Omega-3 Fatty Acids Modulate Weibel-Palade Body Degranulation and Actin Cytoskeleton Rearrangement in PMA-Stimulated Human Umbilical Vein Endothelial Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. PMA-Stimulated Degranulation of Weibel-Palade Bodies
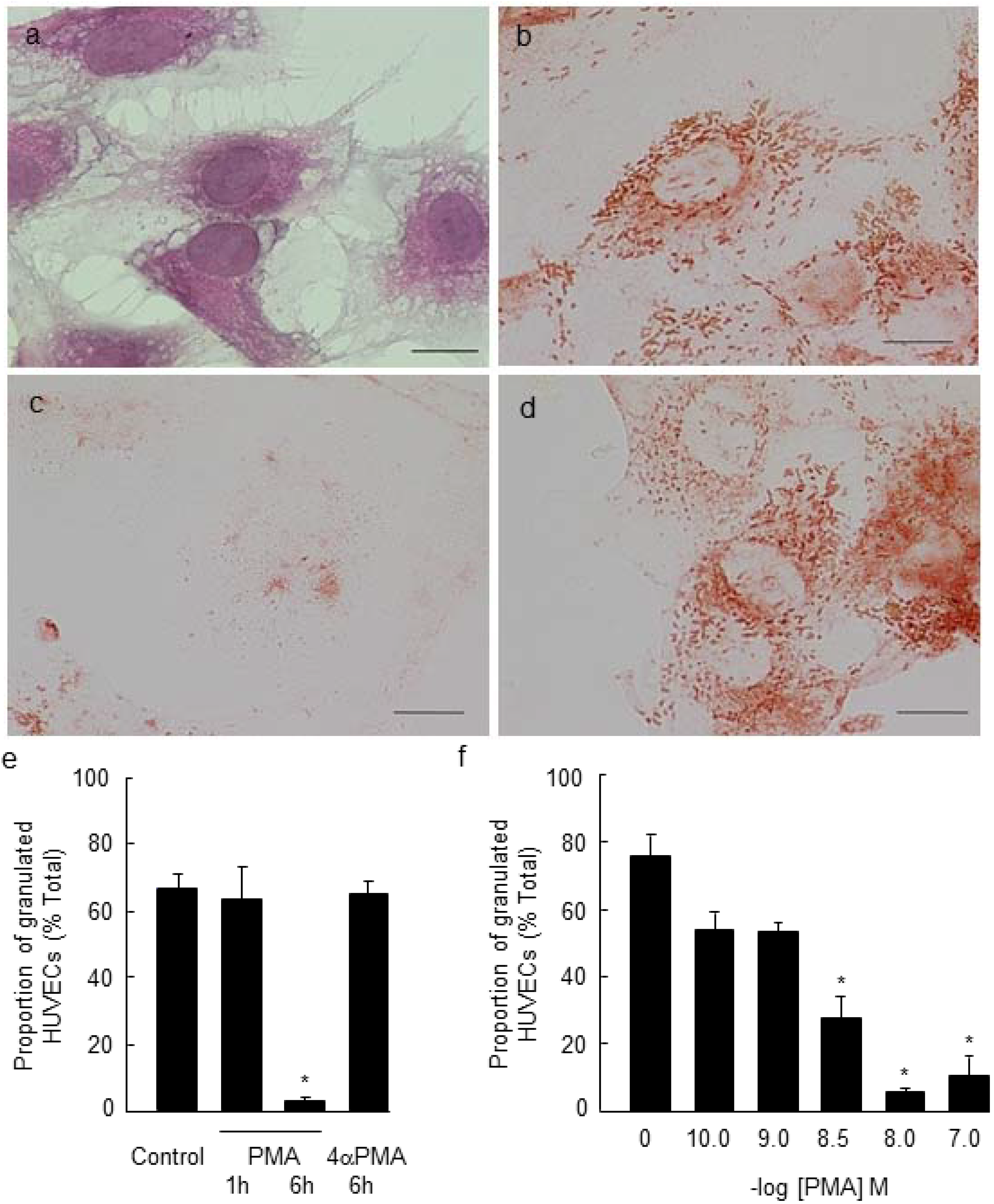
2.2. Effect of Long Chain Omega-3 Fatty Acids on the Pattern of Weibel-Palade Body Degranulation

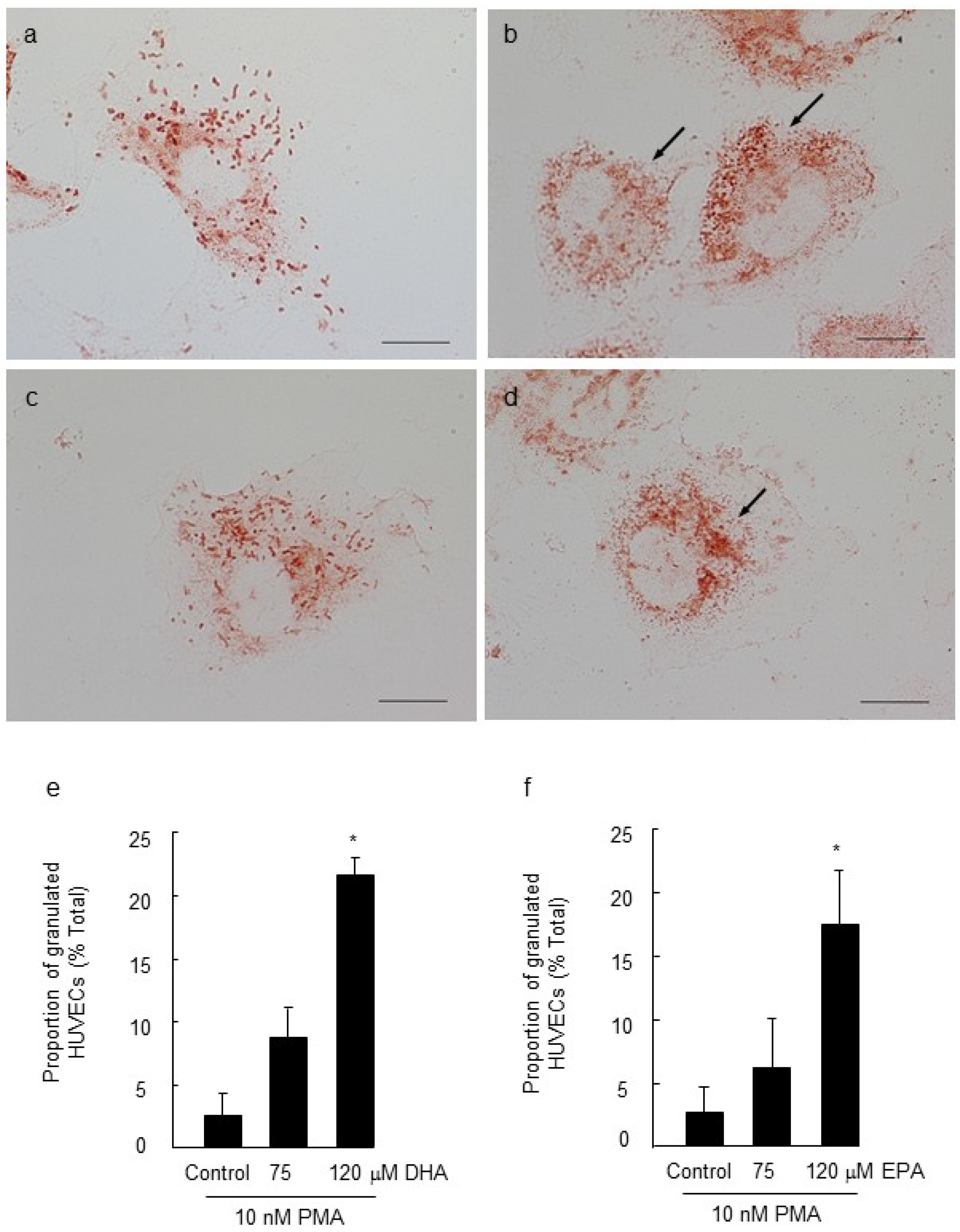
2.3. Effect of LC n-3 PUFAs on Actin Cytoskeletal Rearrangement in PMA Stimulated HUVECs

3. Experimental Section
3.1. Culture of Human Umbilical Vein Endothelial Cells
3.2. Immunocytochemistry Staining for Von Willebrand Factor
3.3. Quantitation of Weibel-Palade Body Degranulation
3.4. Gas Chromatography—Mass Spectrometry Analysis of Cellular LC n-3 PUFA
3.5. Oil Red O Staining for Lipids
3.6. Cellular Actin Remodeling
3.7. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Naya, M.; Tsukamoto, T.; Morita, K.; Katoh, C.; Furumoto, T.; Fujii, S.; Tamaki, N.; Tsutsui, H. Plasma interleukin-6 and tumor necrosis factor-alpha can predict coronary endothelial dysfunction in hypertensive patients. Hypertens. Res. 2007, 30, 541–548. [Google Scholar] [CrossRef]
- Russell, F.D.; Skepper, J.N.; Davenport, A.P. Evidence using immunoelectron microscopy for regulated and constitutive pathways in the transport and release of endothelin. J. Cardiovasc. Pharmacol. 1998, 31, 424–430. [Google Scholar] [CrossRef]
- Michaux, G.; Abbitt, K.B.; Collinson, L.M.; Haberichter, S.L.; Norman, K.E.; Cutler, D.F. The physiological function of von Willebrand’s factor depends on its tubular storage in endothelial Weibel-Palade bodies. Dev. Cell 2006, 10, 223–232. [Google Scholar] [CrossRef]
- Nightingale, T.D.; Pattni, K.; Hume, A.N.; Seabra, M.C.; Cutler, D.F. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood 2009, 113, 5010–5018. [Google Scholar] [CrossRef]
- Metcalf, D.; Nightingale, T.; Zenner, H.; Lui-Roberts, W.; Cutler, D. Formation and function of Weibel-Palade bodies. J. Cell Sci. 2008, 121, 19–27. [Google Scholar] [CrossRef]
- Fiedler, U.; Scharpfenecker, M.; Koidl, S.; Hegen, A.; Grunow, V.; Schmidt, J.M.; Kriz, W.; Thurston, G.; Augustin, H.G. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004, 103, 4150–4156. [Google Scholar] [CrossRef]
- Lowenstein, C.J.; Morrell, C.N.; Yamakuchi, M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc. Med. 2005, 15, 302–308. [Google Scholar] [CrossRef]
- Cleator, J.H.; Qin Zhu, W.; Vaughan, D.E.; Hamm, H.E. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood 2006, 107, 2736–2744. [Google Scholar] [CrossRef]
- Sen, U.; Tyagi, N.; Patibandla, P.K.; Dean, W.L.; Tyagi, S.C.; Roberts, A.M.; Lominadze, D. Fibrinogen-induced endothelin-1 production from endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C840–C847. [Google Scholar] [CrossRef]
- Okruhlicová, L’.; Dlugošová, K.; Mitašiková, M.; Bernátová, I. Ultrastructural characteristics of aortic endothelial cells in borderline hypertensive rats exposed to chronic social stress. Physiol. Res. 2008, 57, S31–S37. [Google Scholar]
- Davis, J.S.; Yeo, T.W.; Piera, K.A.; Woodberry, T.; Celermajer, D.S.; Stephens, D.P.; Anstey, N.M. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit. Care 2010, 14, R89. [Google Scholar] [CrossRef]
- Yeo, T.W.; Lampah, D.A.; Tjitra, E.; Piera, K.; Gitawati, R.; Kenangalem, E.; Price, R.N.; Anstey, N.M. Greater endothelial activation, Weibel-Palade body release and host inflammatory response to Plasmodium vivax, compared with Plasmodium falciparum: A prospective study in Papua, Indonesia. J. Infect. Dis. 2010, 202, 109–112. [Google Scholar] [CrossRef]
- Vischer, U.; Barth, H.; Wollheim, C. Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodelling in cultured endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 883–891. [Google Scholar] [CrossRef]
- Nightingale, T.; White, I.; Doyle, E.; Turmaine, M.; Harrison-Lavoie, K.; Webb, K.; Cramer, L.P.; Cutler, D.F. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J. Cell Biol. 2011, 194, 613–629. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.T.; Kok, F.J. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J. Hypertens. 2002, 20, 1493–1499. [Google Scholar] [CrossRef]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Bønaa, K.; Bjerve, K.; Straume, B.; Gram, I.; Thelle, D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension—A population-based intervention trial from the Tromsø Study. N. Engl. J. Med. 1990, 322, 795–801. [Google Scholar] [CrossRef]
- Mori, T.; Bao, D.; Burke, V.; Puddey, I.; Beilin, L. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 1999, 34, 253–260. [Google Scholar] [CrossRef]
- Morris, M.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef]
- De Caterina, R.; Cybulsky, M.; Clinton, S.; Gimbrone, M.; Libby, P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1994, 14, 1829–1836. [Google Scholar] [CrossRef]
- Stulnig, T.M.; Huber, J.; Leitinger, N.; Imre, E.M.; Angelisova, P.; Nowotny, P.; Waldhausl, W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem. 2001, 276, 37335–37340. [Google Scholar]
- Webb, Y.; Hermida-Matsumoto, L.; Resh, M.D. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 2000, 275, 261–270. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Jenny, N.S.; Mayer-Davis, E.; Jiang, R.; Steffen, L.; Siscovick, D.; Tsai, M.; Herrington, D. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 1238–1243. [Google Scholar] [CrossRef]
- Ye, S.; Tan, L.; Ma, J.; Shi, Q.; Li, J. Polyunsaturated docosahexaenoic acid suppresses oxidative stress induced endothelial cell calcium influx by altering lipid composition in membrane caveolar rafts. Prostaglandins Leukot. Essent. Fatty Acids 2010, 83, 37–43. [Google Scholar] [CrossRef]
- Johansen, O.; Seljeflot, I.; Høstmark, A.T.; Arnesen, H. The Effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1681–1686. [Google Scholar] [CrossRef]
- Seljeflot, I.; Arnesen, H.; Brude, I.R.; Nenseter, M.S.; Drevon, C.A.; Hjermann, I. Effects of omega-3 farry acids and/or antioxidants on endothelial cell markers. Eur. J. Clin. Invest. 1998, 28, 629–635. [Google Scholar] [CrossRef]
- Kanayasu-Toyoda, T.; Morita, I.; Murota, S. Docosapentaenoic acid (22:5, n-3), an elongation metabolite of eicosapentaenoic acid (20:5, n-3), is a potent stimulator of endothelial cell migration on pretreatment in vitro. Prostaglandins Leukot. Essent. Fatty Acids 1996, 54, 319–325. [Google Scholar] [CrossRef]
- McIntosh, A.L.; Huang, H.; Atshaves, B.P.; Wellberg, E.; Kuklev, D.V.; Smith, W.L.; Kier, A.B.; Schroeder, F. Fluorescent n-3 and n-6 very long chain polyunsaturated fatty acids: Three-photon imaging in living cells expressing liver fatty acid-binding protein. J. Biol. Chem. 2010, 285, 18693–18708. [Google Scholar] [CrossRef]
- Salm, P.; Taylor, P.J.; Kostner, K. Simultaneous quantification of total eicosapentaenoic acid, docosahexaenoic acid and arachidonic acid in plasma by high-performance liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2011, 25, 652–659. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar]
- Weylandt, K.H.; Chiu, C.-Y.; Gomolka, B.; Waechter, S.F. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation: omega-3 fatty acids and their resolvin/protectin mediators. Prostaglandins Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Lui-Roberts, W.W.Y.; Collinson, L.M.; Hewlett, L.J.; Michaux, G.; Cutler, D.F. An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells. J. Cell Biol. 2005, 170, 627–636. [Google Scholar] [CrossRef]
- Ma, D.W.; Seo, J.; Davidson, L.A.; Callaway, E.S.; Fan, Y.Y.; Lupton, J.R.; Chapkin, R.S. N-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004, 18, 1040–1042. [Google Scholar]
- Roy, J.; Lefkimmiatis, K.; Moyer, M.P.; Curci, S.; Hofer, A.M. The ω-3 fatty acid eicosapentaenoic acid elicits cAMP generation in colonic epithelial cells via “store-operated” mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G715–G722. [Google Scholar] [CrossRef]
- Klarenbach, S.; Chipiuk, A.; Nelson, R.; Hollenberg, M.; Murray, A. Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: The role of Rho-GTPases. Circ. Res. 2003, 92, 272–278. [Google Scholar] [CrossRef]
- Tonutti, L.; Manzi, L.; Tacconi, M.T.; Bazzoni, G. Eicosapentaenoic acid inhibits endothelial cell migration in vitro. J. Angiogenes. Res. 2010, 2, 12. [Google Scholar] [CrossRef]
- Macarthur, H.; Warner, T.; Wood, E.; Corder, R.; Vane, J. Endothelin-1 release from endothelial cells in culture is elevated both acutely and chronically by short periods of mechanical stretch. Biochem. Biophys. Res. Commun. 1994, 200, 395–400. [Google Scholar] [CrossRef]
- Galbusera, M.; Zoja, C.; Donadelli, R.; Paris, S.; Morigi, M.; Benigni, A.; Figliuzzi, M.; Remuzzi, G.; Remuzzi, A. Fluid shear stress modulates von Willebrand Factor release from human vascular endothelium. Blood 1997, 90, 1558–1564. [Google Scholar]
- Kaye, S.A.; Obrig, T.G. Effect of TNF-α, shiga toxin and calcium ionophore on Weibel-Palade body content of endothelial cells: possible implications for the hemolytic uremic syndrome. Thromb. Res. 1995, 79, 415–421. [Google Scholar] [CrossRef]
- Takanaro, M.; Meneshian, A.; Sheikh, E.; Yamakawa, Y.; Wilkins, K.B.; Hopkins, E.A.; Bulkley, G.B. Rapid upregulation of endothelial P-selectin expression via reactive oxygen species generation. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2054–H2061. [Google Scholar]
- Bhatia, R.; Matsushita, K.; Yamakuchi, M.; Morrell, C.N.; Cao, W.; Lowenstein, C.J. Ceramide triggers Weibel-Palade body exocytosis. Circ. Res. 2004, 95, 319–324. [Google Scholar] [CrossRef]
- Erent, M.; Meli, A.; Moisoi, N.; Babich, V.; Hannah, M.; Skehel, P.; Knipe, L.; Zupancic, G.; Ogden, D.; Carter, T. Rate, extent and concentration dependence of histamine-evoked Weibel-Palade body exocytosis determined from individual fusion events in human endothelial cells. J. Physiol. 2007, 583, 195–212. [Google Scholar] [CrossRef]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481–485. [Google Scholar]
- Ge, X.; Low, B.; Liang, M.; Fu, J. Angiotensin II directly triggers endothelial exocytosis via protein kinase C-dependent protein kinase D2 activation. J. Pharmacol. Sci. 2007, 105, 168–176. [Google Scholar] [CrossRef]
- Tardivel, S.; Gousset-Dupont, A.; Robert, V.; Pourci, M.-L.; Grynberg, A.; Lacour, B. Protective effects of EPA and deleterious effects of DHA on eNOS activity in Ea hy 926 cultured with lysophosphatidylcholine. Lipids 2009, 44, 225–235. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bürgin-Maunder, C.S.; Brooks, P.R.; Russell, F.D. Omega-3 Fatty Acids Modulate Weibel-Palade Body Degranulation and Actin Cytoskeleton Rearrangement in PMA-Stimulated Human Umbilical Vein Endothelial Cells. Mar. Drugs 2013, 11, 4435-4450. https://doi.org/10.3390/md11114435
Bürgin-Maunder CS, Brooks PR, Russell FD. Omega-3 Fatty Acids Modulate Weibel-Palade Body Degranulation and Actin Cytoskeleton Rearrangement in PMA-Stimulated Human Umbilical Vein Endothelial Cells. Marine Drugs. 2013; 11(11):4435-4450. https://doi.org/10.3390/md11114435
Chicago/Turabian StyleBürgin-Maunder, Corinna S., Peter R. Brooks, and Fraser D. Russell. 2013. "Omega-3 Fatty Acids Modulate Weibel-Palade Body Degranulation and Actin Cytoskeleton Rearrangement in PMA-Stimulated Human Umbilical Vein Endothelial Cells" Marine Drugs 11, no. 11: 4435-4450. https://doi.org/10.3390/md11114435
APA StyleBürgin-Maunder, C. S., Brooks, P. R., & Russell, F. D. (2013). Omega-3 Fatty Acids Modulate Weibel-Palade Body Degranulation and Actin Cytoskeleton Rearrangement in PMA-Stimulated Human Umbilical Vein Endothelial Cells. Marine Drugs, 11(11), 4435-4450. https://doi.org/10.3390/md11114435




