Atlantic Salmon (Salmo salar L.) as a Marine Functional Source of Gamma-Tocopherol
Abstract
:1. Introduction
2. Results
2.1. Fish Performance
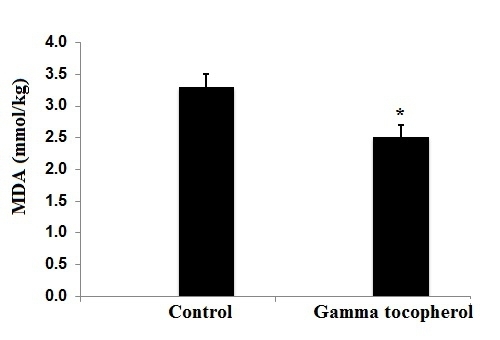
2.2. Tissue Levels of Tocopherols, Malondialdehyde, Antioxidant Enzymes and Fatty Acid Composition
| Tissue | C | gT | p |
|---|---|---|---|
| Fillet | |||
| α-tocopherol | 36.7 ± 3.2 | 45.3 ± 4.4 | 0.86 |
| γ-tocopherol | 5.4 ± 0.3 | 16.3 ± 1.1 | <0.0001 |
| Liver | |||
| α-tocopherol | 912 ± 69 | 937 ± 131 | 0.15 |
| γ-tocopherol | 34.7 ± 2.4 | 112.1 ± 9.9 | <0.0001 |
| Biomarker | C | gT | p | |
|---|---|---|---|---|
| SOD (U/mg protein) | 24.6 | ±2.6 | 18.0 ± 1.2 | 0.04 |
| CAT (U/mg protein) | 46.8 | ±3.3 | 35.8 ± 2.1 | 0.01 |
| GSH (µmol/g) | 2.1 | ±0.1 | 1.9 ± 0.1 | 0.22 |
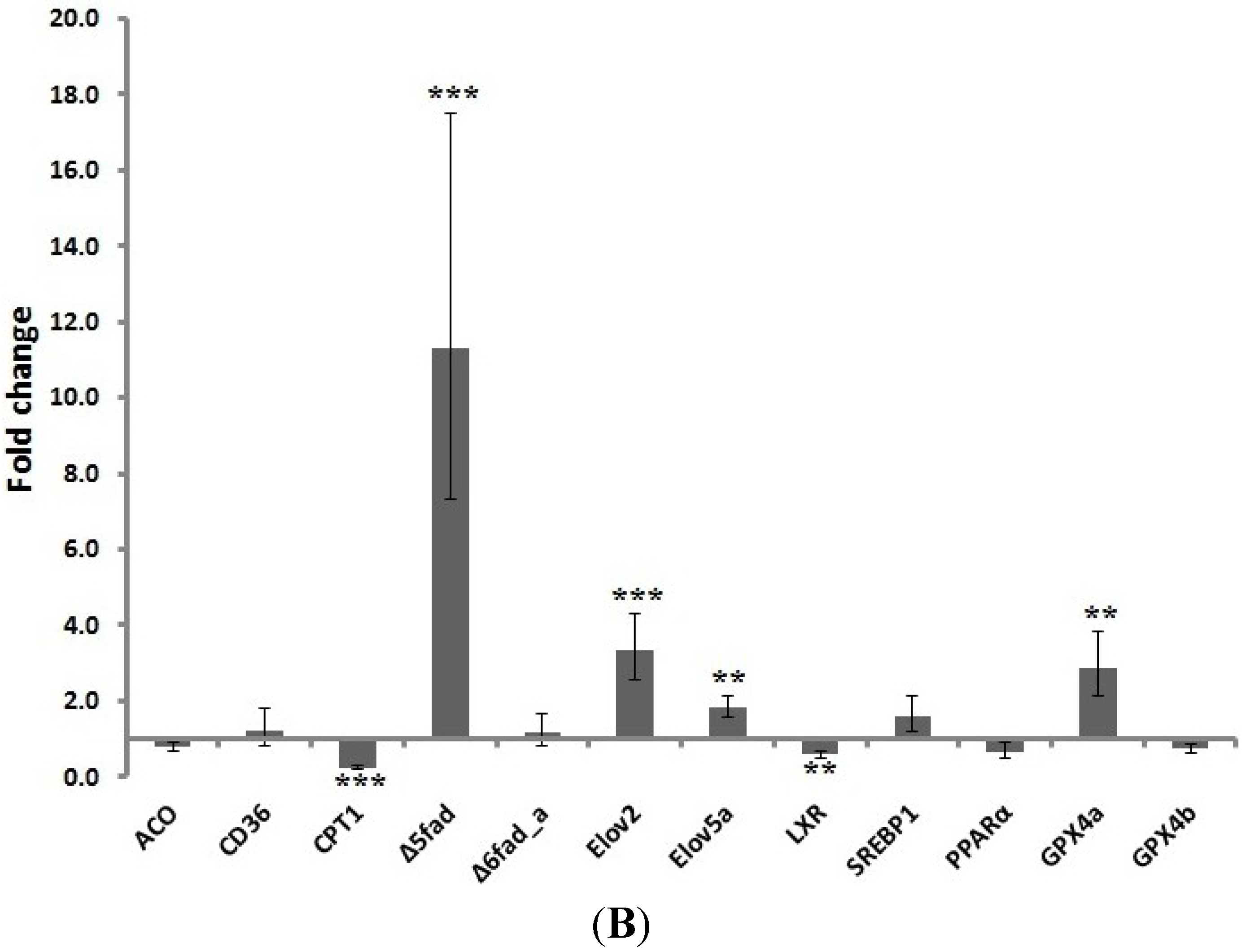
2.3. Gene Expression Analysis
| Fatty Acid | C | gT | p | ||
|---|---|---|---|---|---|
| C14:0 | 1.54 | ±0.01 | 1.40 | ±0.01 | 0.08 |
| C16:0 | 11.86 | ±0.05 | 11.67 | ±0.07 | 0.06 |
| C18:0 | 3.06 | ±0.02 | 3.09 | ±0.05 | 0.70 |
| ∑SFA b | 18.24 | ±0.04 | 20.62 | ±0.12 | 0.10 |
| C16:1n-7 | 1.86 | ±0.04 | 1.80 | ±0.01 | 0.17 |
| C18:1n-9 | 44.59 | ±0.11 | 44.17 | ±0.16 | 0.06 |
| C18:1n-7 | 1.87 | ±0.04 | 1.82 | ±0.06 | 0.53 |
| C20:1n-9 | 2.79 | ±0.02 | 2.66 | ±0.04 | 0.01 |
| ∑MUFA c | 52.32 | ±0.11 | 51.74 | ±0.18 | 0.02 |
| C18:2n-6 | 14.84 | ±0.02 | 14.77 | ±0.08 | 0.49 |
| C20:2n-6 | 1.15 | ±0.01 | 1.05 | ±0.02 | 0.01 |
| C20:4n-6 | 0.31 | ±0.01 | 0.33 | ±0.01 | 0.27 |
| ∑ (n-6) d | 16.82 | ±0.03 | 16.82 | ±0.07 | 0.99 |
| C18:3n-3 | 5.09 | ±0.04 | 5.29 | ±0.06 | 0.02 |
| C20:3n-3 | 0.75 | ±0.02 | 0.81 | ±0.03 | 0.12 |
| C20:5n-3 | 1.55 | ±0.04 | 1.48 | ± 0.05 | 0.33 |
| C22:5n-3 | 0.75 | ± 0.01 | 0.91 | ± 0.08 | 0.09 |
| C22:6n-3 | 3.94 | ±0.08 | 4.13 | ±0.14 | 0.30 |
| ∑ (n-3) e | 13.50 | ±0.11 | 14.30 | ±0.25 | 0.01 |
| n-3/n-6 | 0.80 | ±0.01 | 0.85 | ±0.01 | 0.01 |
| Fatty Acid | C | gT | p | ||
|---|---|---|---|---|---|
| C14:0 | 0.90 | ±0.02 | 0.76 | ±0.04 | 0.01 |
| C16:0 | 11.38 | ±0.55 | 12.06 | ±0.52 | 0.39 |
| C18:0 | 5.64 | ±0.18 | 6.91 | ±0.23 | 0.0008 |
| ∑SFA b | 18.24 | ±0.55 | 20.62 | ±0.65 | 0.01 |
| C16:1n-7 | 1.25 | ±0.06 | 1.03 | ±0.12 | 0.13 |
| C18:1n-9 | 36.19 | ±1.47 | 33.29 | ±2.53 | 0.34 |
| C18:1n-7 | 1.94 | ±0.03 | 1.70 | ±0.05 | 0.003 |
| C20:1n-9 | 3.84 | ±0.19 | 3.59 | ±0.19 | 0.37 |
| ∑MUFA c | 44.18 | ±1.62 | 40.60 | ±2.81 | 0.28 |
| C18:2n-6 | 10.18 | ±0.29 | 8.89 | ±0.30 | 0.008 |
| C20:2n-6 | 2.06 | ±0.09 | 2.01 | ±0.13 | 0.75 |
| C20:4n-6 | 1.81 | ±0.14 | 2.36 | ±0.31 | 0.13 |
| ∑ (n-6) d | 14.89 | ±0.19 | 13.57 | ±0.22 | 0.0005 |
| C18:3n-3 | 2.15 | ±0.13 | 1.85 | ±0.09 | 0.08 |
| C20:3n-3 | 2.41 | ±0.13 | 2.67 | ±0.20 | 0.3 |
| C20:5n-3 | 2.67 | ±0.25 | 2.75 | ±0.32 | 0.85 |
| C22:5n-3 | 1.12 | ±0.07 | 1.19 | ±0.15 | 0.65 |
| C22:6n-3 | 13.03 | ±0.92 | 15.39 | ±1.69 | 0.24 |
| ∑ (n-3) e | 22.35 | ±1.22 | 24.89 | ±2.16 | 0.32 |
| n-3/n-6 | 1.50 | ±0.08 | 1.83 | ±0.14 | 0.08 |

3. Discussion
4. Experimental Section
4.1. Animals and Diets
4.2. Sampling
| Ingredients | As Fed Basis (g/kg) |
| Wheat | 50.0 |
| Wheat gluten | 168.1 |
| Faba beans dehulled | 93.8 |
| Soy protein concentrate | 310.0 |
| Fishmeal NA | 100.0 |
| Palm oil | 27.1 |
| Linseed oil | 11.7 |
| Rapeseed oil | 163.0 |
| Fish oil NA | 38.9 |
| Astaxanthin 10% | 0.4 |
| Vitamin and mineral mix | 37.1 |
| Analyzed composition | |
| Moisture, % | 6.6 |
| Total fat, % | 26.7 |
| Crude protein, % | 45.6 |
| Ash, % | 4.6 |
| α-tocopherol (ppm) | 192 |
| γ-tocopherol (ppm) | 15 |
| Fatty acids | Total fatty acids (g/100 g) |
| C16:0 | 10.35 |
| ∑SFA a | 15.27 |
| C18:1n-9 | 41.17 |
| ∑MUFA b | 49.10 |
| C18:2n-6 | 17.39 |
| ∑ (n-6) | 18.03 |
| C18:3n-3 | 7.61 |
| C20:5n-3 | 1.93 |
| C22:6n-3 | 1.87 |
| ∑ (n-3) | 12.45 |
| n-3/n-6 | 0.69 |
4.3. Chemical and Enzyme Analysis
4.4. Gene Expression Analysis
4.5. Statistical Analysis
| Genes | Primer Sequence Fw | Primer Sequence Rv | Reference |
|---|---|---|---|
| Δ6fad_a | CCCCAGACGTTTGTGTCAG | CCTGGATTGTTGCTTTGGAT | [42] |
| ACO | AAAGCCTTCACCACATGGAC | TAGGACACGATGCCACTCAG | [42] |
| β-actin | ACATCAAGGAGAAGCTGTGC | GACAACGGAACCTCTCGTTA | [42] |
| EF-1α | CTGCCCCTCCAGGACGTTTCAA | CACCGGGCATAGCCGATTCC | [42] |
| Δ5 fad | GTGAATGGGGATCCATAGCA | AAACGAACGGACAACCAGA | [28] |
| Elov2 | CGGTACAAAATGTGCTGGT | TCTGTTTGCCGATAGCCATT | [28] |
| Elov5a | ACAAGACAGGAATCTCTTTCAGATTAA | TCTGGGGTTACTGTGCTATAGTGTAC | [43] |
| LXR | GCCGCCGCTATCTGAAATCTG | CAATCCGGCACCAATCTGTAGG | [43] |
| CD36 | GGATGAACTCCCTGCATGTGA | TGAGGCCAAAGTACTCGTCGA | [44] |
| SREBP1 | CACTACTAGCCCCATGTTTTGATTG | CAGCCACTCTCTAAACACACCAA | |
| PPARα | GCTCCTTGGATGTCCCTGAGT | GCATCTAGAACGGTGGATCCTT | |
| Nrf2 | GGTTTCCAGACTTCTCTCTCAGTGT | GAACATGGCAAGACCGAGCC | |
| GPX4a | GTACGCTGAGAAAGGTTTACGC | TTGATGCCATTTCCCAGG | [30] |
| GPX4b | ATCACCAACGTTGCCTCTAAAT | CCTTGATTTCCACCTCTGTACC | [30] |
| CPT1 | CCTGTACCGTGGAGACCTGT | CAGCACCTCTTTGAGGAAGG | [42] |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dalen, J.E.; Devries, S. Diets to prevent coronary heart disease 1957–2013: What have we learned? Am. J. Med. 2014, 127, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Garry, J.M.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, M.Y.; Patten, G.S. Role of ω3 long-chain polyunsaturated fatty acids in reducing cardio-metabolic risk factors. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [PubMed]
- Faizan, M.; Stubhaug, I.; Menoyo, D.; Esatbeyoglu, T.; Wagner, A.E.; Struksnæs, G.; Koppe, W.; Rimbach, G. Dietary alpha-tocopherol affects tissue vitamin E and malondialdehyde levels but does not change antioxidant enzymes and fatty acid composition in farmed Atlantic salmon (Salmo salar L.). Int. J. Vitam. Nutr. Res. 2013, 83, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; Moehring, J.; Huebbe, P.; Lodge, J.K. Gene-regulatory activity of alpha-tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimbach, G.; Minihane, A.M.; Majewicz, J.; Fischer, A.; Pallauf, J.; Virgli, F.; Weinberg, P.D. Regulation of cell signalling by vitamin E. Proc. Nutr. Soc. 2002, 61, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Barella, L.; Muller, P.Y.; Schlachter, M.; Hunziker, W.; Stöcklin, E.; Spitzer, V.; Meier, N.; de Pascual-Teresa, S.; Minihane, A.M.; Rimbach, G. Identification of hepatic molecular mechanisms of action of alpha-tocopherol using global gene expression profile analysis in rats. Biochim. Biophys. Acta 2004, 24, 66–74. [Google Scholar] [CrossRef]
- Porsgaard, T.; Høy, C.E. Absorption by rats of tocopherols present in edible vegetable oils. Lipids 2000, 35, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [PubMed]
- Christen, S.; Woodall, A.A.; Shigenaga, M.K.; Southwell-Keely, P.T.; Duncan, M.W.; Ames, B.N. Gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: Physiological implications. Proc. Natl. Acad. Sci. USA 1997, 94, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef] [PubMed]
- Christen, S.; Jiang, Q.; Shigenaga, M.K.; Ames, B.N. Analysis of plasma tocopherols alpha, gamma, and 5-nitro-gamma in rats with inflammation by HPLC coulometric detection. J. Lipid Res. 2002, 43, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Hamre, K. Metabolism, interactions, requirements and functions of vitamin E in fish. Aquac. Nutr. 2011, 17, 98–115. [Google Scholar] [CrossRef]
- Tsaknis, J.; Lalas, S.; Evmorfopoulos, E. Determination of malondialdehyde in traditional fish products by HPLC. Analyst 1999, 124, 843–845. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010, 8. [CrossRef]
- Faizan, M.; Esatbeyoglu, T.; Bayram, B.; Rimbach, G. A fast and validated method for the determination of malondialdehyde in fish liver using high-performance liquid chromatography with a photodiode array detector. J. Food Sci. 2014, 79. [Google Scholar] [CrossRef] [PubMed]
- Malav, O.P.; Talukder, S.; Gokulakrishnan, P.; Chand, S. Meat analogue: A review. Crit. Rev. Food Sci. Nutr. 2013. [Google Scholar] [CrossRef]
- Huang, H.Y.; Appel, L.J. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J. Nutr. 2003, 133, 3137–3140. [Google Scholar] [PubMed]
- Parazo, M.P.; Lall, S.P.; Castell, J.D.; Ackman, R.G. Distribution of alpha- and gamma-tocopherols in Atlantic salmon (Salmo salar) tissues. Lipids 1998, 33, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M. Modulation of signal transduction by vitamin E. Mol. Asp. Med. 2007, 28, 481–506. [Google Scholar] [CrossRef]
- Valastyan, S.; Thakur, V.; Johnson, A.; Kumar, K.; Manor, D. Novel transcriptional activities of vitamin E: Inhibition of cholesterol biosynthesis. Biochemistry 2008, 47, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Landrier, J.F.; Gouranton, E.; Reboul, E.; Cardinault, N.; El Yazidi, C.; Malezet-Desmoulins, C.; André, M.; Nowicki, M.; Souidi, M.; Borel, P. Vitamin E decreases endogenous cholesterol synthesis and apo-AI-mediated cholesterol secretion in Caco-2 cells. J. Nutr. Biochem. 2010, 21, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Despret, S.; Dinh, L.; Clément, M.; Bourre, J.M. Alteration of delta-6 desaturase by vitamin E in rat brain and liver. Neurosci. Lett. 1992, 145, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Molecular mechanism of alpha-tocopherol action. Free Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Raghunathan, V.A. Modulated phases of phospholipid bilayers induced by tocopherols. Biochim. Biophys. Acta 2012, 1818, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- Mourente, G.; Bell, J.G.; Tocher, D.R. Does dietary tocopherol level affect fatty acid metabolism in fish? Fish Physiol. Biochem. 2007, 33, 269–280. [Google Scholar] [CrossRef]
- Morais, S.; Taggart, J.B.; Guy, D.R.; Bell, J.G.; Tocher, D.R. Hepatic transcriptome analysis of inter-family variability in flesh n-3 long-chain polyunsaturated fatty acid content in Alantic salmon. BMC Genomics 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Silva, T.; Cordeiro, O.; Rodrigues, P.; Guy, D.R.; Bron, J.E.; Taggart, J.B.; Bell, J.G.; Tocher, D.R. Effects of genotype and dietary fish oil replacement with vegetable oil on the intestinal transcriptome and proteome of Atlantic salmon (Salmo salar). BMC Genomics 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Harris, S.M.; Espinoza, H.M.; McClain, V.; Gallagher, E.P. Characterization of phospholipid hydroperoxide glutathione metabolizing peroxidase (GPX4) isoforms in Coho salmon olfactory and liver tissues and their modulation by cadmium. Aquat. Toxicol. 2012, 114, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, E.E.; Exadactylos, A.; Dadali, O.; Golomazou, E.; Klaoudatos, S.; Panagiotaki, P. Molecular cloning of four glutathione peroxidase (GPX) homologs and expression analysis during stress exposure of the marine teleost Sparus aurata. Comp. Biochem. Physiol. B 2014, 168, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nutrient Requirements of Fish and Shrimp; National Research Council: Washington, DC, USA, 2011.
- Torstensen, B.E.; Espe, M.; Sanden, M.; Stubhaug, I.; Waagbø, R.; Hemre, G.-I.; Fontanillas, R.; Nordgarden, U.; Hevrøy, E.M.; Olsvik, P.; et al. Novel production of Atlantic salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 2008, 285, 193–200. [Google Scholar] [CrossRef]
- Grahl-Nielsen, O.; Barnung, T. Variation in the fatty acid profile of marine animals caused by environmental and developmental changes. Mar. Environ. Res. 1985, 17, 218–221. [Google Scholar] [CrossRef]
- Foodstuffs—Determination of Vitamin E by High Performance Liquid Chromatography—Measurement of α-, β-, γ- and δ-Tocopherol; the Netherlands Standardization Institute (NEN): Delft, The Netherlands, 2014; pp. 1–20.
- Segura, J.; Lopez-Bote, C.J. A laboratory efficient method for intramuscular fat analysis. Food Chem. 2014, 145, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Allison, L.A.; Shoup, R.E. Dual electrode liquid chromatography detector for thiols and disulfides. Anal. Chem. 1983, 55, 8–12. [Google Scholar] [CrossRef]
- Han, D.; Sen, C.K.; Roy, S.; Kobayashi, M.S.; Tritschler, H.J.; Packer, I. Protection against glutamate-induced cytotoxicity in C6 glial cells by thiol antioxidants. Am. J. Physiol. 1997, 273, R1771–R1778. [Google Scholar] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. FEBS 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ipharraguerre, I.R.; Tedó, G.; Menoyo, D.; de Diego Cabero, N.; Holst, J.J.; Nofrarías, M.; Mereu, A.; Burrin, D.G. Bile acids induce glucagon-like peptide 2 secretion with limited effects on intestinal adaptation in early weaned pigs. J. Nutr. 2013, 143, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Leaver, M.J.; Villeneuve, L.A.; Obach, A.; Jensen, L.; Bron, J.E.; Tocher, D.R.; Taggart, J.B. Functional genomics reveals increases in cholesterol biosynthetic genes and highly unsaturated fatty acid biosynthesis after dietary substitution of fish oil with vegetable oils in Atlantic salmon (Salmo salar). BMC Genomics 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, M.; Leaver, M.J.; Tocher, D.R. Transcriptional control mechanisms of genes of lipid and fatty acid metabolism in the Atlantic salmon (Salmo salar L.) established cell line, SHK-1. Biochim. Biophys. Acta 2011, 1811, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Schiller Vestergren, A.; Wagner, L.; Pickova, J.; Rosenlund, G.; Kamal-Eldin, A.; Trattner, S. Sesamin modulates gene expression without corresponding effects on fatty acids in Atlantic salmon (Salmo salar L.). Lipids 2012, 47, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7. [Google Scholar] [CrossRef]
- Steibel, J.P.; Poletto, R.; Coussens, P.M.; Rosa, G.J. A powerful and flexible linear mixed model framework for the analysis of relative quantification RT-PCR data. Genomics 2009, 94, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Bestkeeper-excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menoyo, D.; Sanz-Bayón, C.; Nessa, A.H.; Esatbeyoglu, T.; Faizan, M.; Pallauf, K.; De Diego, N.; Wagner, A.E.; Ipharraguerre, I.; Stubhaug, I.; et al. Atlantic Salmon (Salmo salar L.) as a Marine Functional Source of Gamma-Tocopherol. Mar. Drugs 2014, 12, 5944-5959. https://doi.org/10.3390/md12125944
Menoyo D, Sanz-Bayón C, Nessa AH, Esatbeyoglu T, Faizan M, Pallauf K, De Diego N, Wagner AE, Ipharraguerre I, Stubhaug I, et al. Atlantic Salmon (Salmo salar L.) as a Marine Functional Source of Gamma-Tocopherol. Marine Drugs. 2014; 12(12):5944-5959. https://doi.org/10.3390/md12125944
Chicago/Turabian StyleMenoyo, David, Carmen Sanz-Bayón, Anna Hesby Nessa, Tuba Esatbeyoglu, Mohammad Faizan, Kathrin Pallauf, Nuria De Diego, Anika Eva Wagner, Ignacio Ipharraguerre, Ingunn Stubhaug, and et al. 2014. "Atlantic Salmon (Salmo salar L.) as a Marine Functional Source of Gamma-Tocopherol" Marine Drugs 12, no. 12: 5944-5959. https://doi.org/10.3390/md12125944
APA StyleMenoyo, D., Sanz-Bayón, C., Nessa, A. H., Esatbeyoglu, T., Faizan, M., Pallauf, K., De Diego, N., Wagner, A. E., Ipharraguerre, I., Stubhaug, I., & Rimbach, G. (2014). Atlantic Salmon (Salmo salar L.) as a Marine Functional Source of Gamma-Tocopherol. Marine Drugs, 12(12), 5944-5959. https://doi.org/10.3390/md12125944