlations of compounds 1 and 2.
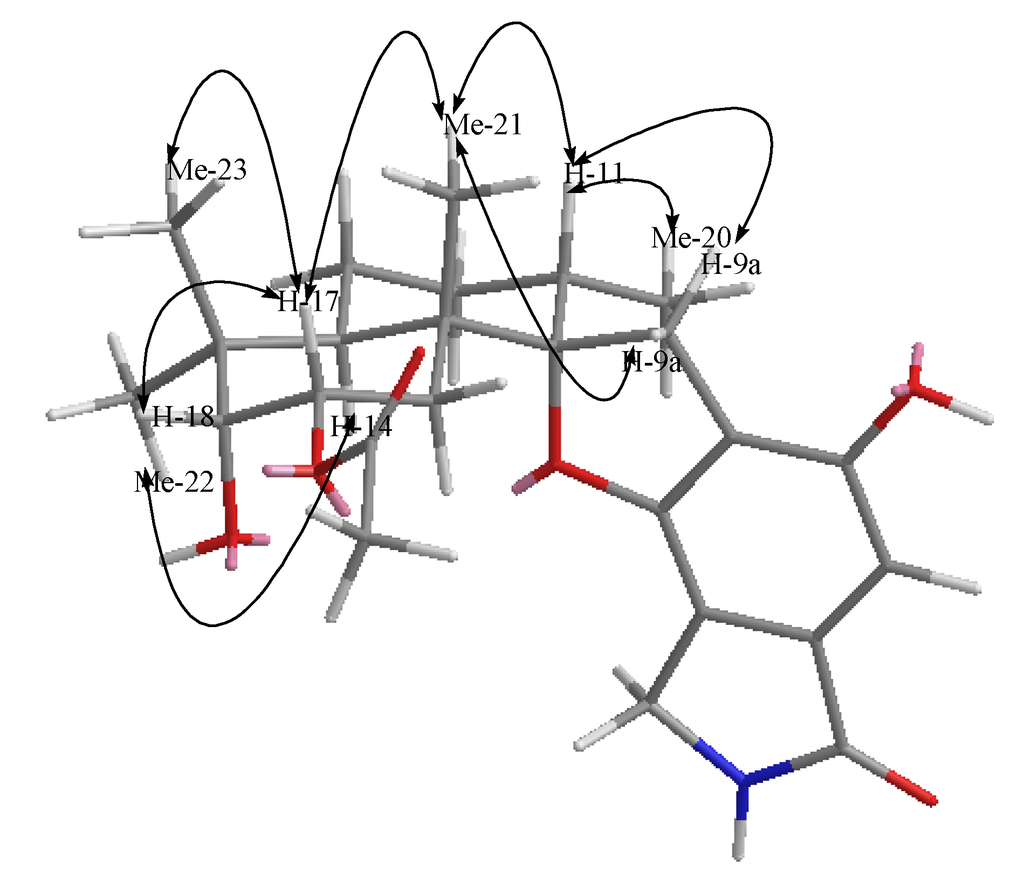
Figure 4.
Key NOESY correlations of compound 1.
Compound 2 was obtained as a yellow powder. The HR-TOF-MS exhibited an ion peak at m/z 778.4319 [M + Na]+ (calcd. 778.4289), corresponding to the molecular formula, C46H61NO8. The characteristic feature of the 13C NMR spectrum of 2 is the presence of 15 pairs of identical or almost equivalent carbon signals, which are assigned to two sesquiterpene units. When comparing the NMR data of 1 (Table 2) with those of stachybotrylactam (8), compound 2 was deduced to be the coupling of two units of spirodihydrobenzofuranlactam/lactone with one stachybotrylactam substructure. The remaining half of the molecule was proved by detailed analysis of 1D and 2D NMR spectra of 2 to have the same sesquiterpene part in its structure (Figure 3). From the analysis of the molecule formula, C46H61NO8, the remaining unit was deduced to be a spirodihydrobenzofuranlactone or spirodihydrobenzofuran instead of spirodihydrobenzofuranlactam. The presence of the oxygenated methine (δC 85.8, δH 7.24) and oxygenated methylene (δC 74.0, δH 5.21, 5.00) implied that the structure of 2 possessed a dihydroisobenzofuran unit in one of the two spirocyclic drimane domains. Detailed analyzes of 2D NMR spectra (1H 1H COSY, HSQC and HMBC) permitted the construction of the two units, and their connection (Figure 3). The HMBC peaks from the proton signal at δH 7.24 (br d, J = 2.1 Hz, H-8′) in the dihydroisobenzofuran unit to the carbon at δC 44.3 (t, C-8) in the spirodihydrobenzofuranlactam indicated that the linkage position of the two units was from N to C-8 as drawn. The relative configuration of the sesquiterpene parts of both monomers of 2 was deduced from the analysis of the NOESY spectrum to be the same as those in compounds 1 and the known stachybotrylactam (8) (see Supplementary Information). Therefore, the structure of this compound was elucidated as a new spirocyclic drimane coupled by two drimane fragment building blocks and given the trivial name stachyin B. Most dimeric spirodihydrobenzofuranlactams isolated from natural resources are those whose the monomers are connected by a N and N connecting unit, such as N–C(OOH)CH2CH2CH2CH2–N in compounds 11 and 12 [16]. The structure of 2 is the first example of spirocyclic drimane coupled by a spirodihydrobenzofuranlactam unit and a spirodihydroisobenzofuran unit, and the connecting position being an N–C instead of an N and N connecting unit.

Table 2.
NMR data (500 MHz) for compound 2 in CD3OD.
| Position | Unit A | Position | Unit B | ||
|---|---|---|---|---|---|
| δC a,b, mult. | δH c, mult. (J in Hz) | δC a,b, mult. | δH c, mult. (J in Hz) | ||
| 1 | 171.1, C | - | 1′ | 74.0, CH2 | 5.21 dd (12.2, 2.1), 5.00, d (12.2) |
| 2 | 134.8, C | - | 2′ | 143.2, C | - |
| 3 | 102.4, CH | 6.77, s | 3′ | 100.2, CH | 6.29, s |
| 4 | 155.3, C | - | 4′ | 156.6, C | - |
| 5 | 119.3, C | - | 5′ | 113.8, C | - |
| 6 | 157.6 d, C | - | 6′ | 157.7 d, C | - |
| 7 | 114.4, C | - | 7′ | 109.1, C | - |
| 8 | 44.3, CH2 | 4.30, d (16.8), 3.86, d (16.8) | 8′ | 85.8, CH | 7.24, br d (2.1) |
| 9 | 33.0, CH2 | 3.23, d (17.0), 2.85, d (17.0) | 9′ | 32.4, CH2 | 3.15 d (16.2), 2.76 d (16.2) |
| 10 | 99.6, C | - | 10′ | 99.6, C | - |
| 11 | 38.5, CH | 1.84, m | 11′ | 38.0, CH | 1.72, m |
| 12 | 32.2, CH2 | 1.54, 1.44, overlap | 12′ | 32.0, CH2 | 1.23, 0.80, 2m |
| 13 | 22.0, CH2 | 1.54, 1.39, overlap | 13′ | 22.0, CH2 | 1.54, 1.39, overlap |
| 14 | 41.4, CH | 2.05, m | 14′ | 41.2, CH | 1.99, m |
| 15 | 43.4, C | - | 15′ | 43.5, C | - |
| 16 | 25.3, CH2 | 1.80, 1.15, overlap | 16′ | 25.3, CH2 | 1.80, 1.15, overlap |
| 17 | 26.1 e, CH2 | 1.96, 1.55, overlap | 17′ | 26.0 e, CH2 | 1.96, 1.55, overlap |
| 18 | 76.5, CH | 3.34, overlap | 18′ | 76.7, CH | 3.34, overlap |
| 19 | 38.0, C | - | 19′ | 38.0, C | - |
| 20 | 16.0, CH3 | 0.67, d (6.5) | 20′ | 16.1, CH3 | 0.49, d (6.5) |
| 21 | 16.5, CH3 | 1.10, s | 21′ | 16.5, CH3 | 1.02, s |
| 22 | 29.0 f, CH3 | 0.98, s | 22′ | 28.8 f, CH3 | 0.96, s |
| 23 | 23.1 g, CH3 | 0.89, s | 23′ | 23.0 g, CH3 | 0.87, s |
a Recorded at 125 MHz; b multiplicities inferred from DEPT and HMQC experiments; c recorded at 500 MHz; d,e,f,g interchangeable.
Compounds 3 and 4 were obtained both as yellow powder. From the detailed analyzes of their MS and NMR spectrum, the structures of 3 and 4 were deduced to be the same compounds patented [22]. No names have been given for these two compounds by the authors, but sum formula and a graphic presentation of the structures were shown. However, no relative configuration and detailed NMR data were reported [13]. Here, we reported the NMR data (Table 3), 2D NMR correlations (Figure 3) and the relative configuration (Chart 1) of the two compounds.
Furthermore, six known spirocyclic drimanes, chartarlactam O [23], chartarlactam K [23], F1839A (7) [21], stachybotrylactam (8) [3], stachybotramide (9) [3] and 2α-acetoxystachybotrylactam acetate (10) [1], and two known spirocyclic drimanes featured by two sesquiterpene-spirobenzofuran structural units connected by a lysine residue, stachybocin B (11) [16] and stachybocin A (12) [16], and a known sesquiterpene, ilicicolin B (13) [24] were identified by comparison of their spectroscopic data with those reported in the literature [3,16,21,23,24].

Table 3.
NMR data (500 MHz) for compound 3 and 4 in CD3OD.
| Position | 3 | 4 | ||
|---|---|---|---|---|
| δC a,b, mult. | δH c, mult. (J in Hz) | δC a,b, mult. | δH c, mult. (J in Hz) | |
| 1 | 171.8, C | - | 171.7, C | - |
| 2 | 134.4, C | - | 134.4, C | - |
| 3 | 102.2, CH | 6.72, s | 102.1, CH | 6.63, s |
| 4 | 155.2, C | - | 155.1, C | - |
| 5 | 119.2, C | - | 119.0, C | - |
| 6 | 157.6, C | - | 157.5, C | - |
| 7 | 114.6, C | - | 114.6, C | - |
| 8 | 45.7, CH2 | 4.64, d (16.8), 4.30, d (16.8) | 46.2, CH2 | 4.58, d (16.8), 4.29, d (16.8) |
| 9 | 32.2, CH2 | 3.27, d (16.9), 2.88, d (16.9) | 32.2, CH2 | 3.24, d (16.9), 2.83, d (16.9) |
| 10 | 99.8, C | - | 99.7, C | - |
| 11 | 38.5, CH | 1.88, m | 38.5, CH | 1.88, m |
| 12 | 33.0, CH2 | 1.62, 1.57, 2 m | 33.0, CH2 | 1.62, 1.55, 2 m |
| 13 | 22.1, CH2 | 1.51, 1.59, 2 m | 22.1, CH2 | 1.53, 1.59, overlap |
| 14 | 41.4, CH | 2.16, dd (11.7, 2.5) | 41.4, CH | 2.13, m |
| 15 | 43.5, C | - | 43.4, C | - |
| 16 | 25.4, CH2 | 1.86, 1.10, 2 m | 25.4, CH2 | 1.88, 1.12, 2 m |
| 17 | 26.0, CH2 | 2.00, 1.56, 2 m | 26.0, CH2 | 2.00, 1.55, 2 m |
| 18 | 76.5, CH | 3.37, t (2.5) | 76.5, CH | 3.36, t (2.5) |
| 19 | 38.6, C | - | 38.6, C | - |
| 20 | 16.0, CH3 | 0.76, d (6.6) | 15.9, CH3 | 0.70, d (6.6) |
| 21 | 16.6, CH3 | 1.08, s | 16.6, CH3 | 1.07, s |
| 22 | 29.0, CH3 | 1.01, overlap | 29.0, CH3 | 1.01, s |
| 23 | 23.0, CH3 | 0.91, s | 23.0, CH3 | 0.92, s |
| 1′ | 53.0, CH | 5.02, dd (11.4, 4.4) | 57.0, CH | 5.27, dd (11.1, 5.1) |
| 2′ | 39.4, CH2 | 1.98 overlap | 36.6, CH2 | 3.55, dd (14.8, 5.1), 3.26 (11.1, 5.0) |
| 3′ | 26.3, CH | 1.51 overlap | 138.7, C | - |
| 4′ | 21.4, CH3 | 1.01, d (6.9) | 129.6, CH | 7.28, dd (8.0, 2.1) |
| 5′ | 23.5, CH3 | 1.02, d (6.9) | 129.6, CH | 7.25, td (8.0, 2.1) |
| 6′ | 172.0 d, C | - | 127.7, CH | 7.17, tt (8.0, 2.1) |
| 7′ | - | - | 129.6, CH | 7.25, td (8.0, 2.1) |
| 8′ | - | - | 129.6, CH | 7.28, dd (8.0, 2.1) |
| 9′ | - | - | 174.0 d, C | - |
a Recorded at 125 MHz; b multiplicities inferred from DEPT and HMQC experiments; c recorded at 500 MHz. d inferred from HMBC.
2.3. Biological Activities
Among the isolates, stachyin B (2) inhibited the growth of the Gram-positive test strains Bacillus subtilis, Staphylococcus epidermidis and the methicillin-resistant Staphylococcus aureus (MRSA) with IC50 values in the range of 1–1.7 µM (Table 4). Stachybocin A (12) and B (11) exhibited IC50 values in the range of 1.8–4.4 µM against the three Gram-positive strains. The activities observed for ilicicolin B (13) were in a similar range with IC50 values of 0.7–3.2 µM. Chloramphenicol was used as positive control for B. subtilis as well as for the human pathogenic strains, S. epidermidis and S. aureus, and revealed IC50 values of 1.45 (±0.13) µM, 1.81 (±0.04) µM, and 2.46 (±0.4) µM, respectively. The results indicate, that the new compound 2 and the known Stachybotrys sp. metabolites 11, 12, and 13 showed comparable activities with chloramphenicol. Among the antimicrobial active compounds 2, 11, 12, and 13, only stachyin B (2) and ilicicolin (13) showed weak cytotoxic activities with IC50 values in the range of 13–30 µM. The new compound 2 exhibited similar IC50 values in comparison to the positive control tamoxifen citrate, which revealed an IC50 value of 16.45 (±0.15) µM for the mouse fibroblasts cell line NIH-3T3 and of 17.87 (±0.5) µM for the carcinoma cell line HepG2.

Table 4.
Antibiotic and cytotoxic activities of the compounds 1–13. The IC50 values are given in µM. Compounds 1 and 3–10 exhibited IC50 values >50 µM in all assays.
| No. | Name | Antibiotic Activity | Cytotoxic Activity | |||
|---|---|---|---|---|---|---|
| B. subtilis | S. epidermidis | S. aureus MRSA | NIH-3T3 | HepG2 | ||
| 2 | stachyin B | 1.42 (±0.07) | 1.02 (±0.09) | 1.75 (±0.09) | 13.01 (±0.46) | 14.27 (±1.54) |
| 11 | stachybocin B | 1.77 (±0.32) | 4.44 (±0.28) | 3.94 (±0.53) | >50 | >50 |
| 12 | stachybocin A | 2.03 (±0.23) | 2.84 (±0.35) | 3.71 (±0.22) | >50 | >50 |
| 13 | ilicicolin B | 1.06 (±0.11) | 3.18 µM (±0.33) | 0.74 (±0.12) | 30.00 (±1.20) | >50 |
The Gram-negative test strain Klebsiella pneumoniae as well as the fungal strains Candida albicans, Trichophyton rubrum, and Septoria tritici were not inhibited by the compounds 1–13. All compounds exhibited no worth mentioning activities in the enzymatic assays. The IC50 values are >50 µM for the acetylcholinesterase and >10 µM for the glycogen-synthase-kinase 3β and phosphodiesterase 4B2.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were recorded on a Perkin Elmer 241 polarimeter (PerkinElmer Inc., Rodgau, Germany). The IR spectra were run on a Perkin Elmer spectrometer (PerkinElmer Inc.) with an ATR unit. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were measured at 25 °C on a Bruker AVANCE DRX 500 NMR spectrometer (Bruker Daltonics, Bremen, Germany) with TMS as internal standard. The signals of the residual solvent protons and the solvent carbons were used as internal references (δH 3.31 ppm and δC 49.0 ppm for methanol-d4). High-resolution mass spectra were acquired on a benchtop time-of-flight spectrometer (micrOTOF II, Bruker Daltonics) with positive electrospray ionization (ESI).
Analytical reversed phase HPLC-UV/MS experiments were performed using a C18 column (Phenomenex Onyx Monolithic C18, 100 × 3.00 mm, Phenomenex Inc., Aschaffenburg, Germany) and applying an H2O/acetonitrile (ACN) gradient with 0.1% formic acid added to both solvents (gradient: 0 min 5% ACN, 4 min 60% ACN, 6 min 100% ACN; flow 2 mL/min) on a VWR Hitachi Elite LaChrom system (VWR, Darmstadt, Germany) with an L-2450 diode array detector, an L-2130 pump, and an L-2200 autosampler (VWR, Darmstadt, Germany). This HPLC system was coupled to an ESI-ion trap detector with positive ionization (Esquire 4000, Bruker Daltonics) for mass detection.
The preparative HPLC was conducted with a VWR HPLC-UV system (VWR International LaPrep, VWR) equipped with a pump P110, an UV detector P311, a Smartline 3900 autosampler (Knauer, Berlin, Germany), a LABOCOL Vario-2000 fraction collector (LABOMATIC, Weil am Rhein, Germany) and a Phenomenx Gemini-NX column (C18, 10 µ, 110 A, 100 × 50 mm, Phenomenex Inc.) was used. An H2O/acetonitrile (ACN) gradient with 0.1% formic acid added to both solvents was applied (gradient: 0 min 30% ACN with a flow of 40 mL/min; 0.5 min 30% ACN, 17.5 min 60% ACN, 22 min 100% ACN, 26 min 30% ACN; flow 100 mL/min).
Semi-preparative HPLC was carried out using a HPLC-UV system (VWR Hitachi Elite LaChrom system, VWR) consisting of an L-1230 pump, an L-2450 diode array detector, an L-2200 autosampler, a Phenomenex Luna column (Silica (2), 5 µ, 100 A, 250 × 10 mm, Phenomenex Inc.) and a Phenomenex Gemini-NX column 1 (C18, 10 µ, 110 A, 100 × 50 mm, Phenomenex Inc.), respectively. The specification of the columns, solvents, and gradients as applied for the purification of the compounds 1–13 as well as the respective retension times and yields are listed in Table 5.

Table 5.
Purification steps and yields of the compounds 1–13.
| Compound | Purification Step | Column | Gradient | Flow (mL/min) | UV Detection at (nm) | tR (min) | Yield (mg) |
|---|---|---|---|---|---|---|---|
| 1 | - | NP | 0 min 20% C, 20 min 70% C | 5 | 224 | 8.8 | 7.6 |
| 2 | 1st | RP | 0 min 62% B, 20 min 63% B | 15 | 215 | 6.0 | 4.5 |
| - | 2nd | NP | 0 min 20% C, 20 min 30% C | 5 | - | - | - |
| 3 | 1st | RP | 0 min 50% B, 20 min 60% B | 15 | 219 | 9.5 | 14.6 |
| 4 | 1st | NP | 0 min 5% C, 20 min 15% C | 5 | 215 | 14.8 | 4.3 |
| - | 2nd | NP | 0 min 15% C, 20 min 35% C | 5→8 | - | - | - |
| 5 | - | NP | 0 min 20% C, 20 min 70% C | 5 (8 min) | 224 | 10.7 | 8.2 |
| - | - | - | - | 8 (12 min) | - | - | - |
| 6 | - | NP | 0 min 20% C, 20 min 70% C | 5 | 224 | 7.2 | 62.9 |
| 7 | - | NP | 0 min 30% C, 20 min 60% C | 5 | 218 | 6.4 | 19 |
| 8 | - | NP | 0 min 15% C, 20 min 20% C | 5 | 215 | 12 | 71.0 |
| 9 | - | NP | 0 min 20% C, 20 min 70% C | 5 | 215 | 13.6 | 10.5 |
| 10 | 1st | RP | isocratic: 40% B, 20 min | 15 | 215 | 10.5 | 6.7 |
| - | 2nd | NP | 0 min 10% C, 20 min 25% C | 5→6 | - | - | - |
| 11 | - | NP | 0 min 20% C, 20 min 70% C | 5→8 | 215 | 12.0 | 7.9 |
| 12 | 1st | RP | 0 min 62% B, 20 min 63% B | 15 | 215 | 8.0 | 14.6 |
| - | 2nd | RP | isocratic: 61% B, 20 min | 15 | - | - | - |
| 13 | 1st | NP | 0 min 0% C, 20 min 50% C | 5 | 215 | 9.4 | 4.9 |
B = acetonitrile supplemented with 0.1% formic acid; C = isopropanol supplemented with 0.1% formic acid; NP = Phenomenex Luna column (Silica (2), 5 µ, 100 A, 250 × 10 mm); RP = Phenomenex Gemini-NX column 1 (C18, 10 µ, 110 A, 100 × 50 mm).
3.2. I Fungal Material and Cultivation Conditions
A strain of the order Hypocreales named Stachybotrys sp. MF347, was isolated from a driftwood sample collected at Helgoland (North Sea, Germany) and cultivated on GPY medium (0.1% glucose, 0.05 peptone, 0.01% yeast extract, and 1.5% agar dissolved in sea water). The strain was cultured on WSP30 agar, a modified Wickerham-medium, which consisted of 1% glucose, 0.5% peptone, 0.3% yeast extract, 0.3% malt extract, 3% sodium chloride, and 1.5% agar (pH = 6.8) [25] to yield cell material for storage. MF347 was stored at the Kultursammlung Mariner Pilze Kiel (KSMP-Kiel) using two methods, liquid nitrogen and the Microbank System at −80 °C (Mast Diagnostika, Reinfeld, Germany). The identification based on morphological criteria was performed using scanning electron microscopy. Young WSP30 agar colonies were cut in 1cm2 samples, transferred through an ethanol series (30%, 50%, 70%, 90%, 3 × 100%; every 15 min) and subsequently critical-point-dried in liquid carbon dioxide (Balzers CPD030, Oerlikon Balzers Coating Germany GmbH, Bingen am Rhein, Germany). Samples were sputter-coated with gold-palladium (Balzers SCD004, Oerlikon Balzers Coating Germany GmbH) and analyzed with a ZEISS DSM 940 (ZEISS, Oberkochen, Germany) scanning electron microscope.
3.3. Fermentation, Extraction and Isolation of the Compounds
Strain MF347 was cultured on WSP30 agar plates at 22 °C for 28 days. This pre-culture was used for the inoculation of 12 × 2 L Erlenmeyer flasks containing 750 mL WSP30TM medium (1% glucose, 0.5% peptone, 0.3% yeast extract, 0.3% malt extract, 3% tropic marine salt (pH = 6.8)) each. After incubation for 22 days at 28 °C in the dark as static cultures, extracts of the cultures were obtained. The mycelium was separated from the culture broth. 150 mL ethanol were added to the mycelium of each flask and homogenized. After a centrifugation step at 10,000 rpm for 10 min, the ethanol was removed by evaporation. The remaining aquatic phases of all 12 flasks were combined and extracted twice with 100 mL ethyl acetate. The organic phase was used for evaporation. The resulting residue was dissolved in 20 mL methanol to get the extract of the mycelium. The fermentation broth was extracted with ethyl acetate (400 mL per each flask). 100 mL deionized water were added to the organic phase. The upper phases of all flasks were combined and used for evaporation. The residue was dissolved in 5 mL methanol to get the extract of the culture broth. Both extracts were subjected to analytical HPLC-UV/MS. For the purification of the compounds 1–13, both extracts were combined.
By preparative HPLC 14 fractions were collected. 10 fractions were purified using semi-preparative HPLC. The specification of the columns, solvents, and gradients as applied for the purification of the compounds 1–13 as well as the respective retention times and yields are listed in Table 5.
Stachyin A (1): yellow powder; [α]24D −15° (c 0.1, MeOH); UV (MeOH) λmax (log є) 218 (4.03), 262 (3.91), 297 (4.12) nm; IR νmax 3288, 1673, 1463, 1330, 1252, 1082, 1008, 988, 880, 771 cm−1; 1H NMR and 13C NMR, see Table 1; ESIMS m/z 466 [M + Na]+; HR-FTICRMS m/z 466.2181 [M + Na]+ (calcd. for C25H33NO6Na, 466.2200).
Stachyin B (2): yellow powder; [α]24D −45° (c 0.1, MeOH); UV (MeOH) λmax (log є) 218 (3.95), 260 (3.90), 302 (4.01) nm; IR νmax 2969, 1675, 1609, 1461, 1390, 1350, 1078, 1068, 940, 737 cm−1; 1H NMR and 13C NMR, see Table 2; ESIMS m/z 778 [M + Na]+; HR-TOF-MS m/z 778.4319 [M + Na]+ (calcd. for C46H61NO8Na, 778.4289).
3.4. Biological Activities Assays
The antimicrobial activities of 1–13 against the bacterium Bacillus subtilis (DSM 347) and the human pathogenic yeast Candida albicans (DSM 1386) were determined according to Ohlendorf et al., 2012 [26].The bioassays using the clinically relevant bacterial strains Staphylococcus epidermidis (DSM 20044), the methicillin-resistant Staphylococcus aureus (MRSA) (DSM 18827), and Klebsiella pneumoniae DSM 30104 were performed as described by Silber et al., 2013 [27]. S. aureus and K. pneumoniae were investigated in the same manner as S. epidermidis. Trichophyton rubrum, a dermatophyte, and the phytopathogenic fungus Septoria tritici were tested according to Jansen et al., 2013 [28]. The inhibitory activity against phosphodiesterase (PDE-4B2) and the cytotoxic activity against HepG2 (human hepatocellular liver carcinoma cell line) and NIH-3T3 (mouse fibroblasts cell line) were determined according to Schulz et al., 2011 [29], apart from the cell concentration with 10,000 HepG2 cells per vial. The determination of the acetylcholinesterase (AchE) inhibitory activity was performed according to Ohlendorf et al., 2012 [26]. The glycogen synthase kinase-3β (GSK-3β) inhibition was tested as described by Baki et al., 2007 [30]. The concentration of the compounds used in the initial bioassays was 100 µM (antibiotic tests), 50 µM (cytotoxic tests), and 10 µM (enzymatic tests), respectively. To determine the IC50 values concentrations of the compounds ranging from 0.1–50 µM were analyzed twice in duplicates. Positive controls were carried out with chloramphenicol (bacteria), nystatin (yeast), clotrimazole (fungi), tamoxifen citrate (cell lines), rolipram (PDE-4B2), huperzine (AchE), and TDZD-8 (GSK-3β).
4. Conclusions
Although many compounds isolates from the genus Stachybotrys were reported to be toxins [3,4], they showed various biological effects, such as immunosuppressive activity [13] and antihyperlipidemic [23]. Antibiotic activities of spirodihydrobenzofuranlactam and spirodihydrobenzofuranlactone were rarely reported. The spirocyclic drimanes with two sesquiterpene-spirobenzofuran structural units 2, 11, and 12 showed antibacterial activity against the clinically relevant methicillin-resistant Staphylococcus aureus (MRSA), whereas spirocyclic drimanes with one sesquiterpene-spirobenzofuran structural unit 1, 3–10 exhibit no activities. It is tentatively implied that the structural feature of two sesquiterpene-spirobenzofuran units with either a N-C or a N-N linkage of spirocyclic drimanes is important for antibiotic activity. This is the first example of spirocyclic drimane coupled by a spirodihydrobenzofuranlactam unit and a spirodihydroisobenzofuran unit, and the connecting position being N-C. Stachyin A (1) and B (2) are structurally interesting, which would provide opportunities to design and synthesize new analogs that could improve the antibiotic and cytotoxic activities of these new compounds.
Acknowledgments
The authors gratefully thank G. Kohlmeyer-Yilmaz, M. Höftmann as well as F. Sönnichsen (Otto Diels Institute of Organic Chemistry, Christian-Albrechts-Universität zu Kiel, Germany) for running and processing NMR experiments. The authors are also very grateful to A. Erhard and R. Koppe for excellent technical assistance. Thanks to J. B. Speakman from BASF for providing Septoria tritici and support in its cultivation. Thanks to NSFC (81273386)’s support to Bin Wu.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crous, P.W.; Gams, W.; Stalpers, J.A.; Robert, V.; Stegehuis, G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004, 50, 19–22. [Google Scholar]
- Domsch, K.-H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi; Academic Press Ltd.: London, UK, 1980; Volume 1. [Google Scholar]
- Jarvis, B.B.; Salemme, J.; Morais, A. Stachybotrys toxins. 1. Nat. Toxins 1995, 3, 10–16. [Google Scholar] [CrossRef]
- Li, D.-W.; Yang, C.S. Taxonomic history and current status of Stachybotrys chartarum and related species. Indoor Air 2005, 15, 5–10. [Google Scholar]
- Gupta, N.; Das, S.; Sabat, J.; Basak, U.C. Isolation, identification and growth of Stachybotrys sp. obtained from mangrove ecosystem of Bhitarkanika, Orissa. Int. J. Plant Sci. 2007, 2, 64–68. [Google Scholar]
- Abdullah, S.K.; Al-Saadoon, A.H.; Al-Salihy, M.H. Fungi from the tidal zone of Khawr AL-Zubair Canal Southern Iraq. Marsh Bull. 2007, 2, 18–31. [Google Scholar]
- Boland, G.J.; Grund, D.W. Fungi from the salt marshes of Minas Basin, Nova Scotia. Proc. N. S. Inst. Sci. 1979, 29, 393–402. [Google Scholar]
- Xu, X.; de Guzman, F.S.; Gloer, J.B.; Shearer, C.A. Stachybotrins A and B: Novel bioactive metabolites from a brackish water isolate of the fungus Stachybotrys sp. J. Org. Chem. 1992, 57, 6700–6703. [Google Scholar] [CrossRef]
- Sponga, F.; Cavaletti, L.; Lazzarini, A.; Borghi, A.; Ciciliato, A.; Losi, D.; Marinelli, F. Biodiversity and potentials of marine-derived microorganisms. J. Biotechnol. 1999, 70, 65–69. [Google Scholar] [CrossRef]
- Osterhage, C. Isolation, Structure Determination and Biological Activity Assessment of Secondary Metabolites from Marine-Derived Fungi. Ph.D. Thesis, Carol Wilhelmina Technical University, Braunschweig, Germany, 2001; p. 186. [Google Scholar]
- Toledo-Hernández, C.; Bones-González, A.; Ortiz-Vázquez, O.E.; Sabat, A.M.; Bayman, P. Fungi in the sea fan Gorgonia ventalina: Diversity and sampling strategies. Coral Reef. 2007, 26, 725–730. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.-H.; Sun, L.-C.; Chen, Z.-H.; Zhang, J.; Bao, B. Isolation of fibrinolytic active compound from marine fungi and initial identification of the strain. Nat. Prod. Res. Dev. 2012, 24, 57–61. [Google Scholar]
- Kaise, H.; Shinohara, M.; Miyazaki, W.; Izawa, T.; Nakano, Y.; Sugawara, M.; Sugiura, K. Structure of K-76, a complement inhibitor produced by Stachybotrys complementi nov. sp. K-76. J. Chem. Soc. Chem. Commun. 1979, 16, 726–727. [Google Scholar]
- Ayer, W.A.; Miao, S. Secondary metabolites of the aspen fungus Stachybotrys cylindrospora. Can. J. Chem. 1992, 71, 487–493. [Google Scholar] [CrossRef]
- Nakamura, M.; Ito, Y.; Ogawa, K.; Michisuji, Y.; Sato, S.; Takada, M.; Hayashi, M.; Yaginuma, S.; Yamamoto, S. Stachybocins, novel endothelin receptor antagonists, produced by Stachybotrys sp. M6222. I. Taxonomy, fermentation, isolation and characterization. J. Antibiot. 1995, 48, 1389–1395. [Google Scholar] [CrossRef]
- Ogawa, K.; Nakamura, M.; Hayashi, M.; Yaginuma, S.; Yamamoto, S.; Furihara, K.; Shiin-Ya, K.; Seto, H. Stachybocins, novel endothelin receptor antagonists, produced by Stachybotrys sp. M6222. II. Structure determination of stachybocins A, B and C. J. Antibiot. 1995, 48, 1396–1400. [Google Scholar] [CrossRef]
- Vázqueza, M.J.; Vegad, A.; Rivera-Sagredoe, A.; Jiménez-Alfarob, M.D.; Dı́ezb, E.; Hueso-Rodrı́guezc, J.A. Novel sesquiterpenoids as tyrosine kinase inhibitors produced by Stachybotrys chartarum. Tetrahedron 2004, 60, 2379–2385. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef]
- Saleema, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef]
- Sakai, K.; Watanabe, K.; Masuda, K.; Tsuji, M.; Hasumi, K.; Endo, A. Isolation, characterization and biological activities of novel triprenyl phenols as pancreatic cholesterol esterase inhibitors produced by Stachybotrys sp. F-1839. J. Antibiot. 1995, 48, 447–456. [Google Scholar] [CrossRef]
- Eder, C.; Kurz, M.; Toti, L. Spirobenzofuranlactam Derivatives, Methods for Their Preparation, and Use Thereof. European Patent EP1572697, 21 February 2007. [Google Scholar]
- Li, Y.; Wu, C.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Chartarlactams A–P, phenylspirodrimanes from the sponge-associated fungus Stachybotrys chartarum with antihyperlipidemic activities. J. Nat. Prod. 2014, 77, 138–147. [Google Scholar] [CrossRef]
- Zhang, P.; Bao, B.; Dang, H.T.; Hong, J.; Lee, H.J.; Yoo, E.S.; Bae, K.S.; Jung, J.H. Anti-inflammatory sesquiterpenoids from a sponge-derived fungus Acremonium sp. J. Nat. Prod. 2009, 72, 270–275. [Google Scholar] [CrossRef]
- Wickerham, L.J. Taxonomy of yeasts. U.S. Dept. Agric. Tech. Bull. 1951, 1029, 1–56. [Google Scholar]
- Ohlendorf, B.; Schulz, D.; Erhard, A.; Nagel, K.; Imhoff, J.F. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J. Nat. Prod. 2012, 75, 1400–1404. [Google Scholar] [CrossRef]
- Silber, J.; Ohlendorf, B.; Labes, A.; Erhard, A.; Imhoff, J.F. Calcarides A–E, antibacterial macrocyclic and linear polyesters from a Calcarisporium strain. Mar. Drugs 2013, 11, 3309–3323. [Google Scholar] [CrossRef]
- Jansen, N.; Ohlendorf, B.; Erhard, A.; Imhoff, J.F. Helicusin E, Isochromophilone X and isochromophilone XI: New chloroazaphilones produced by the fungus Bartalinia robillardoides strain LF550. Mar. Drugs 2013, 11, 800–816. [Google Scholar] [CrossRef]
- Schulz, D.; Beese, P.; Ohlendorf, B.; Erhard, A.; Zinecker, H; Dorador, C.; Imhoff, J.F. Abenquines A–D: Aminoquinone derivatives produced by Streptomyces sp. strain DB634. J. Antibiot. 2011, 64, 763–768. [Google Scholar] [CrossRef]
- Baki, A.; Bielik, A.; Molnar, L.; Szendrei, G.; Keseru, G.M.A. A high throughput luminescent assay for glycogen synthase kinase-3β inhibitors. Assay Drug Develop. Technol. 2007, 5, 75–83. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).