Tetrodotoxin Blockade on Canine Cardiac L-Type Ca2+ Channels Depends on pH and Redox Potential
Abstract
:1. Introduction
2. Results and Discussion
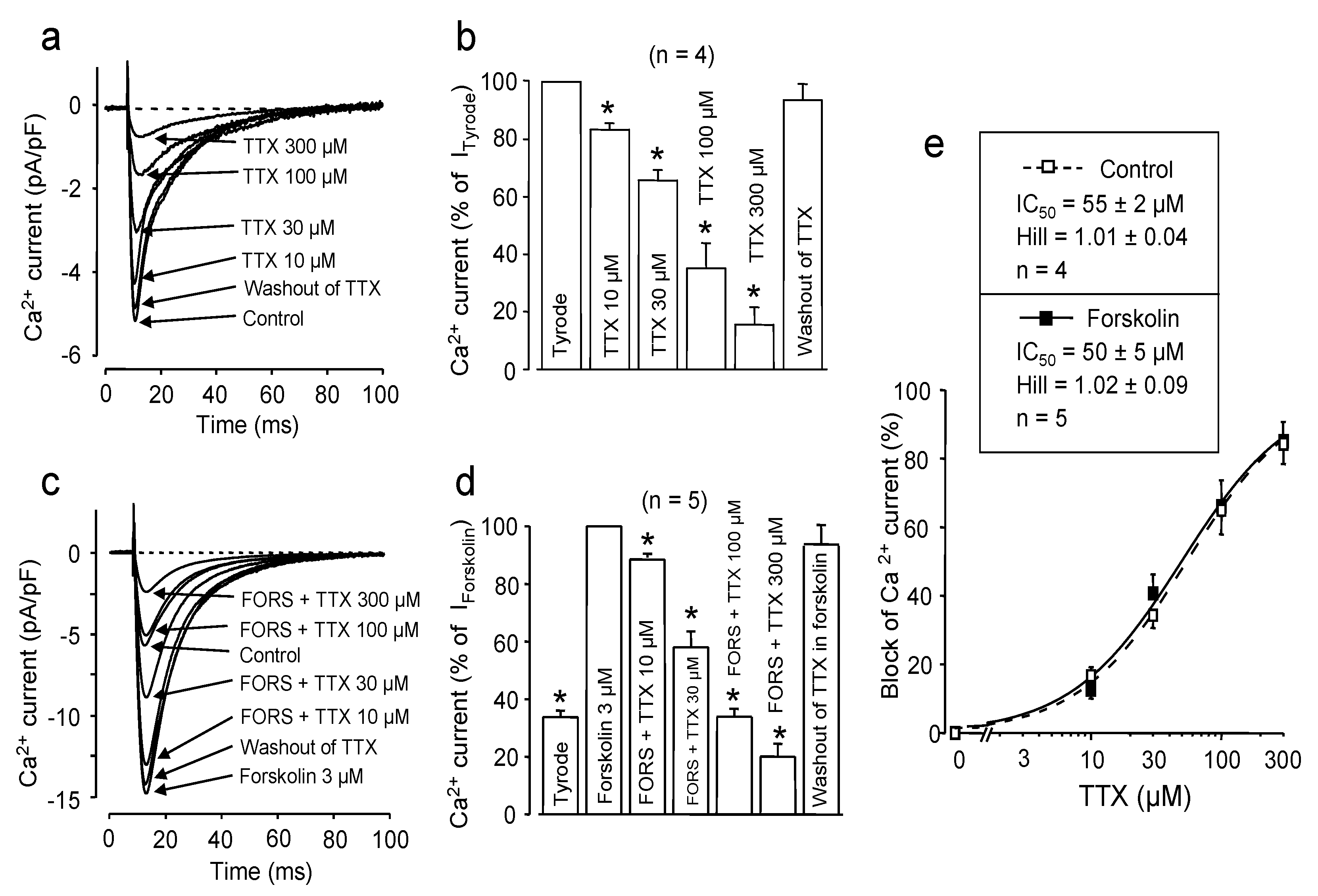
2.1. Effect of Channel Phosphorylation
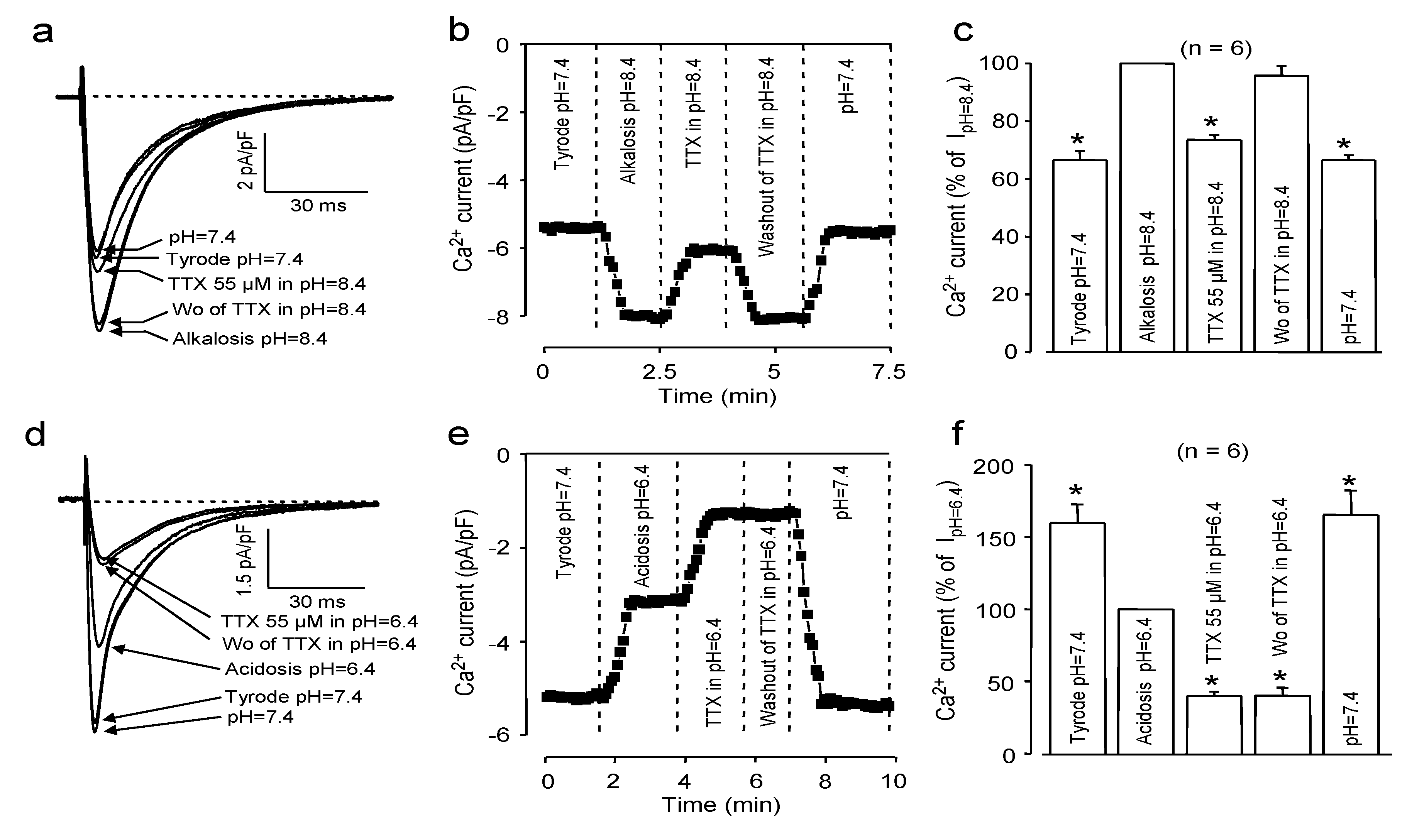
2.2. Effects of Extracellular pH
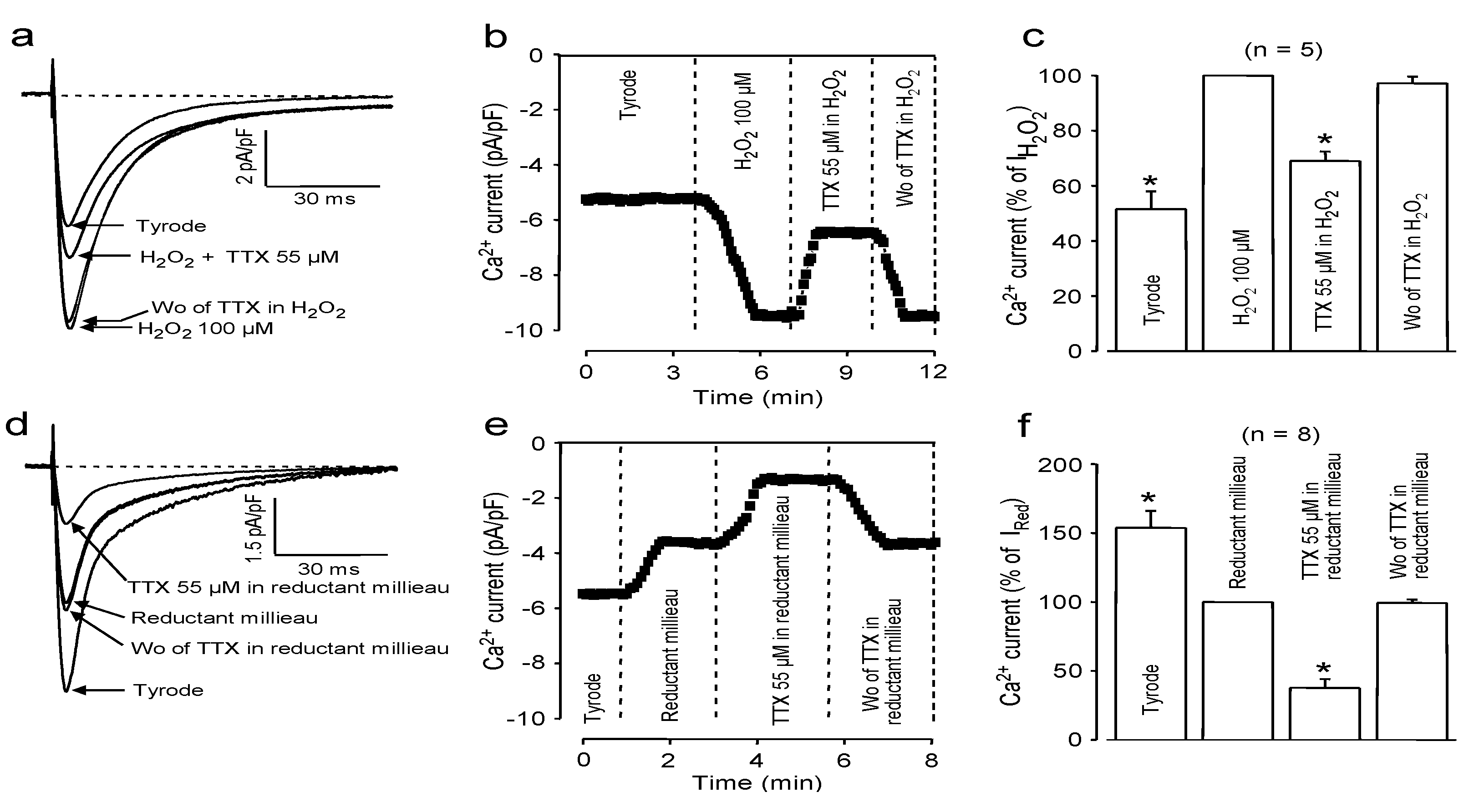
2.3. Effects of Redox Potential Changes
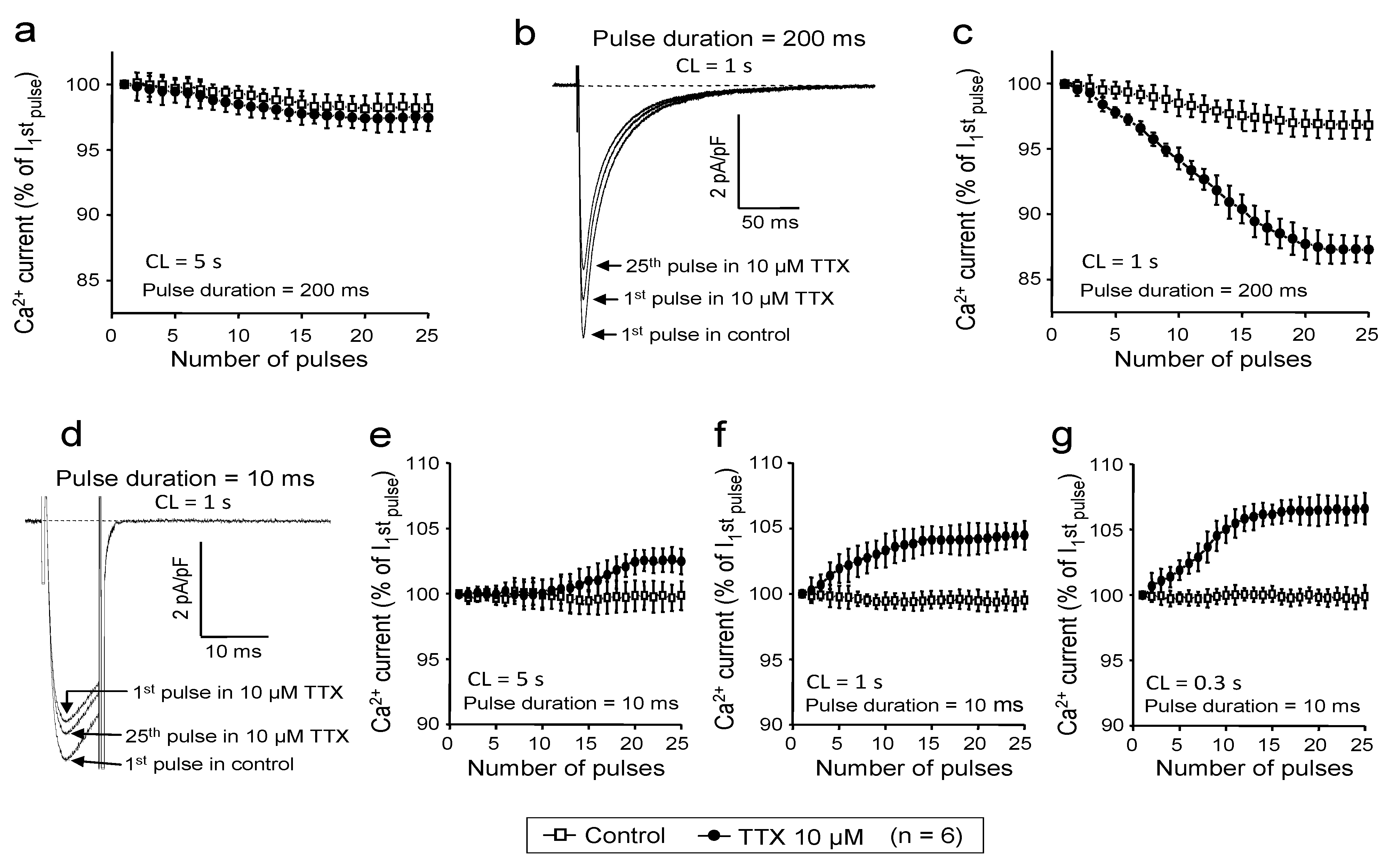
2.4. Use-Dependent Block
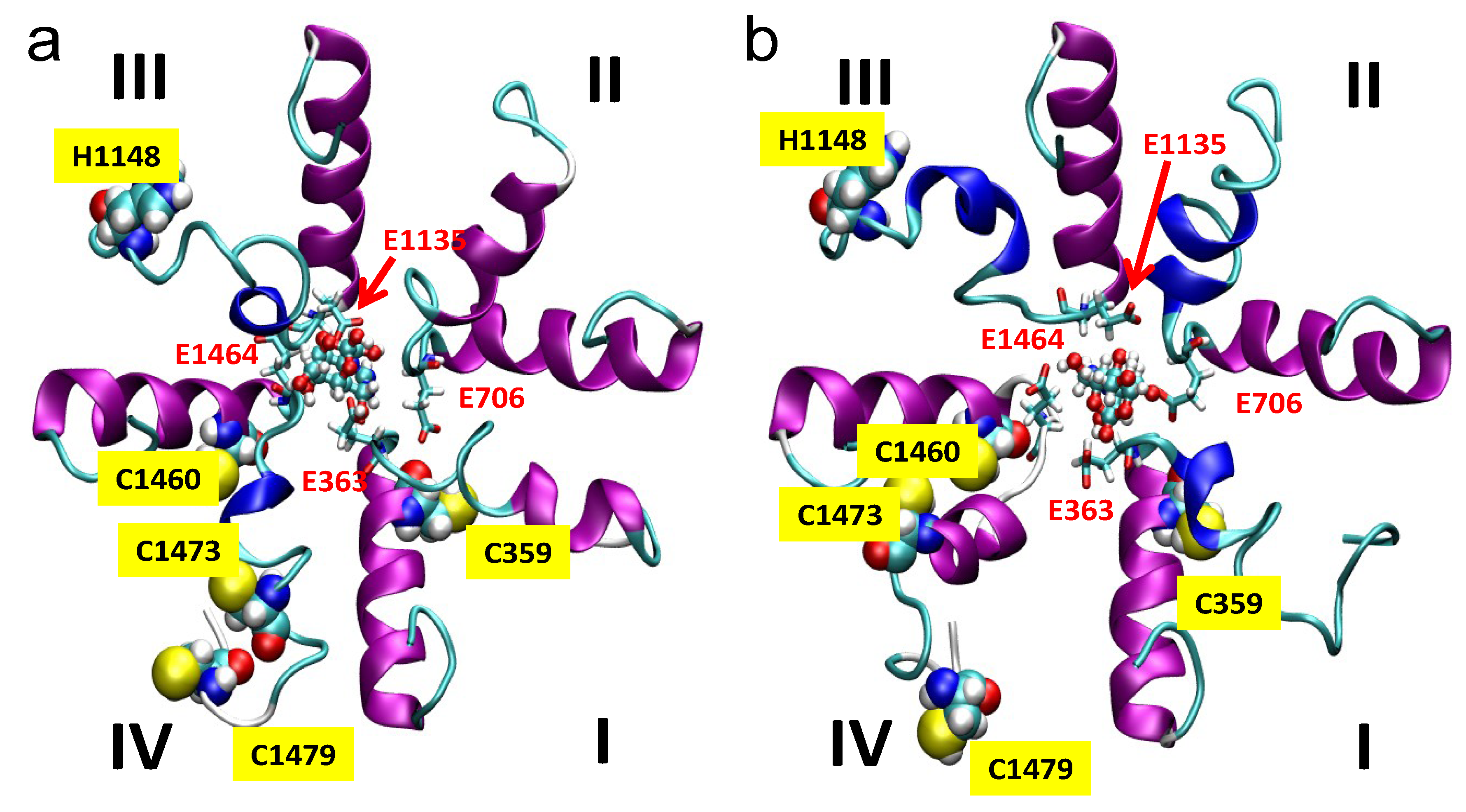
2.5. Simulations
3. Experimental Section
3.1. Isolation of Single Canine Ventricular Myocytes
3.2. Electrophysiology
3.3. Simulation of TTX Binding to Cav1.2 Channels
3.4. Statistics
4. Conclusions
Acknowledgments
References
- Baer, M.; Best, P.M.; Reuter, H. Voltage-dependent action of tetrodotoxin in mammalian cardiac muscle. Nature 1976, 263, 344–345. [Google Scholar] [CrossRef]
- Brown, A.M.; Lee, K.S.; Powell, T. Sodium current in single rat heart muscle cells. J. Physiol. 1981, 318, 479–500. [Google Scholar]
- Vornanen, M.; Hassinen, M.; Haverinen, J. Tetrodotoxin sensitivity of the vertebrate cardiac Na+ current. Mar. Drugs 2011, 9, 2409–2422. [Google Scholar] [CrossRef]
- Chorvatova, A.; Snowdon, R.; Hart, G.; Hussain, M. Effects of pressure overload-induced hypertrophy on TTX-sensitive inward currents in guinea pig left ventricle. Mol. Cell.Biochem. 2004, 261, 217–226. [Google Scholar] [CrossRef]
- Alvarez, J.L.; Salinas-Stefanon, E.; Orta, G.; Ferrer, T.; Talavera, K.; Galán, L.; Vassort, G. Occurrence of a tetrodotoxin-sensitive calcium current in rat ventricular myocytes after long-term myocardial infarction. Cardiovasc. Res. 2004, 63, 653–661. [Google Scholar] [CrossRef]
- Hegyi, B.; Horváth, B.; Bárándi, L.; Papp, F.; Magyar, J.; Bányász, T.; Krasznai, Z.; Szentandrássy, N.; Nánási, P.P. Tetrodotoxin blocks L-type Ca2+ channels in canine ventricular cardiomyocytes. Pflüg. Arch 2012, 464, 167–174. [Google Scholar] [CrossRef]
- Szabó, G.; Szentandrássy, N.; Bíró, T.; Tóth, I.B.; Czifra, G.; Magyar, J.; Bányász, T.; Varró, A.; Kovács, L.; Nánási, P.P. Asymmetrical distribution of ion channels in canine and human left ventricular wall: Epicardium versus midmyocardium. Pflüg. Arch. 2005, 450, 307–316. [Google Scholar] [CrossRef]
- Szentandrássy, N.; Bányász, T.; Bíró, T.; Szabó, G.; Tóth, I.B.; Magyar, J.; Lázár, J.; Varró, A.; Kovács, L.; Nánási, P.P. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc. Res. 2005, 65, 851–860. [Google Scholar] [CrossRef]
- Tikhonov, D.B.; Zhorov, B.S. Modeling P-loops domain of sodium channel: Homology with potassium channels and interaction with ligands. Biophys. J. 2005, 88, 184–197. [Google Scholar] [CrossRef]
- Tikhonov, D.B.; Zhorov, B.S. Possible roles of exceptionally conserved residues around the selectivity filters of sodium and calcium channels. J. Biol. Chem. 2011, 286, 2998–3006. [Google Scholar] [CrossRef]
- Payandeh, J.; Scheuer, T.; Zheng, N.; Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 2011, 475, 353–358. [Google Scholar] [CrossRef]
- Payandeh, J.; Gamal El-Din, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 2012, 486, 135–139. [Google Scholar]
- Tikhonov, D.B.; Zhorov, B.S. Architecture and pore block of eukaryotic voltage-gated sodium channels in view of NavAb bacterial sodium channel structure. Mol. Pharmacol. 2012, 82, 97–104. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Méry, P.F.; Jurevicius, J.; Skeberdis, A.V.; Fischmeister, R. Regulation of myocardial calcium channels by cyclic AMP metabolism. Basic Res. Cardiol. 1996, 91 (Suppl. 2), 1–8. [Google Scholar]
- Fozzard, H.A.; Lipkind, G.M. The tetrodotoxin binding site is within the outer vestibule of the sodium channel. Mar. Drugs 2010, 8, 219–234. [Google Scholar] [CrossRef]
- Satin, J.; Kyle, J.W.; Chen, M.; Bell, P.; Cribbs, L.L.; Fozzard, H.A.; Rogart, R.B. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science 1992, 256, 1202–1205. [Google Scholar] [CrossRef]
- Favre, I.; Moczydlowski, E.; Schild, L. Specificity for block by saxitoxin and divalent cations at a residue which determines sensitivity of sodium channel subtypes to guanidinium toxins. J. Gen. Physiol. 1995, 106, 203–229. [Google Scholar] [CrossRef]
- Stryer, L. Biochemistry, 4th ed; V.H. Freeman and Company: New York, NY, USA, 1995; p. 23. [Google Scholar]
- Tombaugh, G.C.; Somjen, G.G. Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons. J. Physiol. 1996, 493, 719–732. [Google Scholar]
- Saegusa, N.; Moorhouse, E.; Vaughan-Jones, R.D.; Spitzer, K.W. Influence of pH on Ca2+ current and its control of electrical and Ca2+ signaling in ventricular myocytes. J. Gen. Physiol. 2011, 138, 537–559. [Google Scholar] [CrossRef]
- Moczydlowski, E. The molecular mystique of tetrodotoxin. Toxicon 2013, 63, 711–722. [Google Scholar] [CrossRef]
- Ulbricht, W.; Wagner, H.H. The influence of pH on equilibrium effects of tetrodotoxin on myelinated nerve fibres of Rana esculenta. J. Physiol. 1975, 252, 159–184. [Google Scholar]
- Strickholm, A. Ionic permeability of K, Na, and Cl in potassium-depolarized nerve. Dependency on pH, cooperative effects, and action of tetrodotoxin. Biophys. J. 1981, 35, 677–697. [Google Scholar] [CrossRef]
- Guo, J.; Giles, W.R.; Ward, C.A. Effect of hydrogen peroxide on the membrane currents in sinoatrial node cells from rabbit heart. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H992–H999. [Google Scholar]
- Xie, L.H.; Chen, F.; Karagueuzian, H.S.; Weiss, J.N. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ. Res. 2009, 104, 79–86. [Google Scholar]
- Madhvani, R.V.; Xie, Y.; Pantazis, A.; Garfinkel, A.; Qu, Z.; Weiss, J.N.; Olcese, R.J. Shaping a new Ca2+ conductance to suppress early afterdepolarizations in cardiac myocytes. J.Physiol. 2011, 589, 6081–6092. [Google Scholar]
- Zhang, R.; Sun, Y.; Tsai, H.; Tang, C.; Jin, H.; Du, J. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLoS One 2012, 7, e37073. [Google Scholar]
- Lao, Q.Z.; Kobrinsky, E.; Liu, Z.; Soldatov, N.M. oligomerization of Cavβ subunits is essential correlate of Ca2+ channel activity. FASEB J. 2010, 24, 5013–5023. [Google Scholar] [CrossRef]
- Magyar, J.; Bányász, T.; Szigligeti, P.; Körtvély, Á.; Jednákovits, A.; Nánási, P.P. Electrophysiological effects of bimoclomol in canine ventricular myocytes. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 361, 303–310. [Google Scholar] [CrossRef]
- Bányász, T.; Magyar, J.; Körtvély, á.; Szigeti, G.y.; Szigligeti, P.; Papp, Z.; Mohácsi, A.; Kovács, L.; Nánási, P.P. Different effects of endothelin-1 on calcium and potassium currents in canine ventricular cells. Naunyn Schmiedebergs Arch. Pharmacol 2001, 363, 383–390. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflüg. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A.; Simmerling, C. Comparison of multiple amber force fields and development of improved protein backbone parameters. Proteins 2006, 65, 712–725. [Google Scholar] [CrossRef]
- Wang, J.M.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald-an NlogN method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- AMBER, version 12; University of California: San Francisco, CA, USA, 2012.
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lipkind, G.M.; Fozzard, H.A. A structural model of the tetrodotoxin binding site of the Na+ channel. Biophys. J. 1994, 66, 1–13. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hegyi, B.; Komáromi, I.; Kistamás, K.; Ruzsnavszky, F.; Váczi, K.; Horváth, B.; Magyar, J.; Bányász, T.; Nánási, P.P.; Szentandrássy, N. Tetrodotoxin Blockade on Canine Cardiac L-Type Ca2+ Channels Depends on pH and Redox Potential. Mar. Drugs 2013, 11, 2140-2153. https://doi.org/10.3390/md11062140
Hegyi B, Komáromi I, Kistamás K, Ruzsnavszky F, Váczi K, Horváth B, Magyar J, Bányász T, Nánási PP, Szentandrássy N. Tetrodotoxin Blockade on Canine Cardiac L-Type Ca2+ Channels Depends on pH and Redox Potential. Marine Drugs. 2013; 11(6):2140-2153. https://doi.org/10.3390/md11062140
Chicago/Turabian StyleHegyi, Bence, István Komáromi, Kornél Kistamás, Ferenc Ruzsnavszky, Krisztina Váczi, Balázs Horváth, János Magyar, Tamás Bányász, Péter P. Nánási, and Norbert Szentandrássy. 2013. "Tetrodotoxin Blockade on Canine Cardiac L-Type Ca2+ Channels Depends on pH and Redox Potential" Marine Drugs 11, no. 6: 2140-2153. https://doi.org/10.3390/md11062140
APA StyleHegyi, B., Komáromi, I., Kistamás, K., Ruzsnavszky, F., Váczi, K., Horváth, B., Magyar, J., Bányász, T., Nánási, P. P., & Szentandrássy, N. (2013). Tetrodotoxin Blockade on Canine Cardiac L-Type Ca2+ Channels Depends on pH and Redox Potential. Marine Drugs, 11(6), 2140-2153. https://doi.org/10.3390/md11062140



