Territrem and Butyrolactone Derivatives from a Marine-Derived Fungus Aspergillus Terreus
Abstract
:1. Introduction
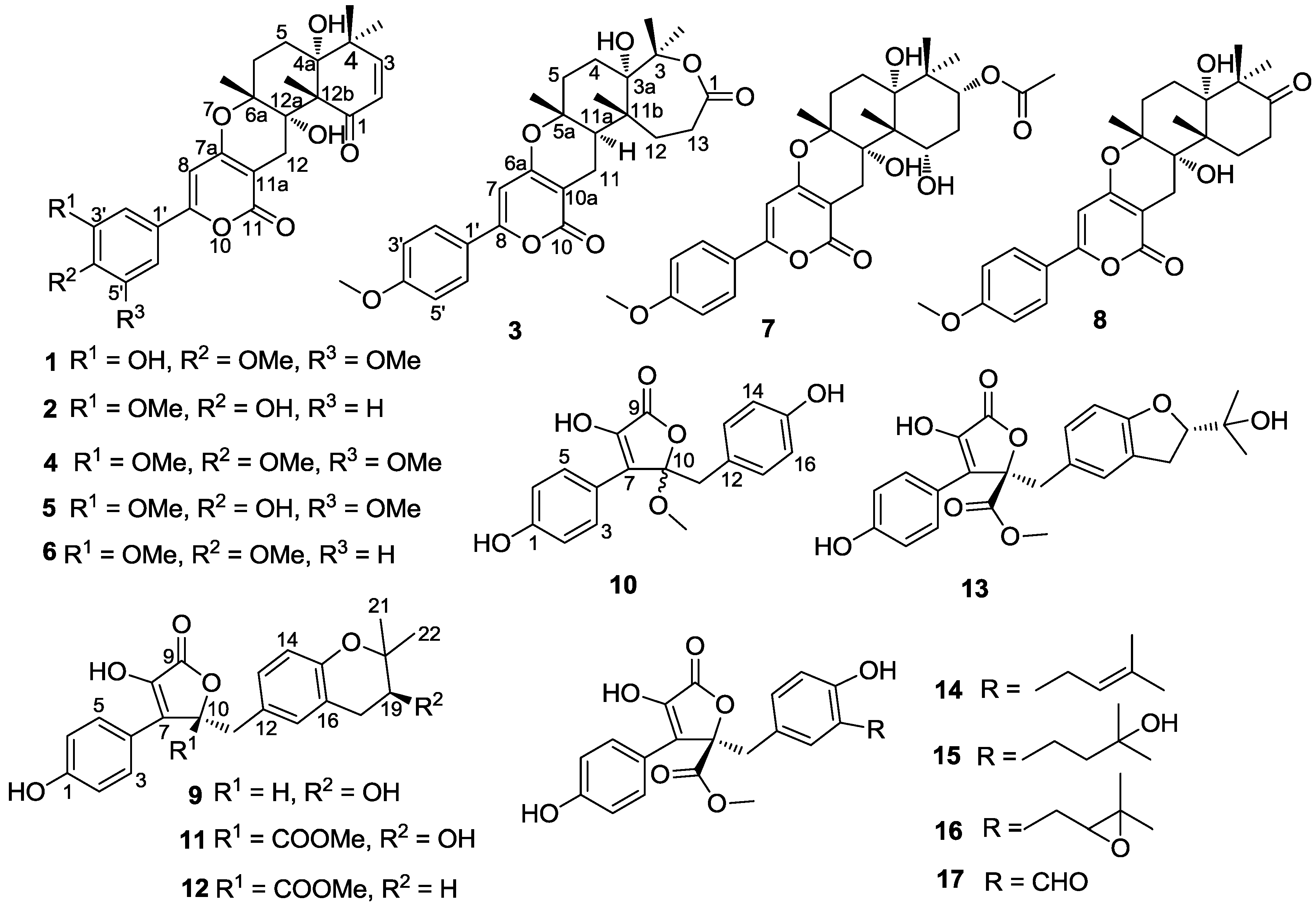
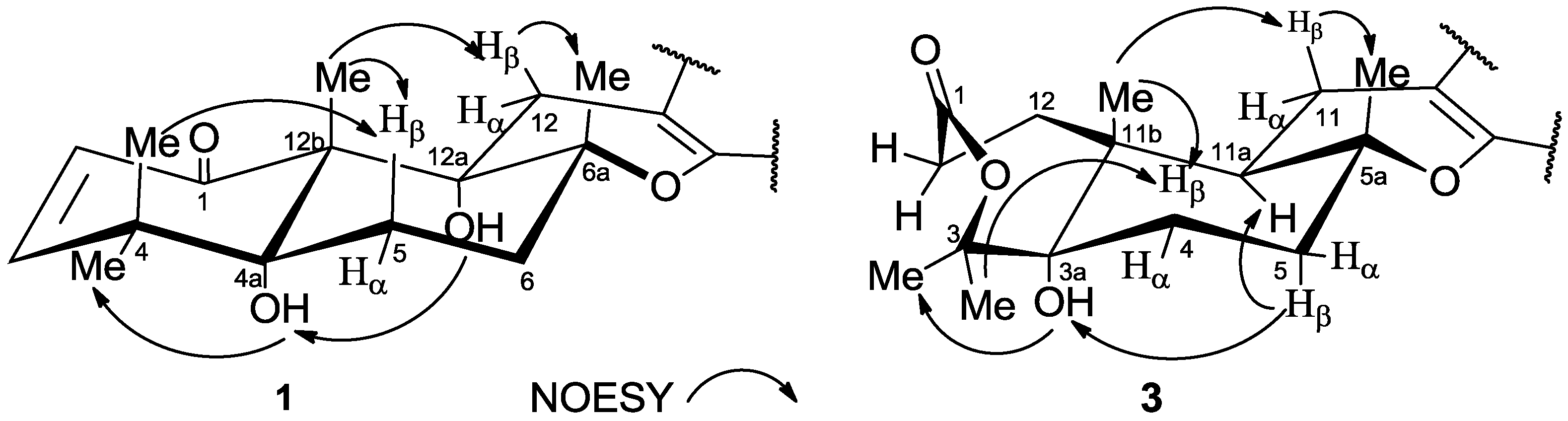
2. Results and Discussion
| Comp. | Anti-AChE IC50 (nM) | Anti-HSV-1 IC50 (μg·mL−1) | Cytotoxicity Against Vero TC0 (μg·mL−1) | Antifouling Against B. Amphitrite EC50 (μg·mL−1) |
|---|---|---|---|---|
| 1 | 4.2 ± 0.6 | NA a | 25 | 12.9 ± 0.5 |
| 2 | 4.5 ± 0.6 | NA a | 200 | NA a |
| 3 | NA a | 16.4 ± 0.6 | 200 | NA a |
| 4 | 4.2 ± 0.6 | NA a | 25 | NA a |
| 5 | 20.1 ± 3.3 | NA a | >25 | NT b |
| 6 | 11.9 ± 2.1 | 6.34 ± 0.4 | 100 | NT b |
| 7 | 5700 ± 800 | NAa | 100 | NT b |
| 8 | 50.0 ± 1.5 | NAa | >25 | NA a |
| 10 | NA a | 21.8 ± 1.8 | 200 | NA a |
| 11 | NA a | NT b | NT b | 22.1 ± 0.8 |
| 12 | NA a | 28.9 ± 1.8 | 100 | 7.4 ± 0.6 |
| 15 | NA a | NT b | NT b | 16.1 ± 0.6 |
| Huperzine A | 39.3 ± 7.6 | NT b | NT b | NT b |
| Acyclovir | NT b | 34.5 ± 0.7 | >1000 | NT b |
3. Experimental Section
3.1. General Experimental Procedure
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Purification
3.5. Enzyme-Based Assay of AChE
3.6. Plaque Reduction Assay
3.7. Barnacle Balanus Amphitrite Larval Settlement Bioassays
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Viegas, C., Jr.; Bolzani, V.S.; Barreiro, E.J.; Fraga, C.A. New anti-Alzheimer drugs from biodiversity: The role of the natural acetylcholinesterase inhibitors. Mini Rev. Med. Chem. 2005, 5, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.T.; Qian, Z.M.; He, X.; Gong, Q.; Wu, K.C.; Jiang, L.R.; Lu, L.N.; Zhu, Z.J.; Zhang, H.Y.; Yung, W.H.; et al. Reducing iron in the brain: A novel pharmacologic mechanism of huperzine A in the treatment of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Gou, S.H.; Fang, X.B.; Cheng, L.; Fleck, C. Current progresses of novel natural products and their derivatives/analogs as anti-Alzheimer candidates: An update. Mini Rev. Med. Chem. 2013, 13, 870–887. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Peng, F.C.; Chiou, C.M.; Ling, K.H. NMR assignments of territrems A B and C and the structure of MB-2 the major metabolite of territrem B by rat liver microsomal fraction. J. Nat. Prod. 1992, 55, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, K.; Shiomi, K.; Yamaguchi, Y.; Arai, N.; Sunazuka, T.; Masuma, R.; Iwai, Y.; Omura, S. Arisugacins C and D, novel acetylcholinesterase inhibitors and their related novel metabolites produced by Penicillium sp FO-4259–11. J. Antibiot. 2000, 53, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Cho, K.M.; Lee, C.K.; Yoo, I.D. Terreulactones A, B, C, and D: Novel acetylcholinesterase inhibitors produced by Aspergillus terreus—II. Physico-chemical properties and structure determination. J. Antibiot. 2003, 56, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kuno, F.; Otoguro, K.; Shiomi, K.; Iwai, Y.; Omura, S. Arisugacins A and B, novel and selective acetylcholinesterase inhibitors from Penicillium sp. FO-4259 .1. Screening, taxonomy, fermentation, isolation and biological activity. J. Antibiot. 1996, 49, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.C. Acetylcholinesterase inhibition by territrem B derivatives. J. Nat. Prod. 1995, 58, 857–862. [Google Scholar] [CrossRef]
- Haritakun, R.; Rachtawee, P.; Chanthaket, R.; Boonyuen, N.; Isaka, M. Butyrolactones from the fungus Aspergillus terreus BCC 4651. Chem. Pharm. Bull. 2010, 58, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, R.R.; D’Souza, C.; Tripathi, A.; Naik, C.G. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry 2009, 70, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Cazar, M.E.; Schmeda-Hirschmann, G.; Astudillo, L. Antimicrobial butyrolactone I derivatives from the ecuadorian soil fungus Aspergillus terreus thorn. Var terreus. World J. Microbiol. Biotechnol. 2005, 21, 1067–1075. [Google Scholar] [CrossRef]
- Haroon, M.H.; Premaratne, S.R.; Choudhry, M.I.; Dharmaratne, H.R.W. A new-glucuronidase inhibiting butyrolactone from the marine endophytic fungus Aspergillus terreus. Nat. Prod. Res. 2013, 27, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Okabe, T.; Ogino, H.; Matsumoto, H.; Suzukitakahashi, I.; Kokubo, T.; Higashi, H.; Saitoh, S.; Taya, Y.; Yasuda, H.; et al. Butyrolactone I, a selective inhibitor of cdk2 and cdc2 kinase. Oncogene 1993, 8, 2425–2432. [Google Scholar] [PubMed]
- Shen, Y.; Zou, J.H.; Xie, D.; Ge, H.L.; Cao, X.P.; Dai, J.G. Butyrolactone and cycloheptanetrione from mangrove-associated fungus Aspergillus terreus. Chem. Pharm. Bull. 2012, 60, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Sunazuka, T.; Handa, M.; Nagai, K.; Shirahata, T.; Harigaya, Y.; Otoguro, K.; Kuwajima, I.; Omura, S. Absolute stereochemistries and total synthesis of (+)-arisugacins A and B, potent, orally bioactive and selective inhibitors of acetylcholinesterase. Tetrahedron 2004, 60, 7845–7859. [Google Scholar] [CrossRef]
- Nagia, M.M.; El-Metwally, M.M.; Shaaban, M.; El-Zalabani, S.M.; Hanna, A.G. Four butyrolactones and diverse bioactive secondary metabolites from terrestrial Aspergillus flavipes MM2: Isolation and structure determination. Org. Med. Chem. Lett. 2012, 2. [Google Scholar] [CrossRef]
- Rao, K.V.; Sadhukhan, A.K.; Veerender, M.; Ravikumar, V.; Mohan, E.V.S.; Dhanvantri, S.D.; Sitaramkumar, M.; Babu, J.M.; Vyas, K.; Reddy, G.O. Butyrolactones from Aspergillus terreus. Chem. Pharm. Bull. 2000, 48, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, R.; Lacey, E.; Tennant, S.; Gill, J.H.; Capon, R.J. Kibdelones: Novel anticancer polyketides from a rare Australian actinomycete. Chemistry 2007, 13, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Cotelle, P.; Cotelle, N.; Teissier, E.; Vezin, H. Synthesis and antioxidant properties of a new lipophilic ascorbic acid analogue. Bioorg. Med. Chem. 2003, 11, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.D.; Cho, K.M.; Lee, C.K.; Kim, W.G. Isoterreulactone A, a novel meroterpenoid with anti-acetylcholinesterase activity produced by Aspergillus terreus. Bioorg. Med. Chem. Lett. 2005, 15, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashid, Z.F.; Hsung, R.P. (+)-Arisugacin A-Computational evidence of a dual binding site covalent inhibitor of acetylcholinesterase. Bioorg. Med. Chem. Lett. 2011, 21, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Degen, S.J.; Mueller, K.L.; Shen, H.C.; Mulder, J.A.; Golding, G.M.; Wei, L.L.; Zificsak, C.A.; Neeno-Eckwall, A.; Hsung, R.P. Synthesis of dihydroxanthone derivatives and evaluation of their inhibitory activity against acetylcholinesterase: Unique structural analogs of tacrine based on the BCD-ring of arisugacin. Bioorg. Med. Chem. Lett. 1999, 9, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Shiota, H.; Naito, T.; Mimura, Y. Sensitivities to other antiviral drugs and thymidine kinase activity of aciclovir-resistant herpes simplex virus type 1. Nippon Ganka Gakkai Zasshi 1994, 98, 513–519. [Google Scholar] [PubMed]
- Qi, S.H.; Xu, Y.; Gao, J.; Qian, P.Y.; Zhang, S. Antibacterial and antilarval compounds from marine bacterium Pseudomonas rhizosphaerae. Ann. Microbiol. 2009, 59, 229–233. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nong, X.-H.; Wang, Y.-F.; Zhang, X.-Y.; Zhou, M.-P.; Xu, X.-Y.; Qi, S.-H. Territrem and Butyrolactone Derivatives from a Marine-Derived Fungus Aspergillus Terreus. Mar. Drugs 2014, 12, 6113-6124. https://doi.org/10.3390/md12126113
Nong X-H, Wang Y-F, Zhang X-Y, Zhou M-P, Xu X-Y, Qi S-H. Territrem and Butyrolactone Derivatives from a Marine-Derived Fungus Aspergillus Terreus. Marine Drugs. 2014; 12(12):6113-6124. https://doi.org/10.3390/md12126113
Chicago/Turabian StyleNong, Xu-Hua, Yi-Fei Wang, Xiao-Yong Zhang, Mu-Ping Zhou, Xin-Ya Xu, and Shu-Hua Qi. 2014. "Territrem and Butyrolactone Derivatives from a Marine-Derived Fungus Aspergillus Terreus" Marine Drugs 12, no. 12: 6113-6124. https://doi.org/10.3390/md12126113
APA StyleNong, X.-H., Wang, Y.-F., Zhang, X.-Y., Zhou, M.-P., Xu, X.-Y., & Qi, S.-H. (2014). Territrem and Butyrolactone Derivatives from a Marine-Derived Fungus Aspergillus Terreus. Marine Drugs, 12(12), 6113-6124. https://doi.org/10.3390/md12126113




