Abstract
Five new compounds, including a benzopyran ribonic glycoside, daldiniside A (1), two isocoumarin ribonic glycosides, daldinisides B (2) and C (3), and two alkaloids, 1-(3-indolyl)-2R,3-dihydroxypropan-1-one (4) and 3-ethyl-2,5-pyrazinedipropanoic acid (5), along with five known compounds (6–10), were isolated from the EtOAc extract of the marine-associated fungus, Daldinia eschscholzii. Their structures were elucidated by extensive physicochemical and spectroscopic properties, besides comparison with literature data. The absolute configurations of compounds 1–3 were corroborated by chemical transformation, GC analysis and X-ray crystallographic analysis. Meanwhile, the absolute configuration of compound 4 and the planar structure of compound 6 were also determined based on the X-ray diffraction analysis. The cytotoxicity of compounds 1–10, antifungal and anti-HIV activities of compounds 1–5 and the in vitro assay for glucose consumption of compounds 1–3 were done in the anti-diabetic model, whereas none showed obvious activity.
1. Introduction
Marine fungi are known as a rich source of structurally diverse and biologically active secondary metabolites, including polyketides, steroids, terpenes and alkaloids. Nevertheless, the potential chemical investigations on marine fungi are limited. In recent decades, bioactive natural products obtained from the marine-derived fungi have attracted the rising attention of organic chemists for discovering new drugs [1].
It was amazing that slight variations of traditional cultivation conditions, such as media compositions, temperature, aeration or the shape of the culturing flask, might lead to the discovery of various types of new natural products by microorganism [2]. As was reported, Daldinia eschscholzii was well-known to produce abundant polyketides as a mantis-associated fungus [3,4], which motivated us to investigate the secondary metabolites produced by the marine-associated fungus, D. eschscholzii. As part of our ongoing research for structurally unique and bioactive natural products from the D. eschscholzii, we obtained a new benzopyran ribonic glycoside (1), two new isocoumarin ribonic glycosides (2 and 3) and two new alkaloids (4 and 5), together with five known derivatives (6–10) from the scaled-up fermentation of the D. eschscholzii. Herein, we describe the isolation, structural elucidation and biological evaluations of these compounds.
2. Results and Discussion
Chemical Structure Elucidation
The EtOAc extract of the solid medium of D. eschscholzii was subjected to extensive chromatographic separations over silica gel CC, RP-C18 silica gel CC, Sephadex LH-20 and semi-preparative HPLC to yield a new benzopyran ribonic glycoside, daldiniside A (1), two new isocoumarin ribonic glycosides, daldinisides B (2) and C (3), and two new alkaloids (4 and 5), along with five known compounds, 2,5-pyrazinedipropanoic acid (6) [5], cyclo-(Phe-Tyr) (7) [6], de-O-methyldiaporthin (8) [7], 4,6,8-trihydroxy-3,4-dihydronaphthalen-1(2H)-one (9) [8] and orcinotriol (10) [9], as shown in Figure 1.
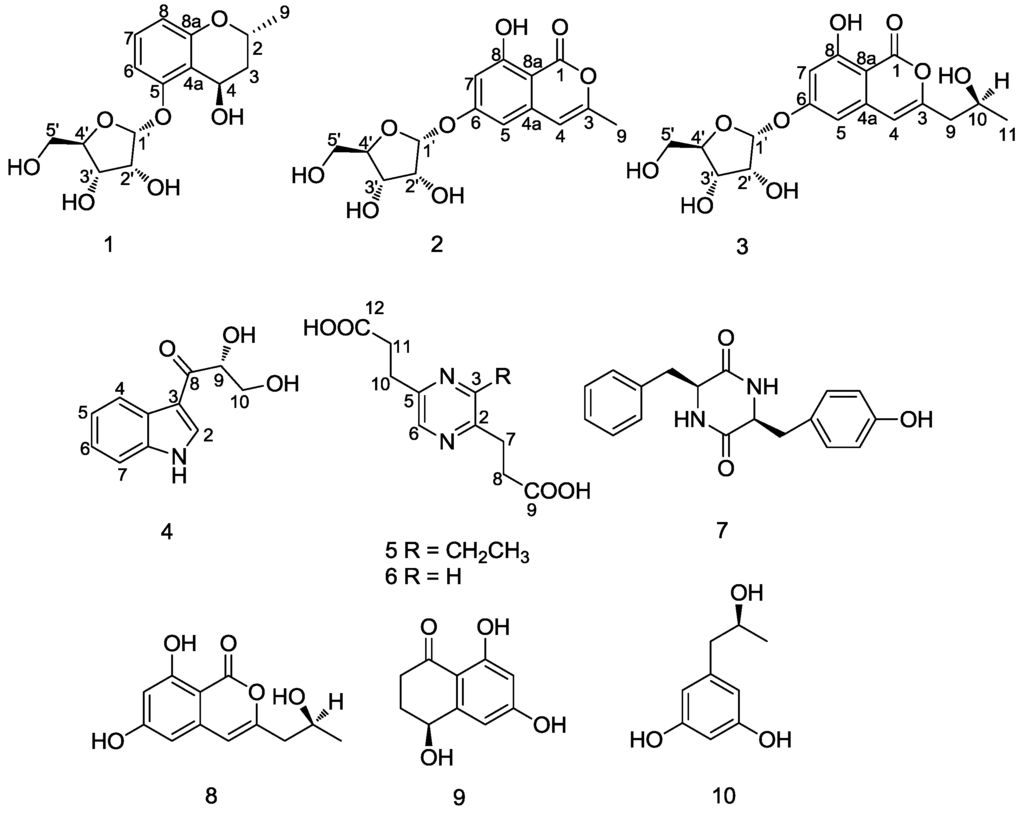
Figure 1.
Structures of compounds 1–10.

Table 1.
1H (400 MHz) and 13C (100 MHz) NMR data for compounds 1–3.
| NO. | 1 a | 2 a | 3 b | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 167.8 | 167.0 | ||||
| 2 | 4.21, m | 68.8 | ||||
| 3 | 1.68, ddd (4.1,12.0, 14.4) 2.01, dt (1.9, 14.4) | 38.9 | 155.9 | 156.7 | ||
| 4 | 5.04, dd (1.9, 4.1) | 60.1 | 6.31, s | 105.8 | 6.40, s | 106.7 |
| 4a | 116.1 | 141.2 | 140.5 | |||
| 5 | 158.6 | 6.58, d (1.8) | 104.5 | 6.70, d (2.1) | 104.3 | |
| 6 | 6.72, d (8.2) | 107.9 | 166.1 | 165.9 | ||
| 7 | 7.12, t (8.2) | 130.5 | 6.62, d (1.8) | 103.9 | 6.89, d (2.1) | 103.7 |
| 8 | 6.49, d (8.2) | 112.0 | 164.5 | 164.1 | ||
| 8a | 157.4 | 101.4 | 101.5 | |||
| 9 | 1.40, d (6.3) | 21.6 | 2.22, s | 19.4 | 2.67, dd (4.9, 14.3) 2.74, dd (7.8, 14.3) | 44.3 |
| 10 | 4.45, m | 65.4 | ||||
| 11 | 1.40, d (6.2) | 24.4 | ||||
| 1′ | 5.70, d (4.5) | 103.4 | 5.74, d (4.4) | 101.8 | 6.17, d (4.2) | 102.1 |
| 2′ | 4.23, dd (4.5, 6.4) | 73.9 | 4.24, dd (4.4, 6.2) | 73.6 | 4.87, m | 74.1 |
| 3′ | 4.10, dd (2.6, 6.4) | 71.4 | 4.12, dd (3.0, 6.2) | 71.2 | 4.85, m | 71.3 |
| 4′ | 4.20, m | 88.5 | 4.15, m | 88.3 | 4.88, m | 89.0 |
| 5′ | 3.64, dd (3.9, 12.1) 3.69, dd (3.6, 12.1) | 63.5 | 3.65, dd (3.8, 12.1) 3.72, dd (3.3, 12.1) | 63.3 | 4.12, dd (3.8, 12.0) 4.17, dd (3.7, 12.0) | 63.4 |
a Measured in CD3OD; b measured in C5D5N.
Compound 1 was obtained as colorless crystal. Its molecular formula was determined as C15H20O7 by HRESIMS at m/z 335.1097 [M + Na]+ (calcd. for C15H20O7Na, 335.1107), indicating the presence of six degrees of unsaturation. The IR spectrum of 1 showed absorptions of hydroxyl (3423 cm−1) and aromatic (1611, 1588 and 1472 cm−1) functionalities. The 1H-NMR spectrum of 1 (Table 1) showed signals at δH 6.72 (1H, d, J = 8.2 Hz), 7.12 (1H, t, J = 8.2 Hz) and 6.49 (1H, d, J = 8.2 Hz), ascribed to one set of the typical 1,2,3-trisubstituted aromatic ring. Additionally, the 1H NMR spectrum of 1 also revealed the signals of one methyl group (δH = 1.40, d, J = 6.3 Hz) and two oxygen-bearing methines at 5.70 (1H, d, J = 4.5 Hz) and 5.04 (1H, dd, J = 1.9, 4.1 Hz). The 13C-NMR spectrum showed one methyl, two methylenes (one oxygenated), nine methines (three aromatic and six oxygenated) and three aromatic quaternary carbons. Furthermore, a series of proton signals at δH 3.64–5.70 and their corresponding carbons at δC 63.5, 71.4, 73.9, 88.5 and 103.4 might suggest a pentose moiety.
Analysis of the key 1H-1H COSY and HMBC correlations (Figure 2) was used to establish the planar structure of 1. In the HMBC spectrum, a diagnostic long-range correlation from the anomeric proton H-1′ to C-5 (δC 158.6) suggested that the sugar moiety was linked to the C-5 of aglycone. The remaining one degree of unsaturation, together with the 1H-1H COSY correlations of H-9/H-2, H-2/H-3 and H-3/H-4 and the HMBC correlations from H-9 to C-2, C-3 and from H-4 to C-2, C-4a, C-5 and C-8a, indicating that a pyranoid ring was linked to C-4a and C-8a, and the methyl and hydroxyl groups were located at C-2 and C-4, respectively. Thus, the planar structure of 1 was established.
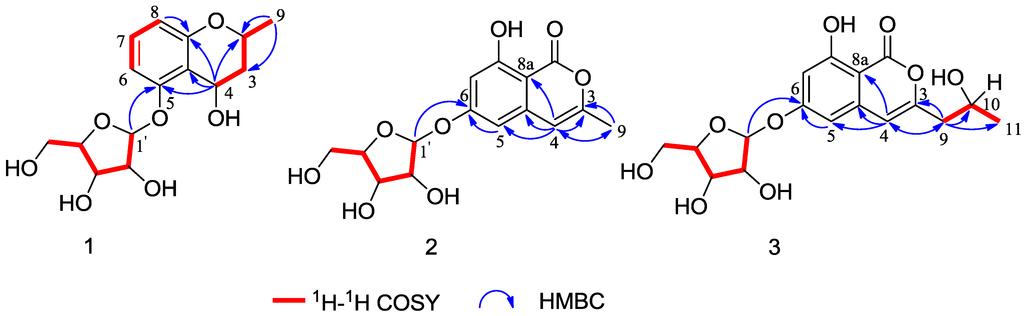
Figure 2.
Selected 1H-1H COSY and HMBC correlations of compounds 1–3.
Acid hydrolysis of 1 gave the sugar motif, and then, it was unambiguously established as d-ribose by chemical transformation and GC analysis. The coupling constant of the anomeric proton at δH 5.70 (H-1′, d, J = 4.5 Hz) in the 1H NMR spectrum of 1 indicated the d-ribose unit to be in the α-configuration [10]. In the NOESY experiment, the correlations of H-2/H-4 or H-9/H-4 were not observed; Thus, it was difficult to determine the configurations at C-2 and C-4. Fortunately, we obtained the crystal of 1, and a single crystal X-ray diffraction experiment was carried out with Cu Kα radiation (Figure 3), allowing an explicit assignment of the absolute structure as 2R and 4R. Hence, the absolute configuration of 1 was elucidated and named daldiniside A.
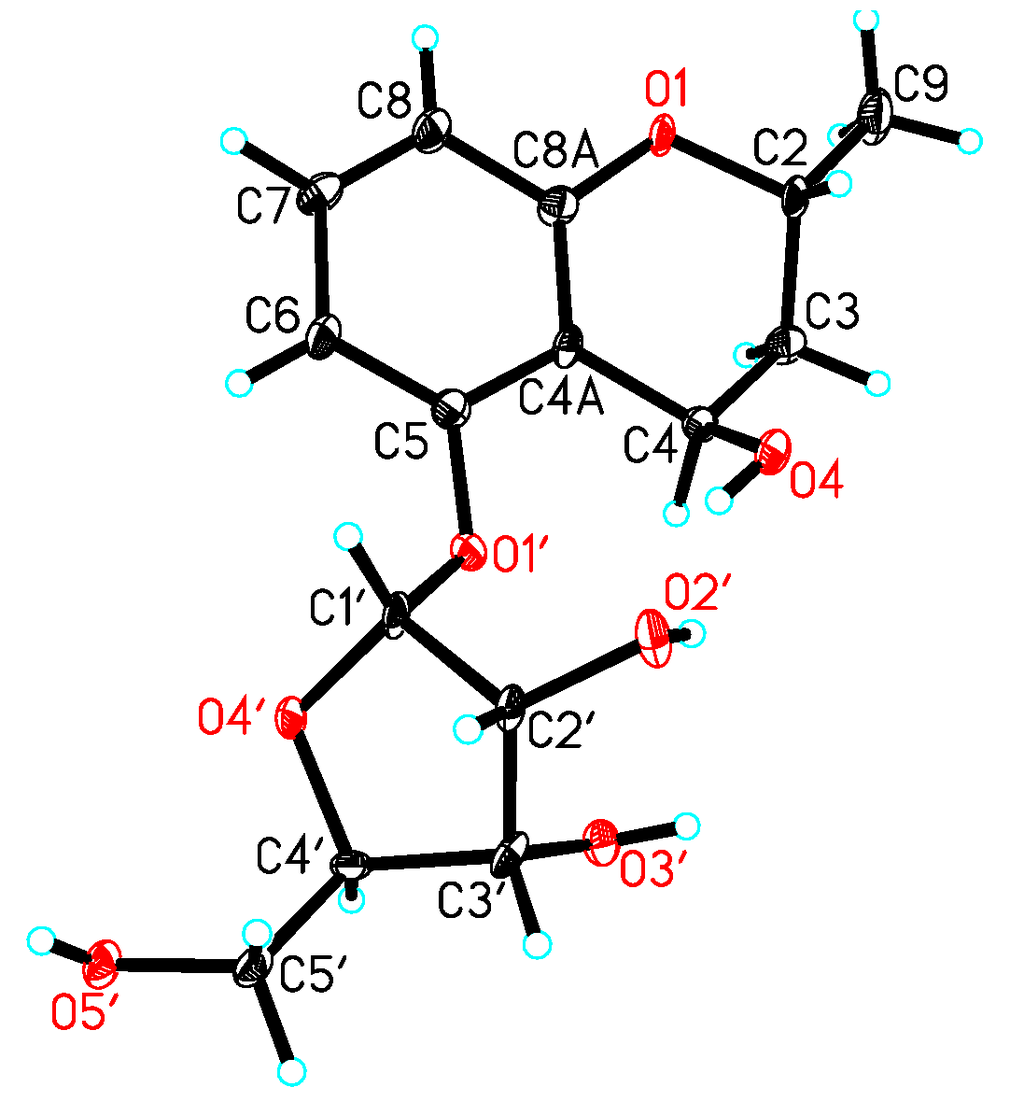
Figure 3.
X-ray structure of compound 1.
Compound 2 was isolated as a yellowish solid with the molecular formula C15H16O8, as deduced by the HRESIMS result ([M + Na]+ at m/z 347.0731, calcd. for C15H16O8Na, 347.0743). The presence of hydroxyl, carbonyl and double bond groups were shown by IR absorption bands at 3429, 1689 and 1573 cm−1, respectively. The α-d-ribose group of 2 was confirmed by NMR experiment (Table 1) and acid hydrolysis. The attachment of the α-d-ribose at C-6 was determined on the basis of the HMBC correlation from H-1′ (δH 5.74) to C-6 (δC 166.1). Apart from the signals of the sugar moiety, the 1H NMR spectrum showed proton signals at δH 6.58 (1H, d, J = 1.8 Hz) and 6.62 (1H, d, J = 1.8 Hz), indicating the presence of a 1,2,3,5-tetrasubstituted aromatic ring. This structural assignment was further established by the HMBC correlations from H-5 to C-6, C-7 and C-8a, and from H-7 to C-5, C-6, C-8 and C-8a. The 13C NMR spectrum showed one ester carbon signal at C-1 (δC 167.8), one olefinic carbon signal at C-4 (δC 105.8) and one methyl carbon signal at C-9 (δC 19.4). The HMBC correlations (Figure 2) from H-9 to C-3, C-4 and from H-4 to C-3, C-4a, C-5, C-8a and C-9 suggested that there existed an isocoumarin unit, in which the hydroxyl and methyl groups were located at C-8 and C-3, respectively. Thus, the structure of 2 was established, namely, daldiniside B.
Compound 3 was determined to be C17H20O9 by the HRESIMS data, which showed a molecular ion at m/z 391.0994 [M + Na]+ (calcd. for C17H20O9Na, 391.1005). The NMR data of 3 were very similar to those of 2 (Table 1), suggesting that they shared the same basic skeleton. Moreover, the signals for a methylene at C-9 (δC 44.3), an oxygenated methine at C-10 (δC 65.4) and a methyl at C-11 (δC 24.4) were observed in the 13C NMR of 3, from which we deduced that a -CH2(9)-CH(10)OH-CH3(11)- group in 3 replaced a -CH3 group in 2. Hence, the planar structure of 3 was determined (Figure 2). To ascertain the absolute configuration at C-10, an acid hydrolysis experiment was carried out. By the chemical transformation and GC analysis, we established the sugar moiety to be α-d-ribose. In addition, the CHCl3 layer was evaporated to dryness, and the NMR data of the residual compound was identical to de-O-methyldiaporthin (: +20.0, c 0.09, MeOH). Therefore, the absolute configuration of 3 was established, namely daldiniside C.
Compound 4 was obtained as a colorless crystal. The molecular formula C11H11NO3 was determined upon analysis of the HRESIMS peak at m/z 228.0628 [M + Na]+ (calcd. for C11H11NO3Na, 228.0637). UV absorption bands at 210, 243, 257 and 300 nm and IR absorption bands at 3394, 3325 and 1607 cm−1 implied the presence of amine, hydroxy and conjugated carbonyl functionalities. In the 1H NMR spectrum (Table 2), the signals at δH 12.03 (1H, s), 8.21 (1H, dd, J = 2.0, 6.6 Hz), 7.19 (1H, m), 7.23 (1H, m) and 7.49 (1H, dd, J = 1.7, 6.8 Hz) indicated the presence of an unsubstituted indole aromatic ring, which was inferred by the 1H-1H COSY correlations of H-4/H-5, H-5/H-6 and H-6/H-7 (Figure 4). The -CO(8)-CH(9)OH-CH2(10)OH- subunit was established by analysis of the 1H-1H COSY correlation of H-9/H-10 and HMBC correlation from H-10 to C-8 and C-9 and linked to the indole moiety by C-3, determined by the HMBC correlations from H-2 to C-3 and C-8. The configuration at C-9 was unequivocally established to be R by the single-crystal X-ray diffraction using Cu Kα radiation (Figure 5). Consequently, the absolute configuration of 4 was established and named 1-(3-indolyl)-2R,3-dihydroxypropan-1-one.

Table 2.
1H and 13C NMR data for compounds 4–6.
| NO. | 4 c | 5 d | 6 c | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 12.03, s | |||||
| 2 | 8.41, s | 134.8 | 152.2 | 153.0 | ||
| 3 | 114.1 | 157.2 | 8.44, d (4.1) | 143.3 | ||
| 3a | 125.9 | |||||
| 4 | 8.21, dd (2.0, 6.6) | 121.4 | ||||
| 5 | 7.19, m | 121.9 | 153.9 | 153.0 | ||
| 6 | 7.23, m | 122.9 | 8.25, s | 141.7 | 8.44, d (4.1) | 143.3 |
| 7 | 7.49, dd (1.7, 6.8) | 112.2 | 3.10, t (7.2) | 29.3 | 2.96, t (7.3) | 29.2 |
| 7a | 136.3 | |||||
| 8 | 195.5 | 2.79, t (7.2) | 32.9 | 2.67, t (7.3) | 32.2 | |
| 9 | 4.69, t (4.5) | 75.8 | 177.1 | 173.8 | ||
| 10 | 3.63, dd (5.5, 11.1) 3.71, dd (4.5, 11.1) | 65.3 | 3.04, t (7.2) | 30.7 | 2.96, t (7.3) | 29.2 |
| 11 | 2.76, t (7.2) | 33.9 | 2.67, t (7.3) | 32.2 | ||
| 12 | 176.9 | 173.8 | ||||
| 13 | 2.88, q (7.5) | 28.3 | ||||
| 14 | 1.29, t (7.5) | 13.1 | ||||
c Measured in DMSO-d6 on a Bruker AM-400 spectrometer; d measured in CD3OD on a Bruker DRX-600 spectrometer.
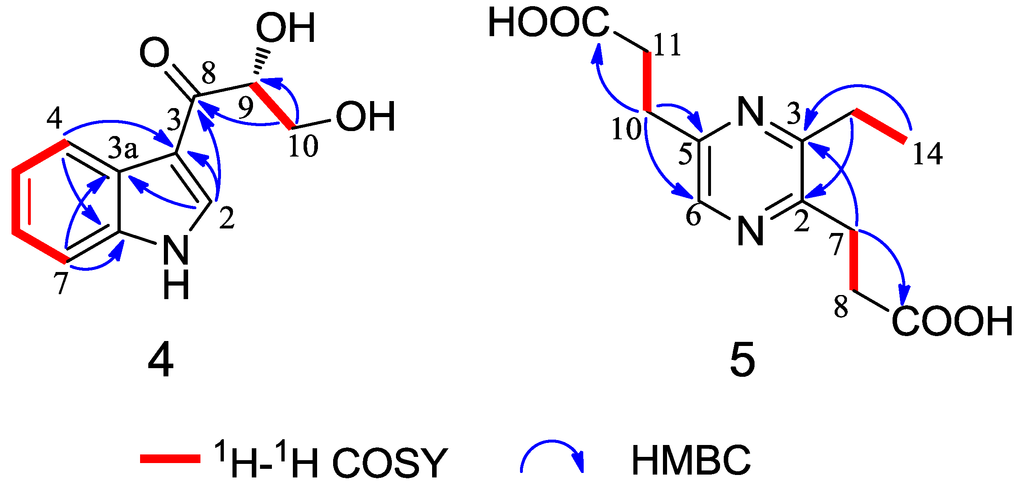
Figure 4.
Selected 1H-1H COSY and HMBC correlations of compounds 4 and 5.
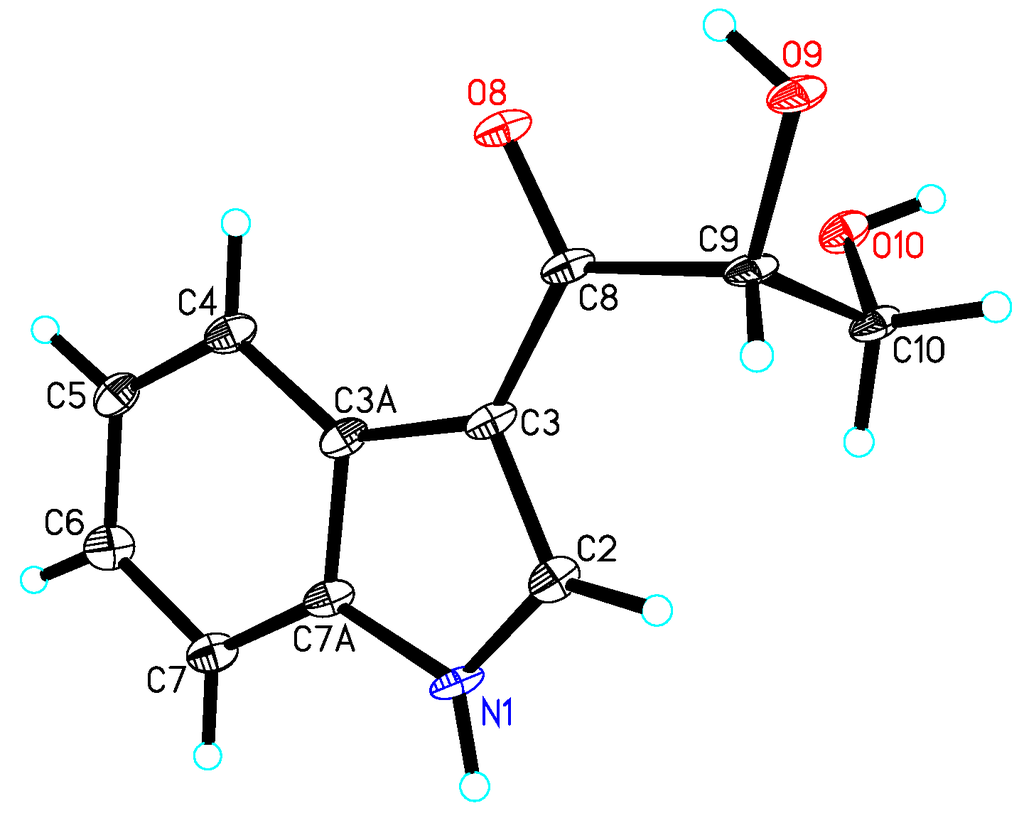
Figure 5.
X-ray structure of compound 4.
Compound 5 was isolated as a yellow oil with the molecular formula C10H12N2O4 as determined by the HRESIMS peak at m/z 275.0998 [M + Na]+ (calcd. for C12H16N2O4Na, 275.1008). In the 2D NMR spectra of 5 (Figure 4), the 1H-1H COSY correlations of H-7/H-8 and H-10/H-11 and the HMBC correlations from H-8 to C-2 and C-9, from H-11 to C-5 and C-12 and from H-6 to C-2 and C-5 indicated the existence of the 2,5-pyrazinedipropanoic acid group. An additional ethyl moiety was located at C-3 by the 1H-1H COSY correlation of H-13/H-14 and a long-rang HMBC correlation from H-14 to C-3. Thus, the structure of 5 was established and named 3-ethyl-2,5-pyrazinedipropanoic acid, whose signals were similar to 6 (Table 2), confirmed by a single-crystal X-ray diffraction using Mo Kα radiation (Figure 6).
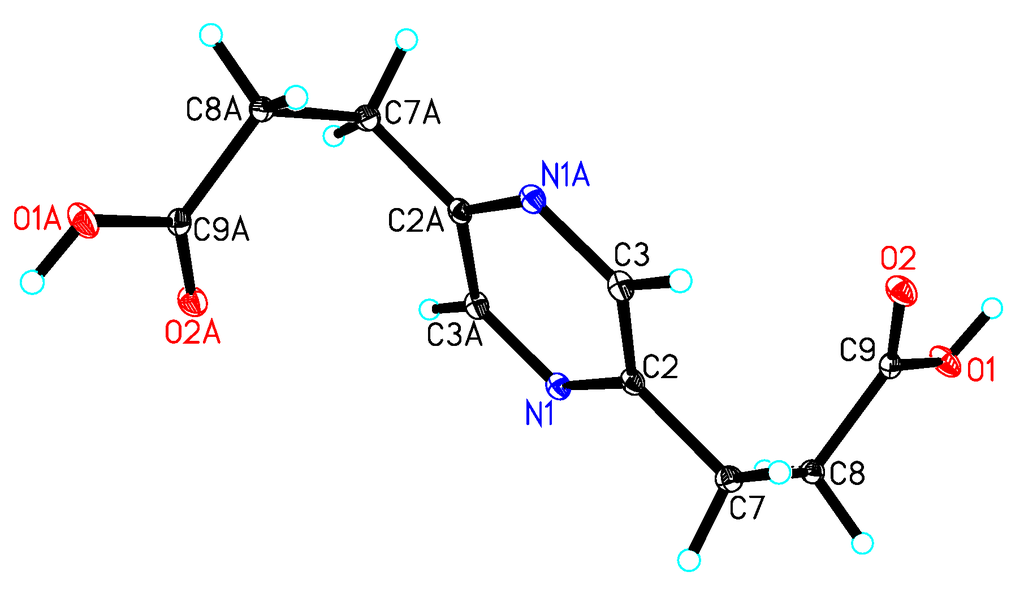
Figure 6.
X-ray structure of compound 6.
3. Experimental Section
3.1. General Experimental Procedures
UV spectra were measured on a Varian Cary 50 spectrophotometer or a Shimadzu UV-2401A spectrophotometer. Optical rotations were recorded on a Perkin-Elmer PE-341LC polarimeter. IR spectra were determined on a Bruker Vertex 70 FT-IR spectrophotometer. The 1H, 13C, and 2D NMR spectroscopic data were recorded on Bruker AM-400 and DRX-600 spectrometers using TMS as the internal standard. HRESIMS data were acquired using an APIQSTAR Pulsar spectrometer. X-ray data were collected using a Bruker APEX DUO diffractometer. Column chromatography was performed on silica gel (100–200 mesh and 200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), RP-C18 silica gel (50 μm, YMC, Kyoto, Japan) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden). Semi-preparative HPLC was conducted on an Agilent 1100 liquid chromatography with a YMC-Pack ODS-A (10 × 250 mm, 5μm, YMC Co., Ltd., Kyoto, Japan) column. GC analysis was performed with an Agilent Technologies 6890N gas chromatography system. Solvents were distilled prior to use, and spectroscopic grade solvents were used. TLC was performed with silica gel 60 F254 (Yantai Chemical Industry Research Institute, Yantai, China) and RP-C18 F254 plates (Merck, Darmstadt, Germany). Peptone (produced by protamine with enzymatic hydrolysis and drying into a pale yellow powder) was purchased from Beijing Shuangxuan Microbial Medium Plant (Product ID: 02-31A, Specification: BR).
3.2. Fungal Material and Fermentation
The strain of fungus D. eschscholzii was isolated from the branches of Scaevola sericea Vahl, collected from the mangrove forest nature reserve in Haikou, Hainan province, China. The fungus was identified by sequence analysis of the ITS region of its rDNA, as described previously [11], and the sequence data have been deposited in NCBI with Accession Number FJ624265. A voucher specimen (MCCC 3J00088) was deposited in a public collection, the Marine Culture Collection of China, MCCC. All of the information and strains collected can be shared at the website http://www.mccc.org.cn/ and the collection center.
The strain D. eschscholzii was cultivated on a potato dextrose agar (PDA) plate at 25 °C for 15 days. The agar was cut into pieces (0.5 × 0.5 cm2) and inoculated into 100 × 500 mL Erlenmeyer (composition: normal rice (100 g), peptone (0.5 g), in distilled water (100 mL)) at 28 °C for 21 days under static conditions.
3.3. Extraction and Isolation
The fermented rice substrate was extracted four times with EtOAc (4 × 25 L) at room temperature. After concentration in vacuo, the total extract (145.0 g) was suspended in water and then extracted exhaustively with petroleum ether and EtOAc, respectively. The EtOAc organic phase was evaporated under reduced pressure to afford a crude extract (77.0 g), which was subjected to silica gel column chromatography (CC) with a CH2Cl2/CH3OH gradient system (1:0, 50:1, 25:1, 10:1, 6:1, 3:1 and 1:1, v/v, each 8 L) to obtain six main fractions (A−F).
Fraction C (CH2Cl2/CH3OH, 10:1; 8.6 g) was subjected to RP-C18 silica gel CC (CH3OH/H2O, 20:80 to 100:0, 12 L) to get five subfractions (C1–C5). Subfraction C2 (CH3OH/H2O, 40:60; 2.8 g) was subjected to Sephadex LH-20 (CH3OH, 1.2 L), then separated by silica gel CC eluted with CH2Cl2–CH3OH (50:1, v/v, 1.9 L) and by semi-preparative HPLC using CH3OH–H2O (2.5 mL/min, CH3OH:H2O = 50:50, v/v) and CH3CN–H2O (2.5 mL/min, CH3CN:H2O = 14:86, v/v) to yield 8 (7.4 mg, tR = 22.0 min) and 9 (2.6 mg, tR = 37.0 min), respectively. Subfraction C3 (CH3OH/H2O, 60:40; 1.2 g) was fractionated by Sephadex LH-20 CC with CH2Cl2/CH3OH (1:1, v/v, 650 mL), silica gel CC (CH2Cl2–CH3OH, 100:1, 1.1 L) and further purified by semi-preparative HPLC using CH3OH–H2O (2.5 mL/min, CH3OH:H2O = 60:40, v/v) to obtain 5 (12.3 mg, tR = 11.7 min).
Fraction D (CH2Cl2/CH3OH, 6:1; 9.5 g) was subjected to RP-C18 silica gel CC eluted with CH3OH–H2O (20:80 to 100:0, 16 L) to afford five subfractions (D1–D5). Subfraction D1 (CH3OH/H2O, 20:80; 2.1 g) was crystallized in CH3OH to yield 6 (30.5 mg) and then chromatographed over Sephadex LH-20 with CH2Cl2/CH3OH (1:1, v/v, 1.3 L), followed by RP-C18 silica gel CC eluted with CH3OH–H2O (10:90 to 20:80, v/v, 4 L) to yield four fractions (D1.1–D1.4). Fraction D1.2 (CH3OH/H2O, 10:90; 505.0 mg) was subjected to silica gel CC (CH2Cl2–CH3OH, 75:1, 750 mL) and purified by semi-preparative HPLC (2.5 mL/min, CH3CN/H2O, 13:87) to yield 10 (14.4 mg, tR = 14.7 min). Fraction D1.3 (CH3OH/H2O, 15:85; 75.0 mg) was subjected to silica gel CC (CH2Cl2–CH3OH, 50:1, 450 mL) and further purified by semi-preparative HPLC (2.5 mL/min, CH3OH/H2O, 20:80) to get 4 (11.4 mg, tR = 40.0 min). Subfraction D2 (CH3OH/H2O, 40:60; 2.2 g) was fractionated by Sephadex LH-20 CC with CH3OH (1.2 L), followed by silica gel CC eluted with CH2Cl2–CH3OH (50:0 to 20:1, v/v, 1.8 L) to obtain three fractions (D2.1–D2.3). Fraction D2.3 (CH2Cl2/CH3OH, 20:1; 300.0 mg) was purified by semi-preparative HPLC (2.5 mL/min, CH3OH/H2O, 30:70) to obtain 3 (250.0 mg, tR = 37.5 min). Fraction D2.2 (CH2Cl2/CH3OH, 30:1; 1.2 g) was successively purified by semi-preparative HPLC using CH3CN−H2O (2.5 mL/min, CH3CN:H2O = 30:70, v/v) to yield 2 (23.0 mg, tR = 11.0 min) and CH3CN–H2O (2.5 mL/min, CH3CN:H2O = 17:83, v/v) to yield 1 (6.5 mg, tR = 36.2 min) and 7 (6.0 mg, tR = 39.0 min), respectively.
Daldiniside A (1): Colorless crystal; : +106.3 (c = 0.16 mg/mL, MeOH); UV (MeOH) λmax (log ε): 206 (4.41), 225 (3.78) and 282 (3.18) nm; IR (KBr) νmax: 3423, 2926, 1611, 1588, 1472, 1263, 1244, 1126, 1088, 1044, 1026 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 335.1097 [M + Na]+ (calcd. for C15H20O7Na, 335.1107).
Daldiniside B (2): Yellowish powder; : +14.1 (c = 0.67 mg/mL, MeOH); UV (MeOH) λmax (log ε): 236 (4.56), 243 (4.57) and 329 (3.69) nm; IR (KBr) νmax: 3429, 2926, 1689, 1644, 1624, 1573, 1506, 1385, 1354, 1235, 1172, 1074, 1043, 694 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 347.0731 [M + Na]+ (calcd. for C15H16O8Na, 347.0743).
Daldiniside C (3): Yellowish powder; : +45.0 (c = 0.13 mg/mL, MeOH); UV (MeOH) λmax (log ε): 203 (3.62), 244 (3.92) and 334 (3.06) nm; IR (KBr) νmax: 3427, 2924, 1686, 1626, 1505, 1384, 1240, 1171, 1127, 1083, 1045, 695 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 391.0994 [M + Na]+ (calcd. for C17H20O9Na, 391.1005).
1-(3-indolyl)-2R,3-dihydroxypropan-1-one (4): Colorless crystal; : +20.0 (c = 0.70 mg/mL, MeOH); UV (MeOH) λmax (log ε): 210 (4.19), 243 (3.82), 257 (3.70) and 300 (3.82) nm; IR (KBr) νmax: 3394, 3325, 1607, 1520, 1442, 1156, 1090, 986, 742, 705 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z 228.0628 [M + Na]+ (calcd. for C11H11NO3Na, 228.0637).
3-ethyl-2,5-pyrazinedipropanoic acid (5): Yellow oil; UV (MeOH) λmax (log ε): 210 (3.94), 243 (3.18) and 279 (3.86) nm; IR (KBr) νmax: 2975, 2935, 1714, 1452, 1392, 1251, 1177, 1122 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z 275.0998 [M + Na]+ (calcd. for C12H16N2O4Na, 275.1008) and m/z 253.1178 [M + H]+ (calcd. for C12H17N2O4, 253.1188).
3.4. X-ray Crystallographic Analysis
Crystal data for 1: C15H20O7, M = 312.31, orthorhombic, a = 5.3212(2) Å, b = 10.3774(4) Å, c = 26.8183(9) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 1480.91(9) Å3, T = 100(2) K, space group P212121, Z = 4, μ(Cu Kα) = 0.943 mm−1, 10,224 reflections measured, 2578 independent reflections (Rint = 0.1191). The final R1 values were 0.1218 (I > 2σ(I)). The final wR(F2) values were 0.3053 (I > 2σ(I)). The final R1 values were 0.1580 (all data). The final wR(F2) values were 0.3760 (all data). The goodness of fit on F2 was 1.445. Flack parameter = 0.0 (7).
Crystal data for 4: C11H11NO3, M = 205.21, monoclinic, a = 4.7449(5) Å, b = 5.4635(5) Å, c = 17.8653(16) Å, α = 90.00°, β = 95.820(5)°, γ = 90.00°, V = 460.75(8) Å3, T = 100(2) K, space group P21, Z = 2, μ(Cu Kα) = 0.903 mm−1, 3,878 reflections measured, 1,498 independent reflections (Rint = 0.0490). The final R1 values were 0.0523 (I > 2σ(I)). The final wR(F2) values were 0.1467 (I > 2σ(I)). The final R1 values were 0.0527 (all data). The final wR(F2) values were 0.1470 (all data). The goodness of fit on F2 was 1.109. Flack parameter = −0.4(4). The Hooft parameter is 0.02(13) for 567 Bijvoet pairs.
Crystal data for 6: C10H12N2O4, M = 224.22, monoclinic, a = 5.6215(9) Å, b = 13.275(2) Å, c = 7.0331(11) Å, α = 90.00°, β = 105.791(2)°, γ = 90.00°, V = 505.04(14) Å3, T = 100(2) K, space group P21/n, Z = 2, μ(Mo Kα) = 0.116 mm−1, 5,086 reflections measured, 1415 independent reflections (Rint = 0.0238). The final R1 values were 0.0368 (I > 2σ(I)). The final wR(F2) values were 0.0918 (I > 2σ(I)). The final R1 values were 0.0380 (all data). The final wR(F2) values were 0.0926 (all data). The goodness of fit on F2 was 1.088.
The crystallographic data for 1 (deposition No. CCDC 989294), 4 (deposition No. CCDC 981181), and 6 (deposition No. CCDC 981180) have been deposited in the Cambridge Crystallographic Data Centre. Copies of the data can be obtained free of charge from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK (fax: +44-1223-336-033; or E-Mail: deposit@ccdc.cam.ac.uk).
3.5. Acid hydrolysis and GC Analysis of 1−3 and Determination of the Absolute Configuration of the Sugar Moiety
Compound 1 (1.2 mg) was hydrolyzed with 2 M aqueous CF3COOH (2.0 mL) at 90 °C for 6 h. The reaction mixture was evaporated to dryness; Then, the residue and l-cysteine methyl ester hydrochloride (2.5 mg) were dissolved in dry pyridine (1.0 mL) and kept at 65 °C for 2 h. The reaction mixture was dried, and then, trimethylsilylimidazole (0.2 mL) was added to the residue, followed by stirring at 65 °C for 1 h [12]. In the end, the resultant solution was extracted with water and n-hexane, and then, the organic phase was submitted to GC analysis by using an HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm, Agilent, Shanghai, China); column temperature, 230 °C; injection temperature, 250 °C; detector FID, detector temperature, 250 °C. A peak at the retention time of 12.61 min for compound 1 was observed. When the corresponding ribose was prepared by the same reaction, the retention times of presilylated d-ribose and l-ribose were 12.66 and 14.09 min, respectively. Hence, the sugar in compound 1 was determined to be d-ribose.
Compounds 2 (1.5 mg) and 3 (9.8 mg) were subjected to a similar treatment as compound 1, and the retention times of ribose were 12.63 and 12.66 min, respectively. Therefore, the sugar in compounds 2 and 3 were determined to be d-ribose. In addition, the reaction mixture of compound 3 was diluted with H2O (1.5 mL) and extracted with CHCl3. The CHCl3 layer was dried to yield the aglycone, whose NMR and optical rotation data were identical to de-O-methyldiaporthin (: +20.0, c 0.09, MeOH) [7].
3.6. Biological Activities
The cytotoxicity of 1–10 against HL-60, SMMC-7721, A-549, MCF-7 and SW-480 was studied using the MTT method [13], and the results showed no obvious inhibitory activity toward the above cancer cells with IC50 > 40 μg/mL. In addition, compounds 1–5 were tested for antifungal activities against Candida albicans (ATCC32354 and ATCC10231) at a concentration of 128 μg/mL and anti-HIV activity according to the described method [14,15]; Unfortunately, none of the compounds exhibited significant activities. Otherwise, the in vitro assay for glucose consumption of compounds 1–3 was done in the anti-diabetic model with DMEM-induced 3T3 fibroblasts [16], whereas none showed obvious activity at the concentration of 20 μg/mL.
4. Conclusions
A new benzopyran glycoside, daldiniside A (1), two new isocoumarin glycosides, daldinisides B (2) and C (3), and two new alkaloids, 1-(3-indolyl)-2R,3-dihydroxypropan-1-one (4) and 3-ethyl-2,5-pyrazinedipropanoic acid (5), together with five known compounds, 2,5-pyrazinedipropanoic acid (6), cyclo-(Phe-Tyr) (7), de-O-methyldiaporthin (8), 4,6,8-trihydroxy-3,4-dihydronaphthalen-1(2H)-one (9) and orcinotriol (10), were discovered from the marine-associated fungus, D. eschscholzii. Natural products embodying the α-d-ribose moiety were quite scarce to be reported. To the best of our knowledge, compounds 2 and 3 were hitherto the first example of isocoumarins containing the α-d-ribose moiety in natural products. Interestingly, the nonenzymatic cyclic dimerization of 5-aminolevulinic acid (5-ALA) might be the key reaction to form a pyrazine nucleus, and a series of derivatives might lead to the formation of 5 and 6 [17].
Supplementary Files
Supplementary File 1Acknowledgments
This project was financially supported by the National Natural Science Foundation of China (31270395, 81102334, 31370372), the Program for New Century Excellent Talents in University, State Education Ministry of China (NCET-2008-0224), and the National Science and Technology Project of China (2011ZX09102-004).
Author Contributions
All listed authors contributed to this work. Performed most of the experiments and wrote this paper: Zheng-Xi Hu. Organized this work and contributed to the structural determination and biological assay of the new compounds: Zeng-Wei Luo and Jin-Wen Zhang. Extraction and isolation: Xiao-Bin Bi. Contributed to the NMR experiments: Guang-Min Yao. Contributed to the X-ray diffraction experiments: Xiao-Nian Li. Advised and assisted Hu’s experiments and also shared the tasks of the manuscript preparation: Jian-Ping Wang and Yong-Bo Xue. The project leader organizing and guiding the experiments and manuscript writing: Yong-Hui Zhang.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [PubMed]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [PubMed]
- Zhang, Y.L.; Ge, H.M.; Zhao, W.; Dong, H.; Xu, Q.; Li, S.H.; Li, J.; Zhang, J.; Song, Y.C.; Tan, R.X. Unprecedented immunosuppressive polyketides from Daldinia eschscholzii, a mantis-associated fungus. Angew. Chem. Int. Ed. 2008, 47, 5823–5826. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, J.; Jiang, N.; Lu, Y.H.; Wang, L.; Xu, S.H.; Wang, W.; Zhang, G.F.; Xu, Q.; Ge, H.M.; et al. Immunosuppressive polyketides from mantis-associated Daldinia eschscholzii. J. Am. Chem. Soc. 2011, 133, 5931–5940. [Google Scholar] [CrossRef] [PubMed]
- McCarron, P.A.; Donnelly, R.F.; Woolfson, A.D.; Andrews, G.P. Analysis of pyrazine 2,5-dipropionic acid in 5-aminolevulinic acid-loaded urological and topical delivery vehicles: Methodology and assay validation. J. Pharm. Biomed. Anal. 2005, 36, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Yang, R.Y.; Guo, Z.Y.; She, Z.G.; Lin, Y.C. A new xanthone derivative from mangrove endophytic fungus No. ZSU-H16. Chem. Nat. Compd. 2010, 46, 15–18. [Google Scholar] [CrossRef]
- Hallock, Y.F.; Clardy, J.; Kenfield, D.S.; Strobel, G. De-O-methyldiaporthin, a phytotoxin from Drechslera siccans. Phytochemistry 1988, 27, 3123–3125. [Google Scholar] [CrossRef]
- Dong, J.Y.; Song, H.C.; Li, J.H.; Tang, Y.S.; Sun, R.; Wang, L.; Zhou, Y.P.; Wang, L.M.; Shen, K.Z.; Wang, C.R.; et al. Ymf 1029A-E, preussomerin analogues from the fresh-water-derived fungus YMF 1.01029. J. Nat. Prod. 2008, 71, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, H.; Tenma, M.; Shimazaki, K.; Kobayashi, J. Three new metabolites from the marine yeast Aureobasidium pullulans. J. Nat. Prod. 1998, 61, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, R.U.; Stevens, J.D. The proton magnetic resonance spectra and tautomeric equilibria of aldoses in deuterium oxide. Can. J. Chem. 1966, 44, 249–262. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.M.; Teuscher, F.; Li, D.L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Nhiem, N.X.; Kiem, P.V.; Minh, C.V.; Kim, N.; Park, S.; Lee, H.Y.; Kim, E.S.; Kim, Y.H.; Kim, S.; Koh, Y. Diarylheptanoids and flavonoids from Viscum album inhibit LPS-Stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. J. Nat. Prod. 2013, 76, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- He, W.J.; Chu, H.B.; Zhang, Y.M.; Han, H.J.; Yan, H.; Zeng, G.Z.; Fu, Z.H.; Olubanke, O.; Tan, N.H. Antimicrobial, cytotoxic lignans and terpenoids from the twigs of Pseudolarix Kaempferi. Planta Med. 2011, 77, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Lu, Y.; Liao, Q.; Wu, Y.; Chen, X. Fangchinoline inhibits human immunodeficiency virus type 1 replication by interfering with gp160 proteolytic processing. PLoS One 2012, 7, e39225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Xu, G.L.; Liu, Y.H.; Rao, Y.; Yu, R.Y.; Zhang, Z.W.; Wang, Y.S.; Tao, L. Anti-diabetic activities of Gegen Qinlian Decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes. Phytomedicine 2013, 20, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.R.; George, S. The nonenzymatic cyclic dimerisation of 5-aminolevulinic acid. Tetrahedron 1992, 48, 7879–7886. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
