Optimal Cleavage and Oxidative Folding of α-Conotoxin TxIB as a Therapeutic Candidate Peptide
Abstract
:1. Introduction

2. Results and Discussion
2.1. Results
2.1.1. Fmoc Synthesis and Cleavage of α-CTx TxIB Resin-Bounded Peptide
| NO. | Recipe |
|---|---|
| Cleavage Cocktail 1 | TFA/phenol/water/TIPS (264/15/15/6 μL, v⁄v⁄v⁄v) |
| Cleavage Cocktail 2 | TFA/water/TIPS (279/15/6 μL, v⁄v⁄v) |
| Cleavage Cocktail 3 | TFA/water (285/15 μL, v⁄v) |
| Cleavage Cocktail 4 | TFA/phenol/water/thioanisole/EDT (247.5/15/15/7.5 μL, v⁄v⁄v⁄v/v) |

2.1.2. Two-step Oxidative Folding of α-CTx TxIB Linear Peptide

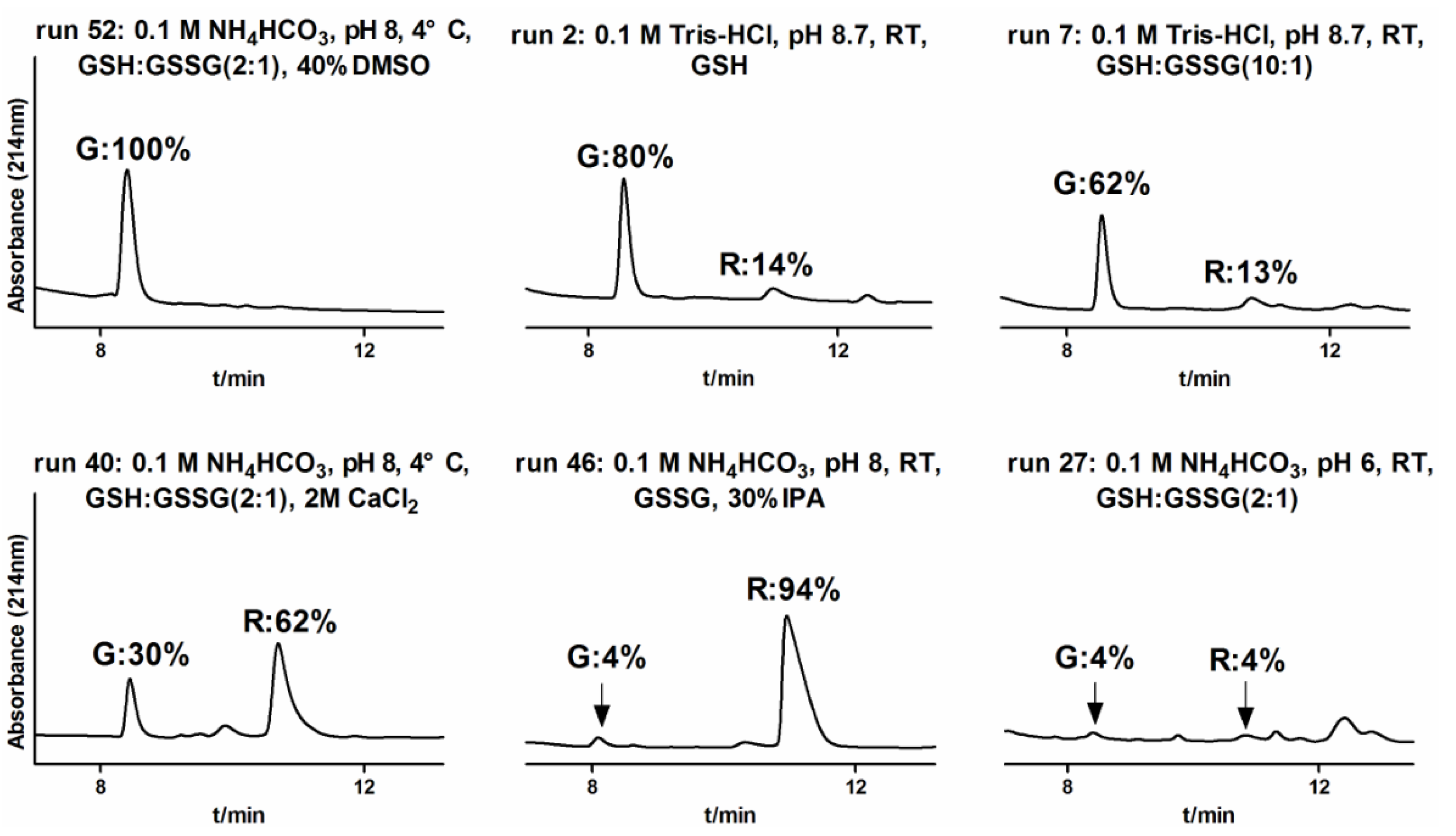
2.1.3. One-Step Oxidative Folding of α-CTx TxIB Linear Peptide
| Oxidation Condition | TxIB | ||||||
|---|---|---|---|---|---|---|---|
| Run | Buffer | Redox Reagent | pH | Temp | Cosolvent/Salt | G(%) | R(%) |
| 52 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | 4 °C | 40% DMSO | 100 | 0 |
| 50 | 0.1 M Tris-HCl | GSH | 8.7 | 4 °C | 40% DMSO | 100 | 0 |
| 51 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | 4 °C | 30% DMSO | 96 | 2 |
| 49 | 0.1 M Tris-HCl | GSH | 8.7 | 4 °C | 30% DMSO | 94 | 3 |
| 32 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | 4 °C | - | 91 | 8 |
| 34 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 9 | 4 °C | - | 90 | 8 |
| 24 | 0.1 M Tris-HCl | GSH | 8.7 | 4 °C | - | 88 | 12 |
| 12 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | RT | - | 88 | 11 |
| 35 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 9 | RT | - | 87 | 12 |
| 20 | 0.1 M Tris-HCl | GSH | 7 | 37 °C | - | 86 | 11 |
| 31 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 7 | 37 °C | - | 86 | 11 |
| 21 | 0.1 M Tris-HCl | GSH | 8 | 4 °C | - | 83 | 14 |
| 33 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | 37 °C | - | 83 | 14 |
| 30 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 7 | RT | - | 83 | 13 |
| 45 | 0.1 M NH4HCO3 | GSH | 8 | RT | 30% IPA | 83 | 7 |
| 11 | 0.1 M NH4HCO3 | GSH:GSSG (1:1) | 8 | RT | - | 82 | 11 |
| 13 | 0.1 M NH4HCO3 | GSH:GSSG (5:1) | 8 | RT | - | 82 | 13 |
| 8 | 0.1 M NH4HCO3 | - | 8 | RT | - | 82 | 13 |
| 25 | 0.1 M Tris-HCl | GSH | 8.7 | 37 °C | - | 82 | 8 |
| 29 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 7 | 4 °C | - | 81 | 15 |
| 2 | 0.1 M Tris-HCl | GSH | 8.7 | RT | - | 80 | 14 |
| 9 | 0.1 M NH4HCO3 | GSH | 8 | RT | - | 80 | 11 |
| 36 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 9 | 37 °C | - | 80 | 15 |
| 3 | 0.1 M Tris-HCl | GSSG | 8.7 | RT | - | 79 | 15 |
| 4 | 0.1 M Tris-HCl | GSH:GSSG(1:1) | 8.7 | RT | - | 77 | 16 |
| 5 | 0.1 M Tris-HCl | GSH:GSSG (2:1) | 8.7 | RT | - | 77 | 17 |
| 39 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | 4 °C | 2 M (NH4)2SO4 | 77 | 21 |
| 22 | 0.1 M Tris-HCl | GSH | 8 | RT | - | 76 | 17 |
| 23 | 0.1 M Tris-HCl | GSH | 8 | 37 °C | - | 76 | 11 |
| 10 | 0.1 M NH4HCO3 | GSSG | 8 | RT | - | 75 | 22 |
| 14 | 0.1 M NH4HCO3 | GSH:GSSG (10:1) | 8 | RT | - | 75 | 11 |
| 19 | 0.1 M Tris-HCl | GSH | 7 | RT | - | 75 | 14 |
| 18 | 0.1 M Tris-HCl | GSH | 7 | 4 °C | - | 72 | 13 |
| 6 | 0.1 M Tris-HCl | GSH:GSSG (5:1) | 8.7 | RT | - | 70 | 15 |
| 54 | 0.1 M NH4HCO3 | 8 | 4 °C | 30% DMSO | 67 | 19 | |
| 1 | 0.1 M Tris-HCl | - | 8.7 | RT | - | 66 | 7 |
| 37 | 0.1 M Tris-HCl | GSH | 8.7 | 4 °C | 2 M (NH4)2SO4 | 64 | 25 |
| 7 | 0.1 M Tris-HCl | GSH:GSSG (10:1) | 8.7 | RT | - | 62 | 13 |
| 48 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | 4 °C | 50% IPA | 60 | 23 |
| 53 | 0.1 M Tris-HCl | 8.7 | 4 °C | 30% DMSO | 54 | 46 | |
| 43 | 0.1 M Tris-HCl | GSH | 8.7 | 4 °C | 30% IPA | 40 | 52 |
| 47 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | 4 °C | 30% IPA | 35 | 58 |
| 17 | 0.1 M Tris-HCl | GSH | 6 | 37 °C | - | 31 | 11 |
| 41 | 0.1 M Tris-HCl | GSH | 8.7 | RT | 30% IPA | 31 | 63 |
| 38 | 0.1 M Tris-HC | GSH | 8.7 | 4 °C | 2 M CaCl2 | 31 | 61 |
| 40 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 8 | 4 °C | 2 M CaCl2 | 30 | 62 |
| 28 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 6 | 37 °C | - | 23 | 17 |
| 16 | 0.1 M Tris-HCl | GSH | 6 | RT | - | 16 | 5 |
| 42 | 0.1 M Tris-HCl | GSSG | 8.7 | RT | 30% IPA | 16 | 73 |
| 44 | 0.1 M Tris-HCl | GSH | 8.7 | 4 °C | 50% IPA | 16 | 84 |
| 27 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 6 | RT | - | 4 | 4 |
| 46 | 0.1 M NH4HCO3 | GSSG | 8 | RT | 30% IPA | 4 | 94 |
| 15 | 0.1 M Tris-HCl | GSH | 6 | 4 °C | - | ND | ND |
| 26 | 0.1 M NH4HCO3 | GSH:GSSG (2:1) | 6 | 4 °C | - | ND | ND |

2.1.4. Optimization of One-Step Oxidative Folding

2.1.5. CD Spectra of α-CTx TxIB Isomers

| Isomer | Secondary structure | |||
|---|---|---|---|---|
| α-helix | β-sheet | β-turns | random coil | |
| globular | 15% | 24% | 28% | 33% |
| ribbon | 12% | 29% | 26% | 33% |
2.2. Discussion
3. Experimental Section
3.1.Materials
3.2.Resin-Bounded Peptide Synthesis and Cleavage
3.3. Two-Step Oxidation Folding
3.4. One-Step Oxidative Folding
3.5. Circular Dichroism (CD) Measurements
4. Conclusions
Abbreviations
| DMSO | dimethyl sulfoxide |
| GSH | reduced glutathione |
| GSSG | oxidized glutathione |
| HPLC | high performance liquid chromatography |
| ESI-MS | electrospray ionization mass spectrum |
| ACN | acetonitrile |
| CDSSTR | a program for estimating protein secondary structure fractions |
| α-CTx | α-conotoxin |
Acknowledgments
Conflicts of Interest
References
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Millar, N.S.; Gotti, C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef]
- Rose, J.E. Multiple brain pathways and receptors underlying tobacco addiction. Biochem. Pharmacol. 2007, 74, 1263–1270. [Google Scholar] [CrossRef]
- Quik, M.; Perez, X.A.; Grady, S.R. Role of α6 nicotinic receptors in CNS dopaminergic function: Relevance to addiction and neurological disorders. Biochem. Pharmacol. 2011, 82, 873–882. [Google Scholar] [CrossRef]
- Luo, S.; Christensen, S.; Zhangsun, D.; Wu, Y.; Hu, Y.; Zhu, X.; Chhabra, S.; Norton, R.S.; McIntosh, J.M. A novel inhibitor of alpha9alpha10 nicotinic acetylcholine receptors from Conus vexillum delineates a new conotoxin superfamily. PLoS ONE 2013, 8, e54648. [Google Scholar]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Han, T.S.; Teichert, R.W.; Olivera, B.M.; Bulaj, G. Conus venoms-a rich source of peptide-based therapeutics. Curr. Pharm. Des. 2008, 14, 2462–2479. [Google Scholar] [CrossRef]
- Luo, S.; Akondi, K.B.; Zhangsun, D.; Wu, Y.; Zhu, X.; Hu, Y.; Christensen, S.; Dowell, C.; Daly, N.L.; Craik, D.J.; et al. Atypical alpha-conotoxin LtIA from Conus litteratus targets a novel microsite of the alpha3beta2 nicotinic receptor. J. Biol. Chem. 2010, 285, 12355–12366. [Google Scholar] [CrossRef]
- Ellison, M.; Haberlandt, C.; Gomez-Casati, M.E.; Watkins, M.; Elgoyhen, A.B.; McIntosh, J.M.; Olivera, B.M. Alpha-RgIA: A novel conotoxin that specifically and potently blocks the alpha9alpha10 nAChR. Biochemistry 2006, 45, 1511–1517. [Google Scholar] [CrossRef]
- Luo, S.; Kulak, J.M.; Cartier, G.E.; Jacobsen, R.B.; Yoshikami, D.; Olivera, B.M.; McIntosh, J.M. alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J. Neurosci. 1998, 18, 8571–8579. [Google Scholar]
- Lopez-Vera, E.; Aguilar, M.B.; Schiavon, E.; Marinzi, C.; Ortiz, E.; Restano Cassulini, R.; Batista, C.V.; Possani, L.D.; Heimer de la Cotera, E.P.; Peri, F.; et al. Novel alpha-conotoxins from Conus spurius and the alpha-conotoxin EI share high-affinity potentiation and low-affinity inhibition of nicotinic acetylcholine receptors. FEBS J. 2007, 274, 3972–3985. [Google Scholar] [CrossRef]
- Klink, R.; de Kerchove d’Exaerde, A.; Zoli, M.; Changeux, J.P. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci. 2001, 21, 1452–1463. [Google Scholar]
- Livett, B.G.; Sandall, D.W.; Keays, D.; Down, J.; Gayler, K.R.; Satkunanathan, N.; Khalil, Z. Therapeutic applications of conotoxins that target the neuronal nicotinic acetylcholine receptor. Toxicon 2006, 48, 810–829. [Google Scholar] [CrossRef]
- Gehrmann, J.; Alewood, P.F.; Craik, D.J. Structure determination of the three disulfide bond isomers of α-conotoxin GI: A model for the role of disulfide bonds in structural stability. J. Mol. Biol. 1998, 278, 401–415. [Google Scholar] [CrossRef]
- Luo, S.; Zhangsun, D.; Wu, Y.; Zhu, X.; Hu, Y.; McIntyre, M.; Christensen, S.; Akcan, M.; Craik, D.J.; McIntosh, J.M. Characterization of a Novel alpha-Conotoxin from Conus textile that Selectively Targets Alpha6/alpha3beta2betab3 Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2013, 288, 894–902. [Google Scholar] [CrossRef]
- Guy, C.A.; Fields, G.B. Trifluoroacetic acid cleavage and deprotection of resin-bound peptides following synthesis by Fmoc chemistry. Methods Enzymol. 1997, 289, 67–83. [Google Scholar] [CrossRef]
- Fields, C.G.; Fields, G.B. Minimization of tryptophan alkylation following 9-fluorenylmethoxycarbonyl solid-phase peptide synthesis. Tetrahedron Lett. 1993, 34, 6661–6664. [Google Scholar] [CrossRef]
- King, D.S.; Fields, C.G.; Fields, G.B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int. J. Pept. Protein Res. 1990, 36, 255–266. [Google Scholar]
- Pearson, D.A.; Blanchette, M.; Baker, M.L.; Guindon, C.A. Trialkylsilanes as scavengers for the trifluoroacetic acid deblocking of protecting groups in peptide synthesis. Tetrahedron Lett. 1989, 30, 2739–2742. [Google Scholar] [CrossRef]
- Bulaj, G.; Olivera, B.M. Folding of conotoxins: formation of the native disulfide bridges during chemical synthesis and biosynthesis of Conus peptides. Antioxid. Redox Signal. 2008, 10, 141–155. [Google Scholar] [CrossRef]
- DeLa Cruz, R.; Whitby, F.G.; Buczek, O.; Bulaj, G. Detergent-assisted oxidative folding of delta-conotoxins. J. Pept. Res. 2003, 61, 202–212. [Google Scholar] [CrossRef]
- Gyanda, R.; Banerjee, J.; Chang, Y.P.; Phillips, A.M.; Toll, L.; Armishaw, C.J. Oxidative folding and preparation of alpha-conotoxins for use in high-throughput structure-activity relationship studies. J. Pept. Sci. 2012, 19, 16–24. [Google Scholar]
- Konermann, L.; Stocks, B.B.; Pan, Y.; Tong, X. Mass spectrometry combined with oxidative labeling for exploring protein structure and folding. Mass Spectrom. Rev. 2010, 29, 651–667. [Google Scholar]
- Salamanca, S.; Chang, J.Y. Unfolding and refolding pathways of a major kinetic trap in the oxidative folding of alpha-lactalbumin. Biochemistry 2005, 44, 744–750. [Google Scholar] [CrossRef]
- Wong, C.T.T.; Taichi, M.; Nishio, H.; Nishiuchi, Y.; Tam, J.P. Optimal Oxidative Folding of the Novel Antimicrobial Cyclotide from Hedyotis biflora Requires High Alcohol Concentrations. Biochemistry 2011, 50, 7275–7283. [Google Scholar] [CrossRef]
- Gunasekera, S.; Daly, N.L.; Clark, R.J.; Craik, D.J. Dissecting the oxidative folding of circular cystine knot miniproteins. Antioxid. Redox signal. 2009, 11, 971–980. [Google Scholar] [CrossRef]
- Leta Aboye, T.; Clark, R.J.; Craik, D.J.; Goransson, U. Ultra-stable peptide scaffolds for protein engineering-synthesis and folding of the circular cystine knotted cyclotide cycloviolacin O2. Chembiochem. 2008, 9, 103–113. [Google Scholar] [CrossRef]
- Steiner, A.M.; Bulaj, G. Optimization of oxidative folding methods for cysteine-rich peptides: a study of conotoxins containing three disulfide bridges. J. Pept. Sci. 2011, 17, 1–7. [Google Scholar] [CrossRef]
- Fuller, E.; Green, B.R.; Catlin, P.; Buczek, O.; Nielsen, J.S.; Olivera, B.M.; Bulaj, G. Oxidative folding of conotoxins sharing an identical disulfide bridging framework. FEBS J. 2005, 272, 1727–1738. [Google Scholar] [CrossRef]
- Bulaj, G.; Buczek, O.; Goodsell, I.; Jimenez, E.C.; Kranski, J.; Nielsen, J.S.; Garrett, J.E.; Olivera, B.M. Efficient oxidative folding of conotoxins and the radiation of venomous cone snails. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. 2), 14562–14568. [Google Scholar] [CrossRef]
- Zhang, R.M.; Snyder, G.H. Factors governing selective formation of specific disulfides in synthetic variants of alpha-conotoxin. Biochemistry 1991, 30, 11343–11348. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Buczek, P.; Bulaj, G. Cosolvent-assisted oxidative folding of a bicyclic alpha-conotoxin ImI. J. Pept. Sci. 2004, 10, 249–256. [Google Scholar] [CrossRef]
- Grishin, A.A.; Wang, C.I.; Muttenthaler, M.; Alewood, P.F.; Lewis, R.J.; Adams, D.J. Alpha-conotoxin AuIB isomers exhibit distinct inhibitory mechanisms and differential sensitivity to stoichiometry of alpha3beta4 nicotinic acetylcholine receptors. J. Biol. Chem. 2010, 285, 22254–22263. [Google Scholar]
- Clark, R.J.; Daly, N.L.; Halai, R.; Nevin, S.T.; Adams, D.J.; Craik, D.J. The three-dimensional structure of the analgesic alpha-conotoxin, RgIA. FEBS Lett. 2008, 582, 597–602. [Google Scholar] [CrossRef]
- Anderson, M.E.; Meister, A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc. Natl. Acad. Sci. USA 1983, 80, 707–711. [Google Scholar] [CrossRef]
- Aboye, T.L.; Clark, R.J.; Burman, R.; Roig, M.B.; Craik, D.J.; Goransson, U. Interlocking disulfides in circular proteins: toward efficient oxidative folding of cyclotides. Antioxid. Redox Signal. 2011, 14, 77–86. [Google Scholar] [CrossRef]
- Kubo, S.; Chino, N.; Kimura, T.; Sakakibara, S. Oxidative folding of omega-conotoxin MVIIC: Effects of temperature and salt. Biopolymers 1996, 38, 733–744. [Google Scholar] [CrossRef]
- Tam, J.P.; Wu, C.R.; Liu, W.; Zhang, J.W. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J. Am. Chem. Soc. 1991, 113, 6657–6662. [Google Scholar] [CrossRef]
- Bulaj, G.; Kortemme, T.; Goldenberg, D.P. Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 1998, 37, 8965–8972. [Google Scholar] [CrossRef]
- Zhang, J.; Diamond, S.; Arvedson, T.; Sasu, B.J.; Miranda, L.P. Oxidative folding of hepcidin at acidic pH. Biopolymers 2010, 94, 257–264. [Google Scholar] [CrossRef]
- Kubo, S.; Tanimura, K.; Nishio, H.; Chino, N.; Teshima, T.; Kimura, T.; Nishiuchi, Y. Optimization of the Oxidative Folding Reaction and Disulfide Structure Determination of Human α-Defensin 1, 2, 3 and 5. Int. J. Pept. Res. Ther. 2008, 14, 341–349. [Google Scholar] [CrossRef]
- Daly, N.L.; Craik, D.J. Acyclic permutants of naturally occurring cyclic proteins Characterization of cystine knot and β-sheet formation in the macrocyclic polypeptide kalata B1. J. Biol. Chem. 2000, 275, 19068–19075. [Google Scholar] [CrossRef]
- Shimizu, S.; Shimizu, K. Alcohol denaturation: thermodynamic theory of peptide unit solvation. J. Am. Chem. Soc. 1999, 121, 2387–2394. [Google Scholar] [CrossRef]
- Welker, E.; Narayan, M.; Wedemeyer, W.J.; Scheraga, H.A. Structural determinants of oxidative folding in proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 2312–2316. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, X.; Wu, Y.; Zhu, F.; Yang, Q.; Wu, Q.; Zhangsun, D.; Luo, S. Optimal Cleavage and Oxidative Folding of α-Conotoxin TxIB as a Therapeutic Candidate Peptide. Mar. Drugs 2013, 11, 3537-3553. https://doi.org/10.3390/md11093537
Wu X, Wu Y, Zhu F, Yang Q, Wu Q, Zhangsun D, Luo S. Optimal Cleavage and Oxidative Folding of α-Conotoxin TxIB as a Therapeutic Candidate Peptide. Marine Drugs. 2013; 11(9):3537-3553. https://doi.org/10.3390/md11093537
Chicago/Turabian StyleWu, Xiaosa, Yong Wu, Furong Zhu, Qiuyuan Yang, Qianqian Wu, Dongting Zhangsun, and Sulan Luo. 2013. "Optimal Cleavage and Oxidative Folding of α-Conotoxin TxIB as a Therapeutic Candidate Peptide" Marine Drugs 11, no. 9: 3537-3553. https://doi.org/10.3390/md11093537
APA StyleWu, X., Wu, Y., Zhu, F., Yang, Q., Wu, Q., Zhangsun, D., & Luo, S. (2013). Optimal Cleavage and Oxidative Folding of α-Conotoxin TxIB as a Therapeutic Candidate Peptide. Marine Drugs, 11(9), 3537-3553. https://doi.org/10.3390/md11093537






