Synthesis and Antiproliferative Activity of 2,5-bis(3′-Indolyl)pyrroles, Analogues of the Marine Alkaloid Nortopsentin
Abstract
:1. Introduction
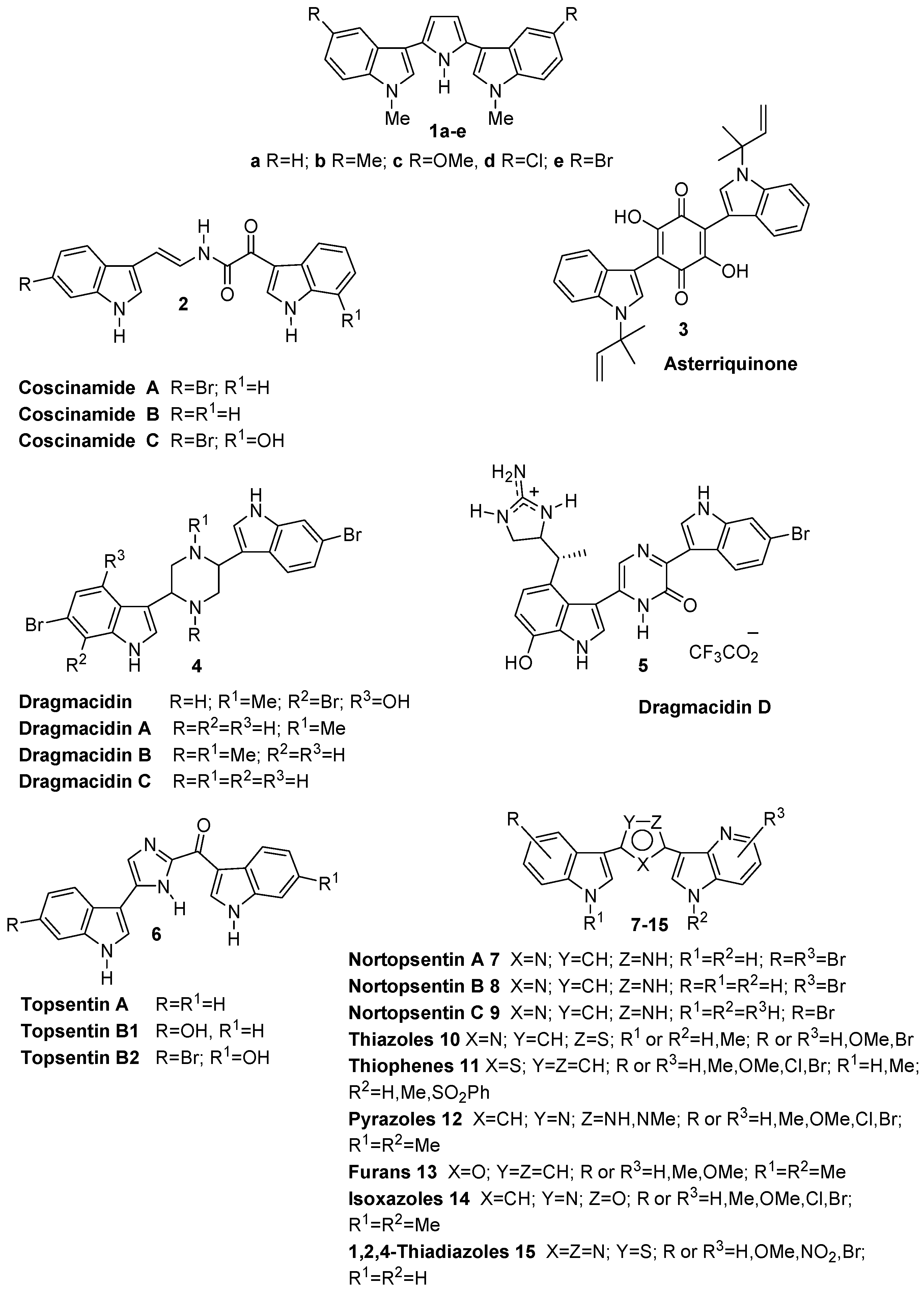
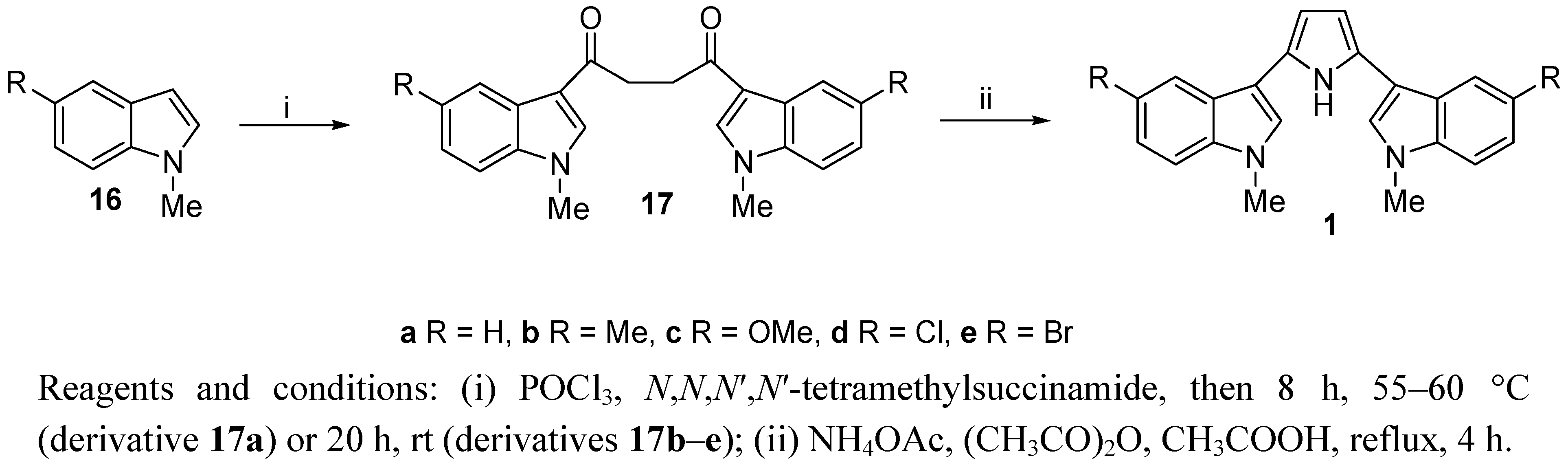
2. Results and Discussion
| Compound | IC50 (μg/mL) | Active/Total a | Tumor Selectivity b,c | |||
|---|---|---|---|---|---|---|
| 1 (μg/mL) | 10 (μg/mL) | 100 (μg/mL) | A | B | ||
| 1a | 0.37 | 8/12 (67%) | 12/12 (100%) | 12/12 (100%) | 1/12 | + |
| 1b | 0.37 | 8/12 (67%) | 12/12 (100%) | 12/12 (100%) | 1/12 | + |
| 1c | 3.4 | 0/12 (0%) | 10/12 (83%) | 12/12 (100%) | 0/12 | − |
| 1d | 3.4 | 1/12 (8%) | 9/12 (75%) | 12/12 (100%) | 1712 | + |
| 1e | 4.4 | 0/12 (0%) | 9/12 (75%) | 12/12 (100%) | 0/12 | − |
| Adr d | 0.007 | 4/12 (33%) | 10/12 (83%) | 11/12 (92%) | 2/12 | ++ |
| Cell line | Tumor | |||||||
|---|---|---|---|---|---|---|---|---|
| histotype | name | 1a | 1b | histotype | name | 1a | 1b | |
| Bladder | BXF | 1218L | 0.72 | 0.32 | Bladder | BXF 1218 | 2.45 | 2.35 |
| BXF | 1352L | 0.68 | 0.41 | BXF 1228 | 2.89 | 2.86 | ||
| BXF | T24 | 1.72 | 0.58 | Colon | CXF 1103 | 4.47 | 18.36 | |
| Colon | CXF | 269L | 1.39 | 0.56 | CXF 1729 | 3.49 | 7.77 | |
| CXF | HCT116 | 3.24 | 1.64 | CXF 1783 | 37.57 | 40.70 | ||
| CXF | HT29 | 5.20 | 2.65 | CXF 280 | 26.18 | 35.70 | ||
| CXF | RKO | 1.50 | 0.63 | CXF 975 | 6.93 | 6.03 | ||
| Gastric | GXA | MKN45 | 0.84 | 0.53 | Gastric | GXF 1172 | 6.27 | >100 |
| GXF | 251L | 1.56 | 0.65 | GXF 251 | 7.10 | 25.50 | ||
| Head&Neck | HNXF | CAL27 | 0.81 | 0.50 | GXF 97 | 2.72 | 3.74 | |
| Liver | LIXF | 575L | 1.84 | 0.61 | Head&Neck | HNXF 536 | 2.45 | 2.67 |
| Lung | LXFA | 289L | 6.34 | 2.39 | HNXF 908 | 2.57 | 4.00 | |
| LXFA | 526L | 1.57 | 0.67 | Lung | LXFA 1012 | 4.76 | 27.34 | |
| LXFA | 629L | 2.28 | 1.36 | LXFA 1584 | 2.07 | 2.73 | ||
| LXFL | 1121L | 0.67 | 0.38 | LXFA 297 | 54.90 | >100 | ||
| LXFL | 529L | 1.85 | 0.67 | LXFA 526 | 2.96 | 3.15 | ||
| LXFL | H460 | 2.08 | 0.89 | LXFA 629 | 2.23 | 3.63 | ||
| Mammary | MAXF | 401NL | 1.24 | 0.64 | LXFA 677 | 23.19 | 27.02 | |
| MAXF | MCF7 | 2.44 | 0.98 | LXFA 923 | 19.50 | 25.68 | ||
| MAXF | MDA231 | 1.18 | 0.49 | LXFE 1422 | 5.90 | 1.72 | ||
| Melanoma | MEXF | 1341L | 0.52 | 0.19 | LXFL 1072 | 3.44 | 3.42 | |
| MEXF | 276L | 0.22 | 0.11 | LXFL 529 | 5.07 | 4.46 | ||
| MEXF | 462NL | 1.31 | 0.55 | LXFL 625 | 24.04 | 38.21 | ||
| Ovarian | OVXF | OVCAR3 | 1.46 | 0.58 | Mammary | MAXF 1322 | 16.80 | 9.49 |
| OVXF | 899L | 5.40 | 2.03 | MAXF 1384 | 34.48 | 33.28 | ||
| Pancreatic | PAXF | PANC1 | 0.74 | 0.41 | MAXF 401 | 5.90 | 11.32 | |
| PAXF | 1657L | 2.68 | 1.02 | Melanoma | MEXF 1539 | 19.90 | 15.31 | |
| PAXF | 546L | 2.70 | 1.16 | MEXF 276 | 1.44 | 1.75 | ||
| Prostate | PRXF | 22RV1 | 1.46 | 0.63 | MEXF 462 | 3.24 | 4.10 | |
| PRXF | DU145 | 4.45 | 1.96 | MEXF 989 | 1.18 | 0.58 | ||
| PRXF | LNCAP | 2.10 | 0.68 | Ovarian | OVXF 1353 | 20.96 | 22.90 | |
| PRXF | PC3M | 0.85 | 0.32 | OVXF 899 | 3.54 | 3.93 | ||
| Plerameso- | PXF | 1118L | 2.50 | 0.88 | Pancreatic | PAXF 546 | 3.52 | 14.63 |
| thelioma | PXF | 1752L | 0.89 | 0.43 | PAXF 736 | 2.99 | 2.10 | |
| PXF | 698L | 1.86 | 0.86 | Prostate | PRXF DU145 | 28.67 | 25.98 | |
| Renal | RXF | 1183L | 1.13 | 0.58 | PRXF PC3M | 2.89 | 2.80 | |
| RXF | 1781L | 1.77 | 0.66 | Pleurameso- | PXF 1752L | 3.71 | 4.05 | |
| RXF | 393NL | 3.14 | 1.34 | thelioma | PXF 541 | 2.20 | 0.37 | |
| RXF | 486L | 3.86 | 1.60 | Renal | RXF 1220 | 6.34 | 3.16 | |
| Sarcoma | SXF | SAOS2 | 0.72 | 0.33 | RXF 486 | 2.90 | 3.90 | |
| SXF | TE671 | 1.60 | 0.53 | RXF 631 | 2.98 | 2.81 | ||
| Uterus | UXF | 1138L | 0.72 | 0.35 | Sarcoma | SXF 1186 | 5.71 | 6.17 |
| SXF 1301 | 3.54 | 23.95 | ||||||
| SXF 627 | 3.40 | 4.06 | ||||||
| geometric mean IC50 | 1.54 | 0.67 | geometric mean IC50 | 5.69 | 7.25 | |||
| Tumor selectivity 1) | 8/42 | 5/42 | Tumor selectivity 1) | 9/44 | 14/44 | |||
| (selective/total) | (19%) | (12%) | (selective/total) | (20%) | (32%) | |||
 | ||||||||
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedure
3.1.2. Synthesis of N,N,N′,N′-Tetramethylsuccinamide
3.1.3. General Procedure for the Preparation of 1,4-bis(Indol-3-yl)butane-1,4-diones (17a–e)
3.1.4. General Procedure for the Preparation of Pyrroles (1a–e)
3.1.4.1. 3,3′-(1H-Pyrrole-2,5-diyl)bis(1-methyl-1H-indole) (1a)
3.1.4.2. 3,3′-(1H-Pyrrole-2,5-diyl)bis(1,5-dimethyl-1H-indole) (1b)
3.1.4.3. 3,3′-(1H-Pyrrole-2,5-diyl)bis(5-methoxy-1-methyl-1H-indole) (1c)
3.1.4.4. 3,3′-(1H-Pyrrole-2,5-diyl)bis(5-chloro-1-methyl-1H-indole) (1d)
3.1.4.5. 3,3′-(1H-Pyrrole-2,5-diyl)bis(5-bromo-1-methyl-1H-indole) (1e)
3.2. Biology
3.2.1. In Vitro Antitumor Activity towards Permanent Growing Human Tumor Cell Lines
3.2.1.1. Cell Lines
3.2.1.2. Cytotoxicity Assay (Monolayer Assay)
3.2.2. Ex-Vivo Antitumor Activity towards Tumor Xenografts
4. Conclusions
Acknowledgments
References
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar]
- Norcross, R.D.; Paterson, I. Total synthesis of bioactive marine macrolidest. Chem. Rev. 1995, 95, 2041–2114. [Google Scholar] [CrossRef]
- Shin, J.; Seo, Y.; Cho, K.W.; Rho, J.R.; Sim, C.J. New bis(indole)alkaloids of the topsentin class from the sponge Spongosorites genitrix. J. Nat. Prod. 1999, 62, 647–649. [Google Scholar] [CrossRef]
- Casapullo, A.; Bifulco, G.; Bruno, I.; Riccio, R. Hamacanthin classes from the Mediterranean marine sponge Raphisia lacazei. J. Nat. Prod. 2000, 63, 447–451. [Google Scholar] [CrossRef]
- Bao, B.; Sun, Q.; Yao, X.; Hong, J.; Lee, C.; Sim, C.J.; Jung, J.H. Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp. J. Nat. Prod. 2005, 68, 711–715. [Google Scholar] [CrossRef]
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef]
- Bokesch, H.R.; Pannell, L.K.; McKee, T.C.; Boyd, M.R. Coscinamides A, B, and C, three new bis indole alkaloids from the marine sponge Coscinoderma sp. Tetrahedron Lett. 2000, 41, 6305–6308. [Google Scholar]
- Shimizu, S.; Yamamoto, Y.; Inagaki, L.; Koshimura, S. Antitumor effect and structure-activity relationship of asterriquinone analogs. Gann 1982, 73, 642–648. [Google Scholar]
- Kohmoto, S.; Kashman, Y.; McConnell, O.J.; Rinehart, K.L., Jr.; Wright, A.; Koehn, F. The first total synthesis of Dragmacidin D. J. Org. Chem. 1988, 53, 3116–3118. [Google Scholar]
- Morris, S.A.; Andersen, R.J. Brominated bis(indole)alkaloids from the marine sponge Hexadella sp. Tetrahedron 1990, 46, 715–720. [Google Scholar] [CrossRef]
- Fahy, E.; Potts, B.C.M.; Faulkner, D.J.; Smith, K. 6-Bromotryptamine derivatives from the gulf of California Tunicate Didemnum candidum. J. Nat. Prod. 1991, 54, 564–569. [Google Scholar] [CrossRef]
- Wright, A.E.; Pomponi, S.A.; Cross, S.S.; McCarthy, P. A new bis-(indole)alkaloids from a deep-water marine sponge of the genus Spongosorites. J. Org. Chem. 1992, 57, 4772–4775. [Google Scholar] [CrossRef]
- Capon, R.J.; Rooney, F.; Murray, L.M.; Collins, E.; Sim, A.T.R.; Rostas, J.A.P.; Butler, M.S.; Carroll, A.R. Dragmacidins: New protein phosphatase inhibitors from a Southern Australian deep-water Marine Sponge Spongosorites sp. J. Nat. Prod. 1998, 61, 660–662. [Google Scholar] [CrossRef]
- Bartik, K.; Braekman, J.-C.; Daloze, D.; Stoller, C. Topsentin, new toxic bis-indole alkaloids from the marine sponge Topsentia genitrix. Can. J. Chem. 1987, 65, 2118–2121. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Kashman, Y.; Cross, S.S.; Lui, M.S.; Pomponi, S.A.; Diaz, M.C. Topsentin, bromotopsentin, and dihydrodeoxybromotopsentin: Antiviral and antitumor bis(indolyl)imidazoles from Caribbean deep-sea sponge of the family Halichondriidae. Structural and synthetic studies. J. Org. Chem. 1988, 53, 5446–5453. [Google Scholar] [CrossRef]
- Alvarez, M.; Salas, M. Marine, nitrogen-containing heterocyclic natural products-structures and syntheses of compounds containing indole units. Heterocycles 1991, 32, 1391–1429. [Google Scholar] [CrossRef]
- Sakem, S.; Sun, H.H. Nortopsentins A, B and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991, 56, 4304–4307. [Google Scholar]
- Kawasaki, I.; Yamashita, M.; Ohta, S. Total Synthesis of Nortopsentins A–D, marine alkaloids. Chem. Pharm. Bull. 1996, 44, 1831–1839. [Google Scholar] [CrossRef]
- Moody, C.J.; Roffey, J.R.A. Synthesis of N-protected Nortopsentins B and D. Arkivoc 2000, 1, 393–401. [Google Scholar]
- Miyake, F.Y.; Yakushijin, K.; Horne, D.A. A concise synthesis of Topsentin A and Nortopsentin B and D. Org. Lett. 2000, 2, 2121–2123. [Google Scholar] [CrossRef]
- Fresneda, P.M.; Molina, P.; Sanz, M.A. Microwave-assisted regioselective synthesis of 2,4-disubstituted imidazoles: Nortopsentin D synthesized by minimal effort. Synlett 2001, 2, 218–221. [Google Scholar]
- Jiang, B.; Gu, X.-H. Syntheses and cytotoxicity evaluation of bis(indolyl)thiazole, bis(indolyl)pyrazinone and bis(indolyl)pyrazine: Analogues of cytotoxic marine bis(indole) alkaloid. Bioorg. Med. Chem. 2000, 8, 363–371. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, C.G.; Xiong, C.; Wang, J. Synthesis and cytotoxicity evaluation of novel indolylpyrimidines and indolylpyrazines as potential antitumor agents. Bioorg. Med. Chem. 2001, 9, 1149–1154. [Google Scholar] [CrossRef]
- Jiang, B.; Xiong, W.-N.; Yang, C.-G. Synthesis and antitumor evaluation of novel monoindolyl-4-trifluoromethylpyridines and bisindolyl-4-trifluoromethylpyridines. Bioorg. Med. Chem. Lett. 2001, 11, 475–477. [Google Scholar] [CrossRef]
- Xiong, W.-N.; Yang, C.-G.; Jiang, B. Synthesis of novel analogues of marine indole alkaloids: Mono(indolyl)-4-trifluoromethylpyridines and bis(indolyl)-4-trifluoromethylpyridines as potential anticancer agents. Bioorg. Med. Chem. 2001, 9, 1773–1780. [Google Scholar] [CrossRef]
- Gu, X.-H.; Wan, X.-Z.; Jiang, B. Syntheses and biological activities of bis(3-indolyl)thiazoles, analogues of marine bis(indole)alkaloid nortopsentin. Bioorg. Med. Chem. Lett. 1999, 9, 569–572. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Martorana, A.; Dattolo, G.; Gia, O.; Dalla Via, L.; Cirrincione, G. Synthesis and antitumor properties of 2,5-bis(3′-indolyl)thiophenes: Analogues of marine alkaloid nortopsentin. Bioorg. Med. Chem. Lett. 2007, 17, 2342–2346. [Google Scholar]
- Diana, P.; Carbone, A.; Barraja, P.; Martorana, A.; Gia, O.; Dalla Via, L.; Cirrincione, G. 3,5-Bis(3′-indolyl)pyrazoles, analogues of marine alkaloid nortopsentin: Synthesis and antitumor properties. Bioorg. Med. Chem. Lett. 2007, 17, 6134–6137. [Google Scholar]
- Diana, P.; Carbone, A.; Barraja, P.; Kelter, H.H.; Fiebig, G.; Cirrincione, G. Synthesis and antitumor activity of 2,5-bis(3′-indolyl)-furans and 3,5-bis(3′-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem. 2010, 18, 4524–4529. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, N.M.; Chang, K.-H.; Gupta, R.; Shah, K. Synthesis and in-vitro anticancer activity of 3,5-bis(indolyl)-1,2,4-thiadiazoles. Bioorg. Med. Chem. Lett. 2011, 21, 5897–5900. [Google Scholar] [CrossRef]
- Jacquemard, U.; Dias, N.; Lansiaux, A.; Bailly, C.; Logé, C.; Robert, J.M.; Lozach, O.; Meijer, L.; Merour, J.Y.; Routier, S. Synthesis of 3,5-bis(2-indolyl)pyridine and 3-[(2-indolyl)-5-phenyl]pyridine derivatives as CDK inhibitors and cytotoxic agents. Bioorg. Med. Chem. 2008, 16, 4932–4953. [Google Scholar]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Parrino, B.; Lopergolo, A.; Pennati, M.; Zaffaroni, N.; Cirrincione, G. Synthesis and antitumor activity of 3-(2-phenyl-1,3-thiazol-4-yl)-1H-indoles and 3-(2-phenyl-1,3-thiazol-4-yl)-1H-7-azaindoles. ChemMedChem 2011, 6, 1300–1309. [Google Scholar] [CrossRef]
- Johnson, M.R. Synthesis of Bronzaphyrin NS. J. Org. Chem. 1997, 62, 1168–1172. [Google Scholar] [CrossRef]
- Park, C.H.; Bergsagel, D.E.; McCulloch, E.A. Mouse myeloma tumor stem cells: A primary cell culture assay. J. Natl. Cancer Inst. 1971, 46, 411–422. [Google Scholar]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar]
- Beachy, P.A.; Karhadkar, S.S.; Berman, D.M. Tissue repair and stem cell renewal in carcinogenesis. Nature 2004, 432, 324–331. [Google Scholar] [CrossRef]
- Fiebig, H.H.; Berger, D.P.; Dengler, W.A.; Wallbrecher, E.; Winterhalter, B.R. Combined in vitro/in vivo test procedure with human tumor xenografts. In Immunodeficient Mice in Oncology; Fiebig, H.H., Berger, D.P., Eds.; Karger: Basel, Switzerland, 1992; pp. 321–351. Contributions to Oncology Volume 42. [Google Scholar]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anti-Cancer Drugs 1995, 6, 522–532. [Google Scholar]
- Fiebig, H.H.; Maier, A.; Burger, A.M. Clonogenic assay with established human tumour xenografts: Correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur. J. Cancer 2004, 40, 802–820. [Google Scholar] [CrossRef]
- Roth, T.; Burger, A.M.; Dengler, W.; Willmann, H.; Fiebig, H.H. Human tumor cell lines demonstrating the characteristics of the patient tumors as useful models for anticancer drug screening. In Relevance of Tumor Models for Anticancer Drug Development; Fiebig, H.H., Burger, A.M., Eds.; Karger: Basel, Switzerland, 1999; pp. 145–156. Contributions to Oncology Volume 54. [Google Scholar]
- Fiebig, H.H.; Dengler, W.A.; Roth, T. Human Tumor Xenografts: Predictivity, Characterization, and Discovery of New Anticancer Agents. In Relevance of Tumor Models for Anticancer Drug Development; Fiebig, H.H., Burger, A.M., Eds.; Karger: Basel, Switzerland, 1999; pp. 29–50. Contributions to Oncology Volume 54. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carbone, A.; Parrino, B.; Barraja, P.; Spanò, V.; Cirrincione, G.; Diana, P.; Maier, A.; Kelter, G.; Fiebig, H.-H. Synthesis and Antiproliferative Activity of 2,5-bis(3′-Indolyl)pyrroles, Analogues of the Marine Alkaloid Nortopsentin. Mar. Drugs 2013, 11, 643-654. https://doi.org/10.3390/md11030643
Carbone A, Parrino B, Barraja P, Spanò V, Cirrincione G, Diana P, Maier A, Kelter G, Fiebig H-H. Synthesis and Antiproliferative Activity of 2,5-bis(3′-Indolyl)pyrroles, Analogues of the Marine Alkaloid Nortopsentin. Marine Drugs. 2013; 11(3):643-654. https://doi.org/10.3390/md11030643
Chicago/Turabian StyleCarbone, Anna, Barbara Parrino, Paola Barraja, Virginia Spanò, Girolamo Cirrincione, Patrizia Diana, Armin Maier, Gerhard Kelter, and Heinz-Herbert Fiebig. 2013. "Synthesis and Antiproliferative Activity of 2,5-bis(3′-Indolyl)pyrroles, Analogues of the Marine Alkaloid Nortopsentin" Marine Drugs 11, no. 3: 643-654. https://doi.org/10.3390/md11030643
APA StyleCarbone, A., Parrino, B., Barraja, P., Spanò, V., Cirrincione, G., Diana, P., Maier, A., Kelter, G., & Fiebig, H.-H. (2013). Synthesis and Antiproliferative Activity of 2,5-bis(3′-Indolyl)pyrroles, Analogues of the Marine Alkaloid Nortopsentin. Marine Drugs, 11(3), 643-654. https://doi.org/10.3390/md11030643




