Synthesis of Glycyrrhetinic Acid-Modified Chitosan 5-Fluorouracil Nanoparticles and Its Inhibition of Liver Cancer Characteristics in Vitro and in Vivo
Abstract
:1. Introduction
2. Results
2.1. Fourier Transformed Infrared Spectroscopy (FT-IR) Spectra and 1H-Nuclear Magnetic Resonance (1H-NMR) of GA-CTS
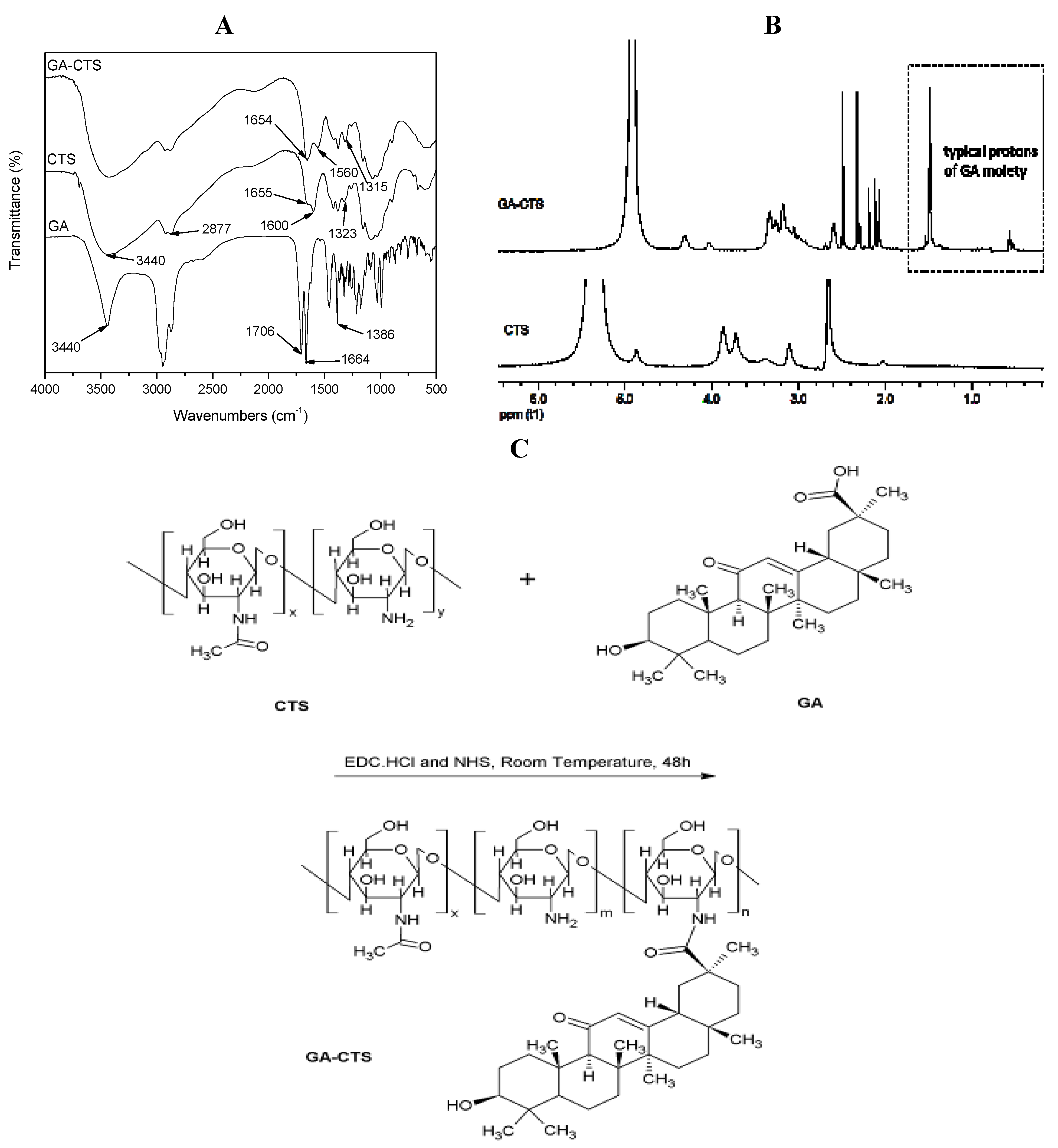
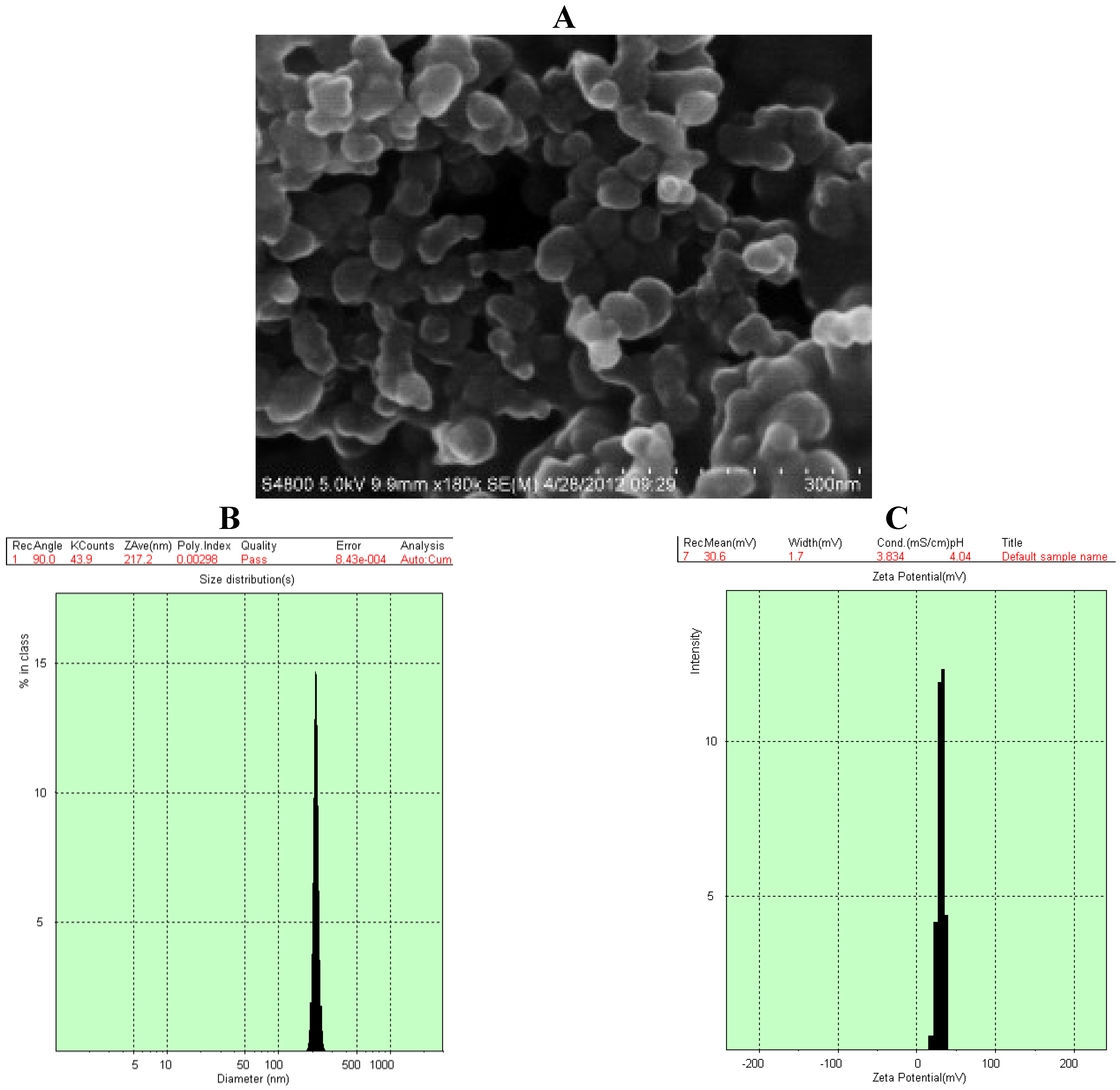
2.2. Characterization of GA-CTS/5-FU Nanoparticles
2.3. Dose- and Time-Dependent Cytotoxicity of GA-CTS Nanoparticles in Tumor Cells
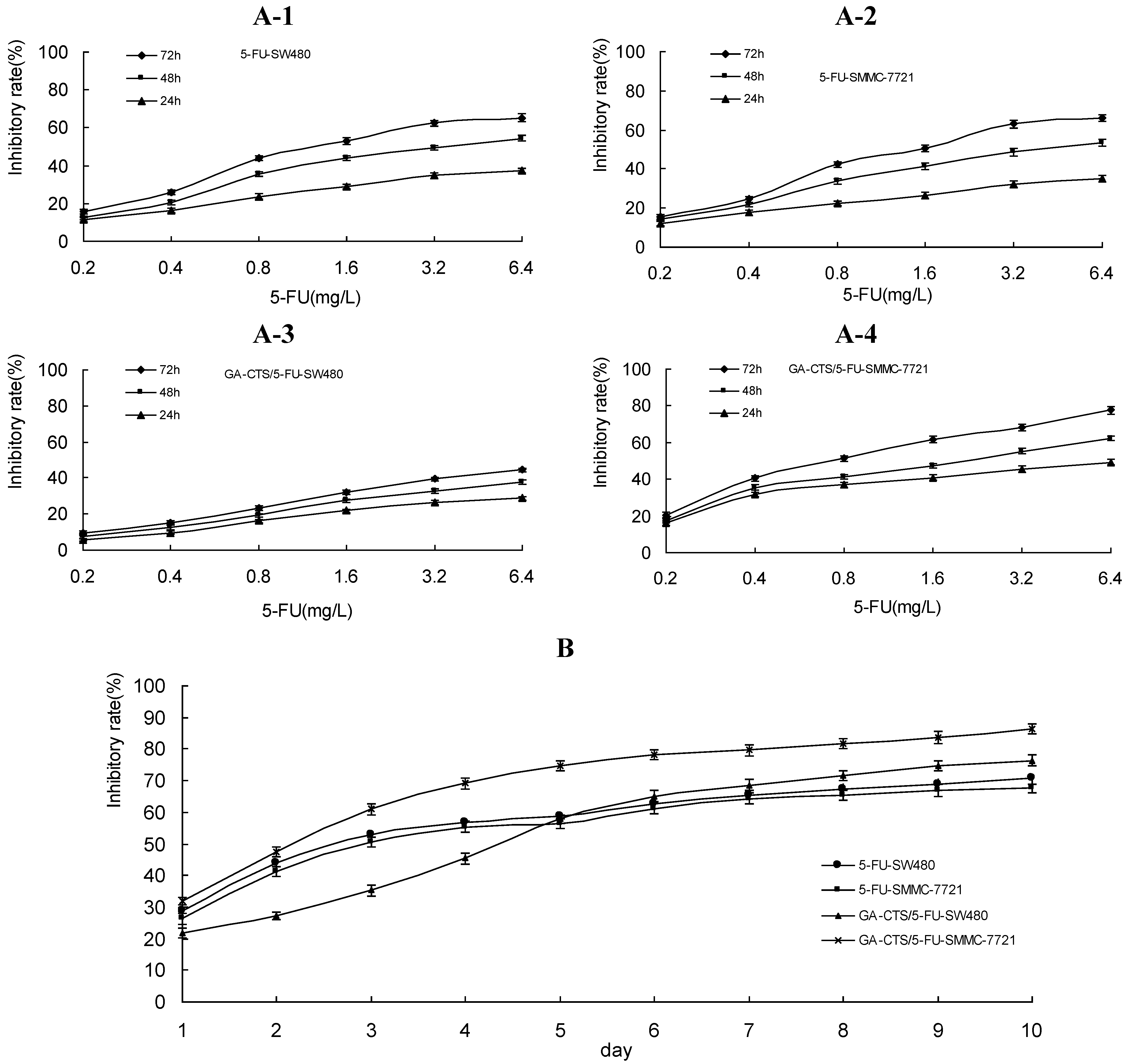
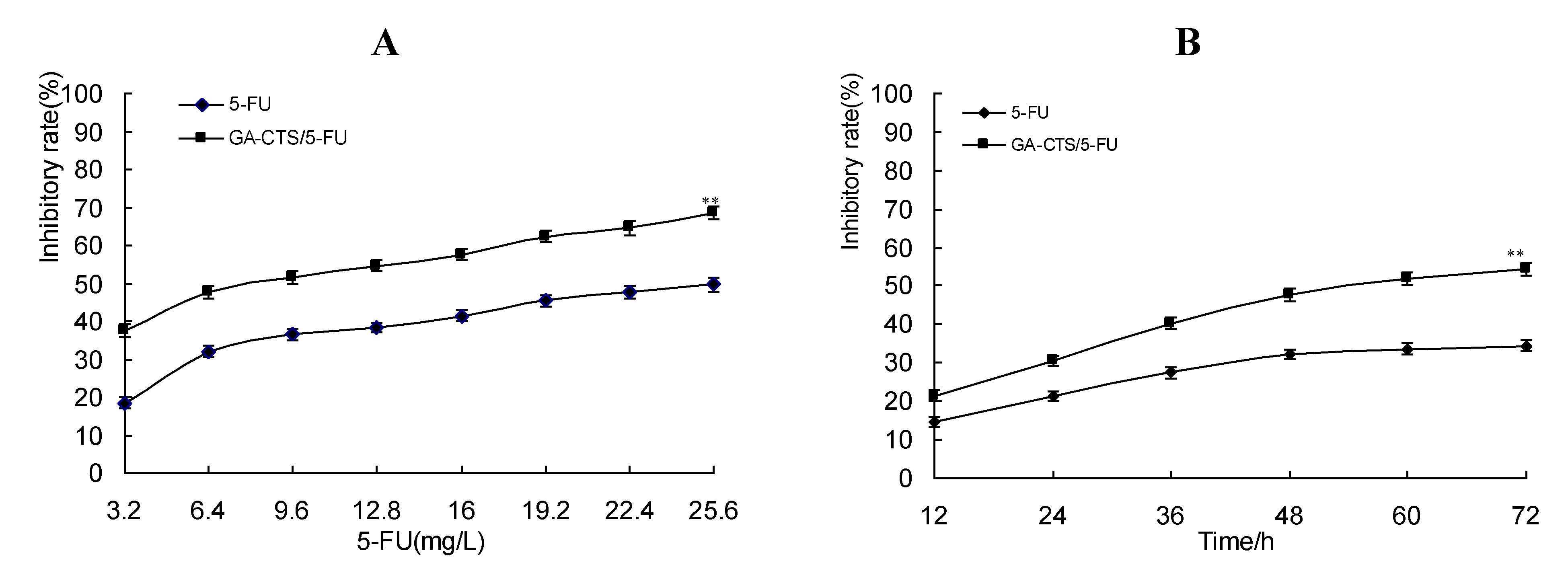
2.4. Cytotoxicity of GA-CTS Nanoparticles in Drug-Resistant Hepatoma Cells
| Parental cell line of SMMC-7721 | 5-FU resistance SMMC-7721 cell line | ||||||
|---|---|---|---|---|---|---|---|
| Concentrations (mg/L) | A Value | Inhibitory rate (%) | IC50 (mg/L) | Concentrations (mg/L) | A Value | Inhibitory rate (%) | IC50 (mg/L) |
| 5-FU | |||||||
| 0 | 0.65 ± 0.06 | 0 | 0.83 ± 0.06 | ||||
| 0.2 | 0.55 ± 0.05 | 14.64 | 3.2 | 0.67 ± 0.05 | 18.34 | ||
| 0.4 | 0.51 ± 0.07 | 21.65 | 6.4 | 0.56 ± 0.06 | 32.10 | ||
| 0.8 | 0.43 ± 0.04 | 33.65 | 3.76 | 12.8 | 0.51 ± 0.05 | 38.24 | 32.34 |
| 1.6 | 0.38 ± 0.03 | 41.25 | 25.6 | 0.42 ± 0.04 | 49.60 | ||
| 3.2 | 0.33 ± 0.03 | 48.65 | 51.2 | 0.39 ± 0.04 | 53.21 | ||
| 6.4 | 0.30 ± 0.04 | 53.64 | 102.4 | 0.29 ± 0.03 | 65.40 | ||
| GA-CTS/5-FU | |||||||
| 0 | 0.63 ± 0.05 | 0 | 0.81 ± 0.04 | ||||
| 0.2 | 0.52 ± 0.04 | 17.35 | 3.2 | 0.51 ± 0.05 | 37.45 | ||
| 0.4 | 0.41 ± 0.04 | 35.46 | 6.4 | 0.42 ± 0.04 | 47.74 | ||
| 0.8 | 0.37 ± 0.03 | 41.52 | 2.05 | 9.6 | 0.39 ± 0.03 | 51.35 | 8.33 |
| 1.6 | 0.33 ± 0.03 | 47.35 | 12.8 | 0.37 ± 0.04 | 54.58 | ||
| 3.2 | 0.28 ± 0.02 | 55.35 | 16.0 | 0.34 ± 0.03 | 57.65 | ||
| 6.4 | 0.24 ± 0.02 | 62.18 | 19.2 | 0.31 ± 0.03 | 62.24 | ||
2.5. Determination of in Vitro and in Vivo Liver Targeting of GA-CTS/5-FU

2.6. In Vivo Anti-Tumor Efficacy of GA-CTS/5-FU

3. Discussion
4. Experimental Section
4.1. Mice and Cell Lines
4.2. GA-CTS Synthesis
4.3. FT-IR Spectroscopy
4.4. 1H-NMR Experiments
4.5. Preparation of GA-CTS/5-FU Nanoparticles
4.6. In Vitro Release of GA-CTS/5-FU Nanoparticles
4.7. Cytotoxicity of GA-CTS/5-FU Nanoparticles
4.8. Establishment of SMMC-7721/5-FU Drug-Resistant Cell Lines and Calculation of Resistance Index
4.9. Inhibition of GA-CTS/5-FU Nanoparticles on SMMC-7721/5-FU Drug-Resistant Cells
4.10. Orthotopic Transplant Mouse Model of Hepatocellular Carcinoma
4.11. Targeting GA-CTS/5-FU Nanoparticles in Hepatocellular Carcinoma In Vivo
4.12. Targeting of GA-CTS Nanoparticles in Hepatic Cancer in Vitro
4.13. Inhibitory Effects of GA-CTS/5-FU Nanoparticles in and Orthotopic Liver Transplantation Mouse Model
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Toriumi, F.; Kubota, T.; Saikawa, Y.; Yoshida, M.; Otani, Y.; Watanabe, M.; Kumai, K.; Kitajima, M. Thymidylate synthetase (TS) genotype and TS/dihydropyrimidine dehydrogenase mRNA level as an indicator in determining chemosensitivity to 5-fluorouracil in advanced gastric carcinoma. Anticancer Res. 2004, 24, 2455–2463. [Google Scholar]
- Johnson, K.R.; Wang, L.; Miller, M.R.; Willingham, M.C.; Fan, W. 5-Fluorouracil interferes with paclitaxel cytotoxicity against human solid tumor cells. Clin. Cancer Res. 1997, 3, 1739–1745. [Google Scholar]
- Fang, J.Y.; Liu, P.F.; Huang, C.M. Decreasing systemic toxicity via transdermal delivery of anticancer drugs. Curr. Drug Metab. 2008, 9, 592–597. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Yan, Z.; Wang, J.; Luo, J.; Liu, Q. Hepatic arterial infusion with irinotecan, oxaliplatin, and floxuridine plus systemic chemotherapy as first-line treatment of unresectable liver metastases from colorectal cancer. Onkologie 2012, 35, 480–484. [Google Scholar]
- Takahashi, Y.; Yamashita, K.; Endo, Y.; Sasaki, T.; Mai, M. Oral administration of uracil-tegafur (UFT) inhibits liver micrometastasis of human colon cancer in an orthotopic nude mouse model and its early detection system. Surg. Today 2004, 34, 855–859. [Google Scholar]
- Oh, I.H.; Min, H.S.; Li, L.; Tran, T.H.; Lee, Y.K.; Kwon, I.C.; Choi, K.; Kim, K.; Huh, K.M. Cancer cell-specific photoactivity of pheophorbide a-glycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials 2013, 34, 6454–6463. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B Biointerfaces 2013, 110C, 313–320. [Google Scholar] [CrossRef]
- Javid, A.; Ahmadian, S.; Saboury, A.A.; Kalantar, S.M.; Rezaei-Zarchi, S. Chitosan Coated Superparamagnetic Iron Oxide Nanoparticles for Doxorubicin Delivery: Synthesis and Anticancer Effect against Human Ovarian Cancer Cells. Chem. Biol. Drug Des. 2013, 82, 296–306. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Cai, J.P.; Ma, X.Y.; Li, Y.; Cheng, J.W.; Wei, R.L. Sustained release of 5-fluorouracil from chitosan nanoparticles surface modified intra ocular lens to prevent posterior capsule opacification: an in vitro and in vivo study. J. Ocul. Pharmacol. Ther. 2013, 29, 208–215. [Google Scholar] [CrossRef]
- Xu, J.; Ma, L.; Liu, Y.; Xu, F.; Nie, J.; Ma, G. Design and characterization of antitumor drug paclitaxel-loaded chitosan nanoparticles by W/O emulsions. Int. J. Biol. Macromol. 2012, 50, 438–443. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, J.; Wang, Z.; Yin, J.; Zheng, Y.; Chen, X. Layer-by-layer assembly of poly(L-glutamic acid)/chitosan microcapsules for high loading and sustained release of 5-fluorouracil. Eur. J. Pharm. Biopharm. 2011, 78, 336–345. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Yu, J.M.; Li, Y.J.; Qiu, L.Y.; Jin, Y. Polymeric nanoparticles of cholesterol-modified glycol chitosan for doxorubicin delivery: Preparation and in vitro and in vivo characterization. J. Pharm. Pharmacol. 2009, 61, 713–719. [Google Scholar] [CrossRef]
- He, Z.Y.; Zheng, X.; Wu, X.H.; Song, X.R.; He, G.; Wu, W.F.; Yu, S.; Mao, S.J.; Wei, Y.Q. Development of glycyrrhetinic acid-modified stealth cationic liposomes for gene delivery. Int. J. Pharm. 2010, 397, 147–154. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, C.N.; Wang, X.H.; Wang, W.; Huang, W.; Cha, R.T.; Wang, C.H.; Yuan, Z.; Liu, M.; Wan, H.Y.; et al. Glycyrrhetinic acid-modified chitosan/poly(ethylene glycol) nanoparticles for liver-targeted delivery. Biomaterials 2010, 31, 4748–4756. [Google Scholar] [CrossRef]
- Huang, W.; Wang, W.; Wang, P.; Tian, Q.; Zhang, C.; Wang, C.; Yuan, Z.; Liu, M.; Wan, H.; Tang, H. Glycyrrhetinic acid-modified poly(ethylene glycol)-b-poly(gamma-benzyl l-glutamate) micelles for liver targeting therapy. Acta Biomater. 2010, 6, 3927–3935. [Google Scholar] [CrossRef]
- Jeong, H.G.; You, H.J.; Park, S.J.; Moon, A.R.; Chung, Y.C.; Kang, S.K.; Chun, H.K. Hepatoprotective effects of 18beta-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P450 2E1 expression. Pharmacol. Res. 2002, 46, 221–227. [Google Scholar] [CrossRef]
- Lallemand, B.; Gelbcke, M.; Dubois, J.; Prevost, M.; Jabin, I.; Kiss, R. Structure-activity relationship analyses of glycyrrhetinic acid derivatives as anticancer agents. Mini Rev. Med. Chem. 2011, 11, 881–887. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.H.; Wang, W.; Zhang, C.N.; Wang, P.; Yuan, Z. Self-assembly and liver targeting of sulfated chitosan nanoparticles functionalized with glycyrrhetinic acid. Nanomedicine 2012, 8, 870–879. [Google Scholar] [CrossRef]
- Huang, W.; Wang, W.; Wang, P.; Zhang, C.N.; Tian, Q.; Zhang, Y.; Wang, X.H.; Cha, R.T.; Wang, C.H.; Yuan, Z. Glycyrrhetinic acid-functionalized degradable micelles as liver-targeted drug carrier. J. Mater. Sci. Mater. Med. 2011, 22, 853–863. [Google Scholar] [CrossRef]
- Negishi, M.; Irie, A.; Nagata, N.; Ichikawa, A. Specific binding of glycyrrhetinic acid to the rat liver membrane. Biochim. Biophys. Acta 1991, 1066, 77–82. [Google Scholar] [CrossRef]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, J.; Jia, N.; Zhao, Y.; Shen, H. Chitosan-coated magnetic nanoparticles as carriers of 5-fluorouracil: Preparation, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2009, 68, 1–6. [Google Scholar] [CrossRef]
- Ligabue, A.; Marverti, G.; Liebl, U.; Myllykallio, H. Transcriptional activation and cell cycle block are the keys for 5-fluorouracil induced up-regulation of human thymidylate synthase expression. PLoS One 2012, 7, e47318. [Google Scholar]
- Nabeya, Y.; Suzuki, T.; Furuya, A.; Koide, N.; Ohkoshi, M.; Takiguchi, M.; Ochiai, T.; Matsubara, H.; Hiwasa, T. Calpain regulates thymidylate synthase-5-fluoro-dUMP complex levels associated with response to 5-fluorouracil in gastric cancer cells. Cancer Sci. 2011, 102, 1509–1515. [Google Scholar] [CrossRef]
- Cheng, M.; He, B.; Wan, T.; Zhu, W.; Han, J.; Zha, B.; Chen, H.; Yang, F.; Li, Q.; Wang, W.; et al. 5-Fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PLoS One 2012, 7, e47115. [Google Scholar]
- Snow, K.; Judd, W. Characterisation of adriamycin- and amsacrine-resistant human leukaemic T cell lines. Br. J. Cancer 1991, 63, 17–28. [Google Scholar] [CrossRef]
- Kuo, M.T.; Bao, J.J.; Curley, S.A.; Ikeguchi, M.; Johnston, D.A.; Ishikawa, T. Frequent coordinated overexpression of the MRP/GS-X pump and gamma-glutamylcysteine synthetase genes in human colorectal cancers. Cancer Res. 1996, 56, 3642–3644. [Google Scholar]
- Banerjee, D.; Mayer-Kuckuk, P.; Capiaux, G.; Budak-Alpdogan, T.; Gorlick, R.; Bertino, J.R. Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim. Biophys. Acta 2002, 1587, 164–173. [Google Scholar] [CrossRef]
- Violette, S.; Poulain, L.; Dussaulx, E.; Pepin, D.; Faussat, A.M.; Chambaz, J.; Lacorte, J.M.; Staedel, C.; Lesuffleur, T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int. J. Cancer 2002, 98, 498–504. [Google Scholar] [CrossRef]
- Hibasami, H.; Iwase, H.; Yoshioka, K.; Takahashi, H. Glycyrrhetic acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int. J. Mol. Med. 2006, 17, 215–219. [Google Scholar]
- Gianni, M.; Koken, M.H.; Chelbi-Alix, M.K.; Benoit, G.; Lanotte, M.; Chen, Z.; de The, H. Combined arsenic and retinoic acid treatment enhances differentiation and apoptosis in arsenic-resistant NB4 cells. Blood 1998, 91, 4300–4310. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, M.; Gao, X.; Wang, Y.; Chen, H.; He, B.; Xu, H.; Li, Y.; Han, J.; Zhang, Z. Synthesis of Glycyrrhetinic Acid-Modified Chitosan 5-Fluorouracil Nanoparticles and Its Inhibition of Liver Cancer Characteristics in Vitro and in Vivo. Mar. Drugs 2013, 11, 3517-3536. https://doi.org/10.3390/md11093517
Cheng M, Gao X, Wang Y, Chen H, He B, Xu H, Li Y, Han J, Zhang Z. Synthesis of Glycyrrhetinic Acid-Modified Chitosan 5-Fluorouracil Nanoparticles and Its Inhibition of Liver Cancer Characteristics in Vitro and in Vivo. Marine Drugs. 2013; 11(9):3517-3536. https://doi.org/10.3390/md11093517
Chicago/Turabian StyleCheng, Mingrong, Xiaoyan Gao, Yong Wang, Houxiang Chen, Bing He, Hongzhi Xu, Yingchun Li, Jiang Han, and Zhiping Zhang. 2013. "Synthesis of Glycyrrhetinic Acid-Modified Chitosan 5-Fluorouracil Nanoparticles and Its Inhibition of Liver Cancer Characteristics in Vitro and in Vivo" Marine Drugs 11, no. 9: 3517-3536. https://doi.org/10.3390/md11093517
APA StyleCheng, M., Gao, X., Wang, Y., Chen, H., He, B., Xu, H., Li, Y., Han, J., & Zhang, Z. (2013). Synthesis of Glycyrrhetinic Acid-Modified Chitosan 5-Fluorouracil Nanoparticles and Its Inhibition of Liver Cancer Characteristics in Vitro and in Vivo. Marine Drugs, 11(9), 3517-3536. https://doi.org/10.3390/md11093517




