1. Introduction
Cardiovascular disease is the leading cause of mortality in the Western world. There is evidence that the consumption of oily fish may significantly decrease cardiovascular disease risk in humans [
1]. Oily fish, such as Atlantic salmon, is an important source of long chain omega 3 fatty acids including eicosapentaenoic acid (EPA; C20:5
n-3) and docosahexaenoic acid (DHA; C22:6
n-3) which may exhibit beneficial cardiovascular effects [
2,
3,
4]. Due to their double bonds, both EPA and DHA are prone to lipid peroxidation. Our studies have demonstrated that Vitamin E (alpha tocopherol) significantly prevents the oxidation of long chain PUFAs in oily fish [
5]. The antioxidant properties of Vitamin E result from its phenolic hydroxyl group which donates hydrogen to peroxyl radicals, resulting in the formation of relatively stable lipid species [
6]. In addition to its antioxidant function, important gene-regulatory properties of Vitamin E have been described [
7,
8].
Vitamin E comprises a mixture of four tocopherols which are 6-chromanol derivatives connected to an aliphatic side-chain. Individual tocopherols are named according to the position and number of the methyl groups on the phenol ring, with the α-, β-, γ- and δ- vitamins containing three, two, two and one methyl groups, respectively. These structural differences determine the biological activity of tocopherols [
6].
Gamma tocopherol (gT) occurs at high concentrations in plant-derived oils including soya- and rapeseed oil [
9,
10]. Studies suggest that gT exhibits biological functions which clearly distinguishes it from alpha tocopherol [
10]. As compared to alpha tocopherol, gT is a more potent scavenger of reactive nitrogen species. In this context, it is postulated that gT acts as a trap for electrophilic nitrogen oxides forming stable carbon-centered adducts through the nucleophilic 5-position [
11].
Chronic inflammatory processes are centrally involved in the pathogenesis of cardiovascular diseases. Interestingly, gT, but not alpha tocopherol, inhibited cyclooxygenase 2 in cultured macrophages, thereby mediating potent anti-inflammatory activity [
12]. Furthermore, Jiang and co-workers observed a suppression of inducible nitric oxide synthase expression by gT in lipopolysaccharide-challenged macrophages [
12]. The anti-inflammatory properties of gT
in vitro have been subsequently confirmed in corresponding
in vivo studies in laboratory rodents [
13].
Plasma concentrations of gT in humans may be inversely associated with cardiovascular disease risk [
10]. Given the unique functional properties and health benefits of gT, it seems plausible to enrich oily fish with gT [
14]. While vegetable oils, which are considered an important source of gT, do not contain significant amounts of long chain polyunsaturated fatty acids, oily fish is a source of EPA and DHA. Gamma-tocopherol in combination with the fish oils EPA and DHA may act synergistically as far as their beneficial cardiovascular activities in humans are concerned. In addition, feeding Atlantic salmon with gT might improve fish antioxidant status thereby preventing the oxidation of EPA and DHA. This could be a feasible nutritional strategy for the salmon feed industry to maintain high tissue EPA and DHA levels in a context of scarce fish oil supply as a raw material for feed production.
In this study we have aimed to investigate if dietary gT affects tocopherol levels, antioxidant status and fatty acid composition of Atlantic salmon as an important source of EPA and DHA and as a potential functional source of gT for human nutrition.
To this end we have measured malondialdehyde (MDA) concentrations as a biomarker of lipid peroxidation and sensory quality [
15] and carried out fatty acid gas chromatography analyses, antioxidant enzyme activity measurements and gene expression analyses of genes involved in fatty acid transport, synthesis and metabolism.
3. Discussion
Marine functional foods may supply the consumer with macro- and micronutrients as well as nutraceuticals, thereby positively affecting cardiovascular health. In general, seafood and especially oily fish, such as Atlantic salmon, seem to be attractive sources to deliver fat soluble vitamins such as tocopherols since Vitamin E absorption is positively correlated with the lipid level of the diet. Furthermore, it has been suggested that tocopherols consumed within a complex food matrix may prevent cardiovascular disease more efficiently as compared to purified Vitamin E supplements [
18].
Our data indicate that feeding Atlantic salmon with gT-rich diets resulted in a significant increase in gT concentrations in fish fillet, thereby improving its nutritional value. Thus, Atlantic salmon could be considered as a potential marine functional source of gT for human nutrition. However, future human trials (e.g., bioavailability studies, intervention studies) are required to test the hypothesis whether the consumption of gT-rich Atlantic salmon results in increased plasma levels of gT that may lead to potential anti-inflammatory and anti-atherogenic effects.
Interestingly, high dietary gT concentrations did not impair alpha tocopherol tissue levels. Thus, under the conditions investigated, no antagonistic interaction between gamma and alpha tocopherol was evident. Contrarily, high dietary alpha tocopherol concentrations have been reported to decrease gT tissue concentrations [
5]. This is possibly related to the induction of cytochrome P450 enzymes which are involved in the metabolism of tocopherols and preferentially degrade the desmethyl vitamers [
19].
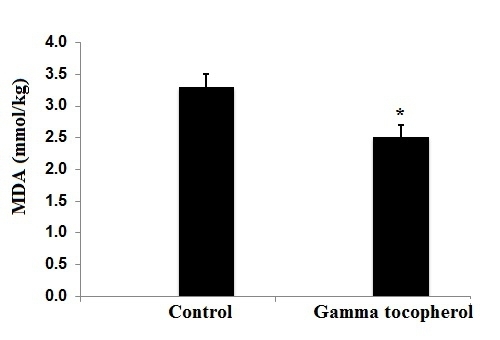
Furthermore, we observed lower hepatic catalase and superoxide dismutase activities which may be indicative of an overall lowered oxidative stress level in the gT-fed salmon. Dietary gT supplementation significantly decreased MDA concentration in fish fillet. MDA is a biomarker of lipid peroxidation and impairs the sensory quality of oily fish [
17]. Current as well as literature data [
20] suggest that dietary gT could possibly improve the sensory quality of oily fish by reducing lipid peroxidation. In this context, future studies are needed to evaluate whether gT affects the shelf life of Atlantic salmon.
The enrichment of fat soluble pro-vitamins and vitamins into the food matrix is a common practice in aquaculture. However, the transfer of micronutrients including Vitamin E from the diet into the animal tissue is always accompanied by a loss in assimilation. In order to quantify the apparent Vitamin E absorption, digestibility experiments need to be conducted which was beyond the scope of our study. Furthermore, Vitamin E transfer may be experimentally determined by its whole body retention. In order to do so, Vitamin E levels need to be quantified in all tissues at the beginning and the end of a feeding trial. However, within this study Vitamin E was only determined in fillet and liver at the end of the study. Thus, it was not possible to pinpoint the exact Vitamin E retention. However, analyzed alpha and gamma tocopherol levels in liver and fillet suggest that the tissue retention of alpha tocopherol was higher as compared to gamma tocopherol which is in line with literature data [
14]. In terms of alpha tocopherol, it has been suggested that around 40% of dietary alpha tocopherol is retained in the fillet of Atlantic salmon [
14]. Since in the fillet of our salmon following dietary supplementation, the gamma tocopherol concentration was 2.8 fold lower as compared to alpha tocopherol a transfer efficiency of around 15% may be assumed.
Irrespective of a loss of retention, our experimental approach leads to a marine product rich in both, omega-3 fatty acids as well as alpha and gamma tocopherol. In this context it has been suggested that the consumption of 200 g Atlantic salmon would be sufficient to fulfill roughly 30% of the dietary Vitamin E recommendation [
5]. Furthermore, it should be taken into account that oily fish does not only provide omega 3 fatty acids but is also an important source of protein, minerals and trace elements.
Besides its antioxidant activity, Vitamin E also plays an important role in signal transduction [
21]. Both isoforms alpha and gamma tocopherol seem to affect lipid homeostasis in different ways. Studies in hepatocytes and Caco-2 cells have shown a decrease in cholesterol synthesis and secretion due to tocopherols via down-regulation of LXR, BCA1, ABCG1, SREBP1c or SREBP-2 transcription factors [
22,
23]. Additionally, there is evidence that alpha tocopherol can modulate the expression and activity of Δ9 and Δ6 fad [
22,
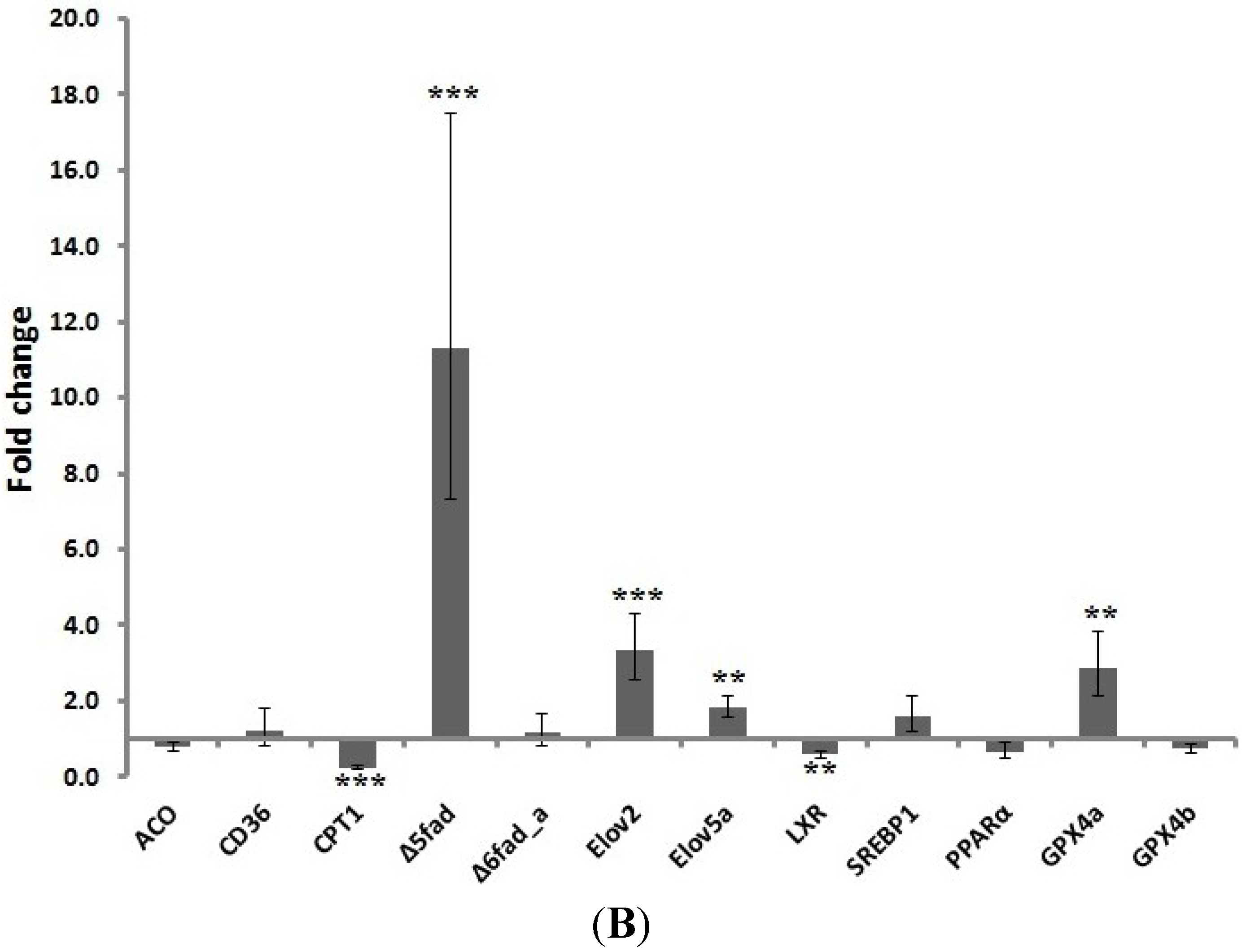
24]. In the present study, salmon fed the gT-enriched diets exhibited a significant hepatic down-regulation of different genes encoding proteins involved in lipid transport (CD36) and beta oxidation (CPT1 and ACO) as well as long chain PUFA synthesis (Δ6fad_a and elov2) which was mediated by decreased
LXR gene expression. Together with the observed trend towards an increase of C18:3
n-3 in the fillet, the decrease in liver C18:1
n-7, C18:2
n-6 and the accumulation of C18:0 may be the result of an overall down-regulation of lipid transport and beta oxidation as well as a decreased elongation and desaturation of C18:0. There are several molecular mechanisms by which tocopherol or its metabolites may affect gene expression and enzyme function [
21,
25]. The structural changes that tocopherol promotes when it is embedded in the membrane can affect lipid homeostasis [
21]. Moreover, gT exerts different structural influence on the membrane than alpha tocopherol [
26]. Studies in fish also suggest an indirect effect of Vitamin E on long chain PUFA synthesis by altering the cellular redox status [
27]. Therefore, the efficient accumulation of both alpha and gamma tocopherol in salmon liver and lowered oxidative stress levels might have contributed to the changes observed in gene expression.
Similarly, CPT1 and transcription factor gene expression was down-regulated in the pyloric caeca of salmon fed gT. However,
Δ5 fad,
Elov2 and
Elov5a expression was increased. Different gene expression patterns of elongases and desaturases have been reported in the liver and pyloric caeca of salmon fed vegetable oils [
28,
29]. Additionally, the expression of the antioxidant enzyme GPX4a, which reduces phospholipid hydroperoxides [
30], in the pyloric caeca of fish fed gT suggests a protective role of gT in lipid peroxidation. Moreover, Malandrakis
et al. [
31] suggested that the GPx status, which was improved by gamma tocopherol in our study, might be considered as an important indicator of fish welfare.