Beneficial Effects of Marine Algal Compounds in Cosmeceuticals
Abstract
:1. Introduction
2. Marine Algae in Cosmeceuticals
2.1. Skin Health Protection and Skin Whitening
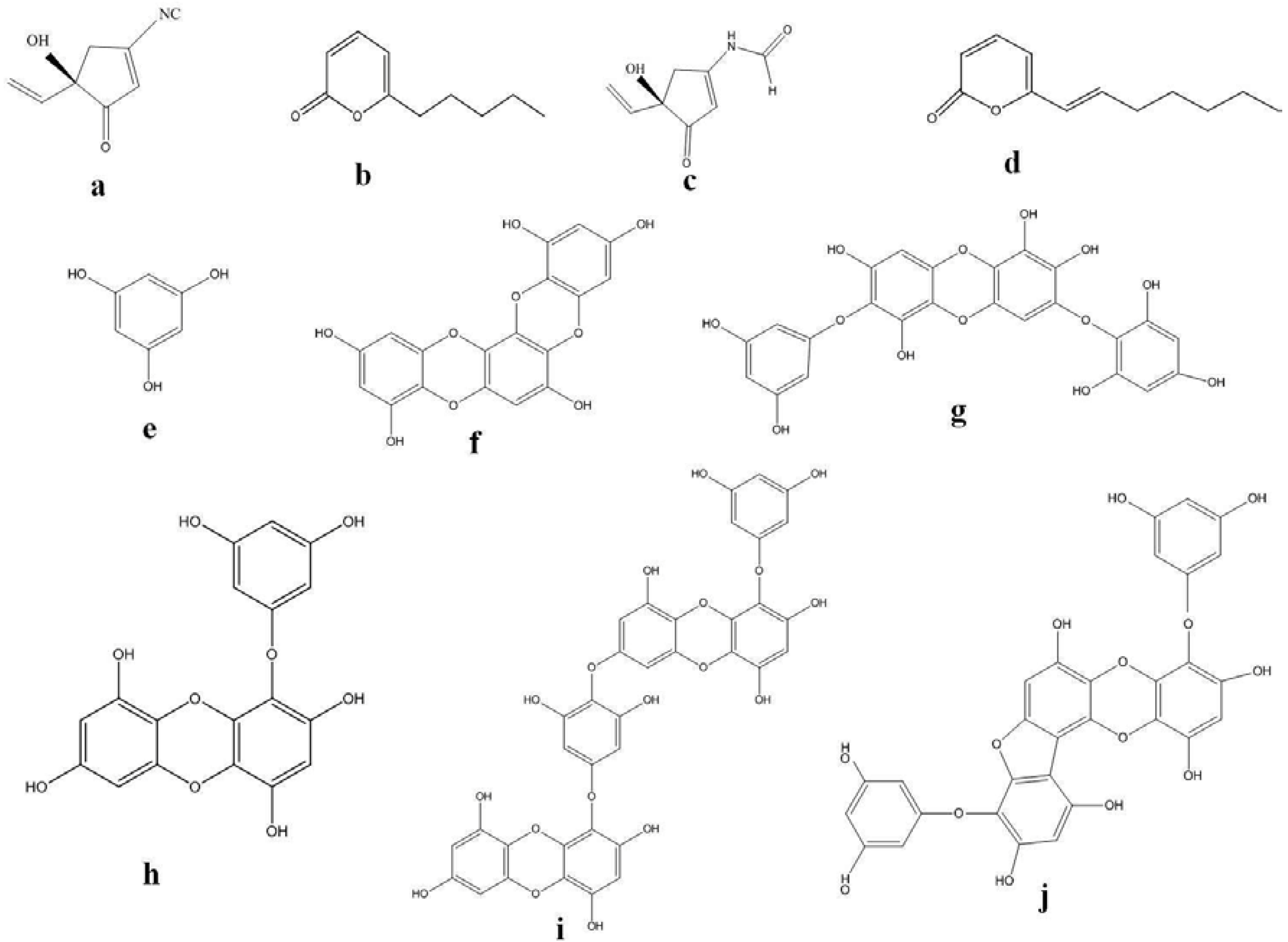
| Serial Number | Medicinal Application | Phlorotannin and Brown Algal Species | References |
|---|---|---|---|
| 1 | Inhibitory effect on histamine release | 6,6′-bieckol— E. cava | [59] |
| Methanolic Extracts— E.arborea | [60] | ||
| Eckol | [61] | ||
| 6,6′-bieckol | |||
| 6,8′-bieckol | |||
| 8,8′-bieckol— E.arborea | |||
| Phlorofucofuroeckol A | |||
| Phlorofucofuroeckol B | |||
| 2 | Inhibitory effect hyaluronidase | Eckol | [62] |
| Phlorofucofuroeckol A— E. bicyclis | |||
| Dieckol E. kurome | |||
| 8,8′-bieckol | |||
| 3 | Inhibitory effect on FcεRI overexpression | Methanolic Extracts— E. cava | [63] |
| 4 | Inhibitory effect on overexpression of IgE | Phlorofucofuroeckol A— E.arborea | [30,61] |
| 5 | Inhibitory effect of MMP-1 expression | Eckol | [64] |
| Dieckol |
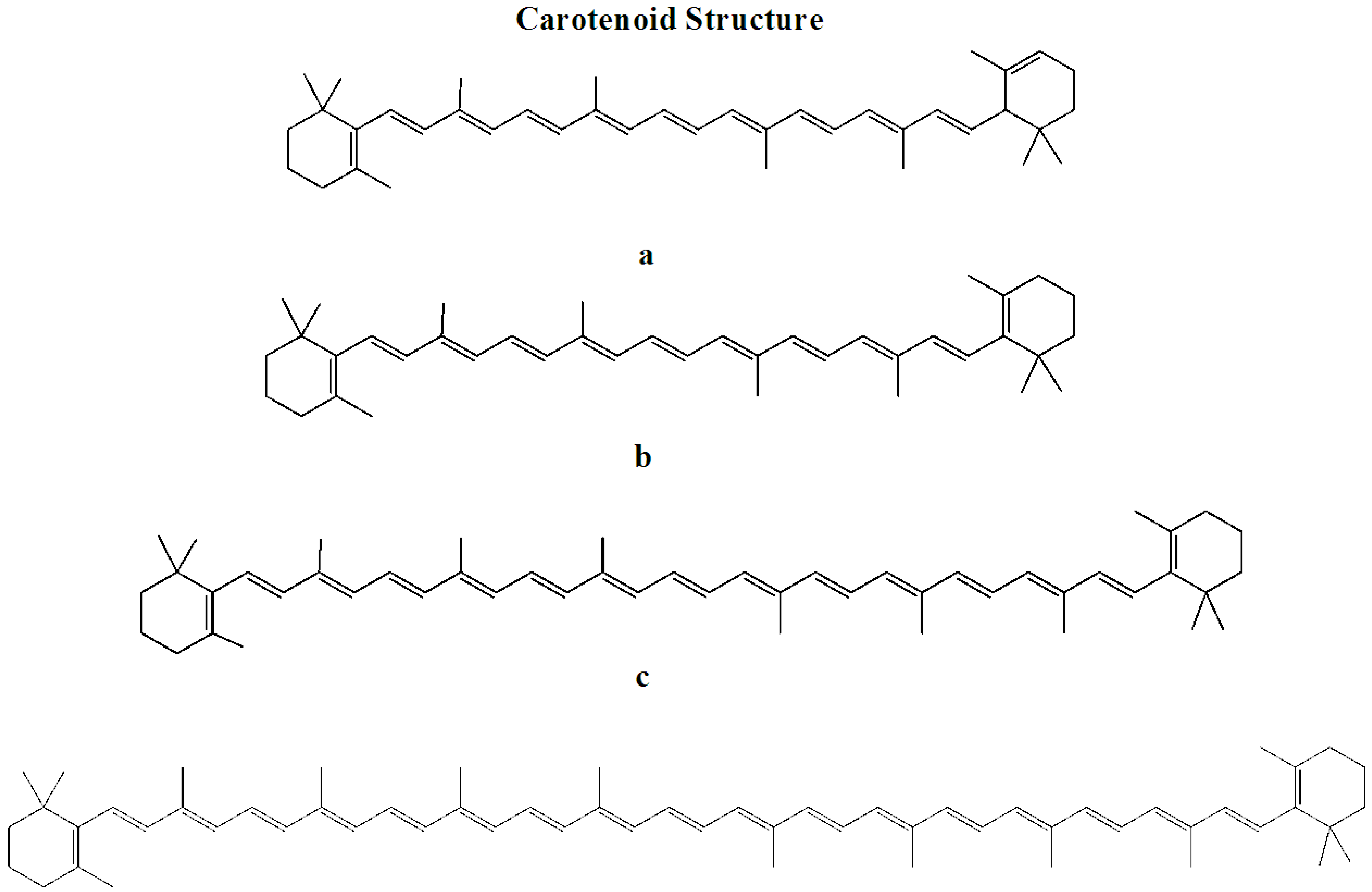
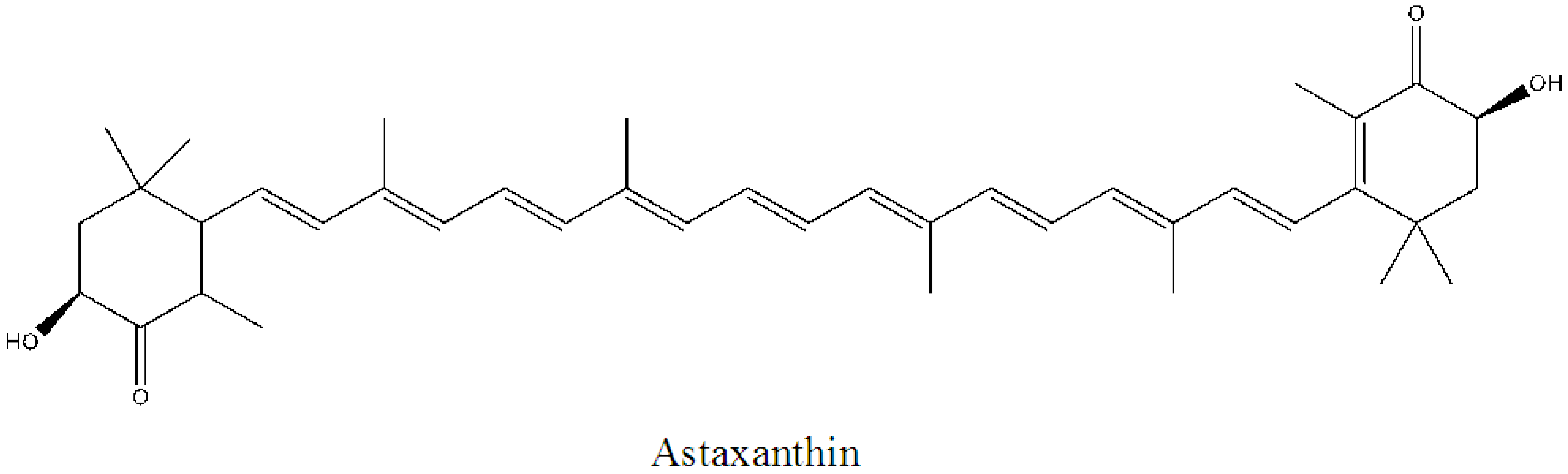
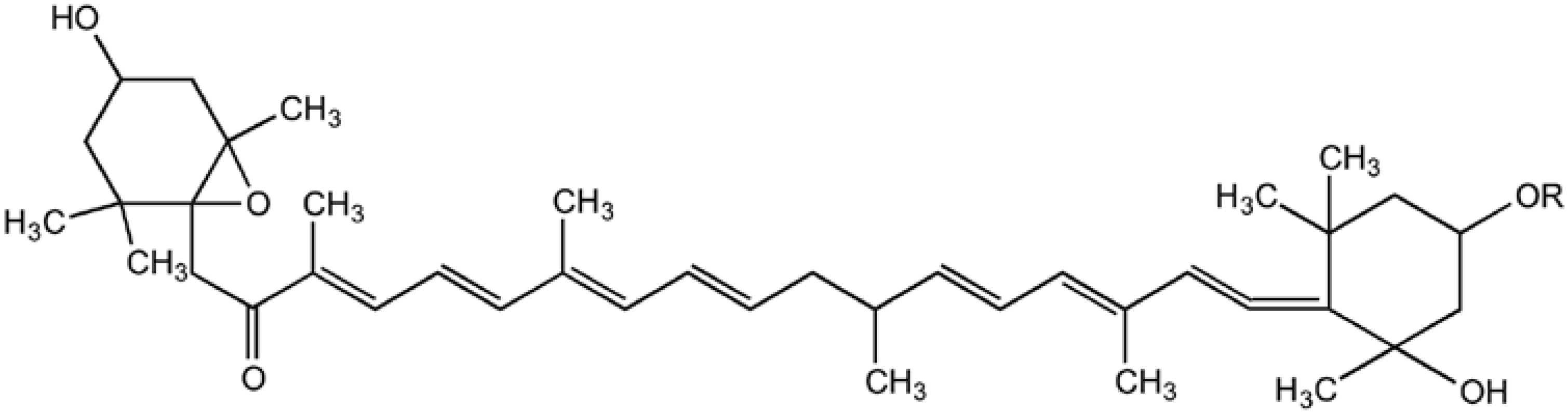
2.2. Prevention of Skin-Related Protozoan Diseases
2.3.Atopic Dermatitis
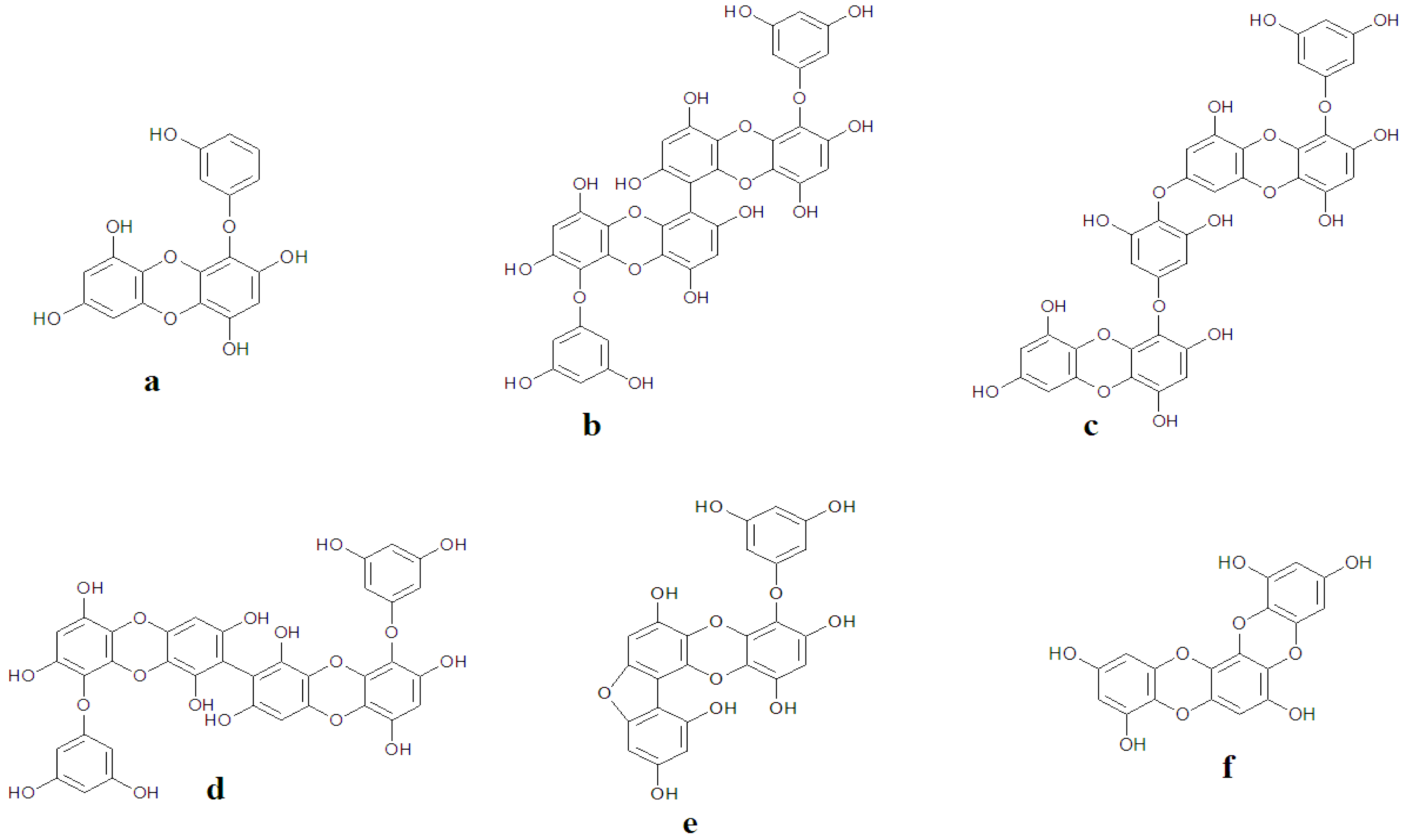
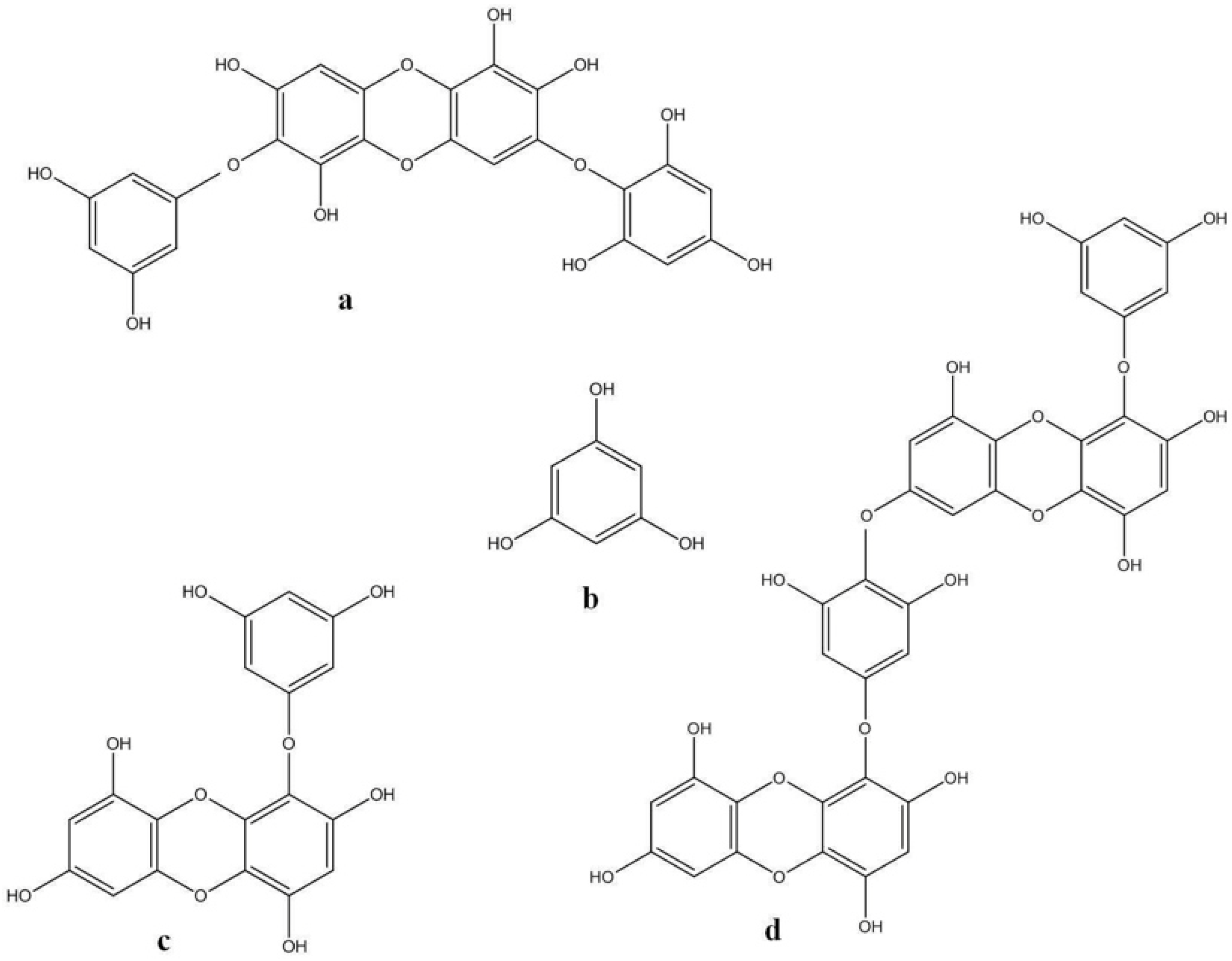
2.4. The Role of Fucoidan in Skin Diseases Treatment
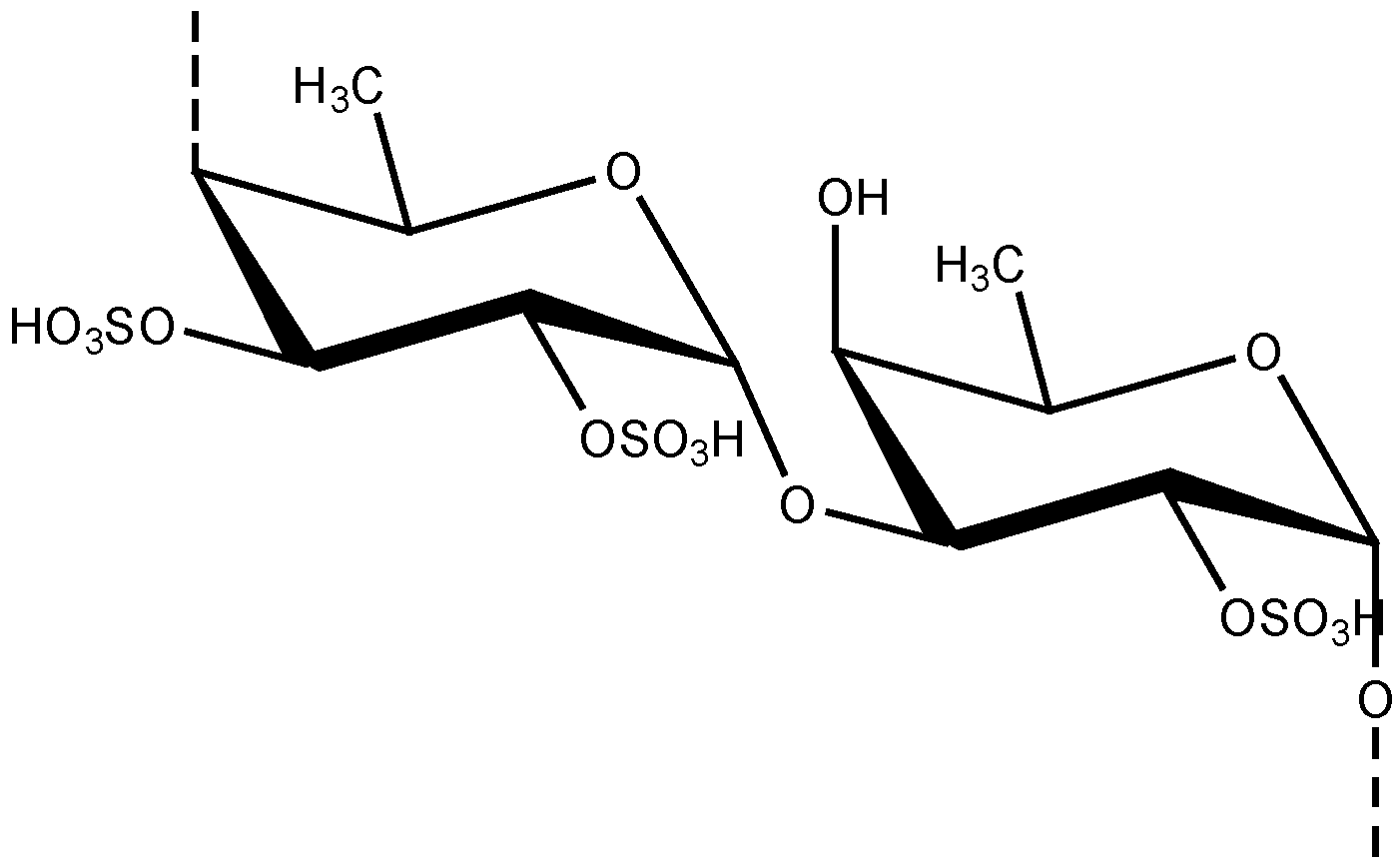
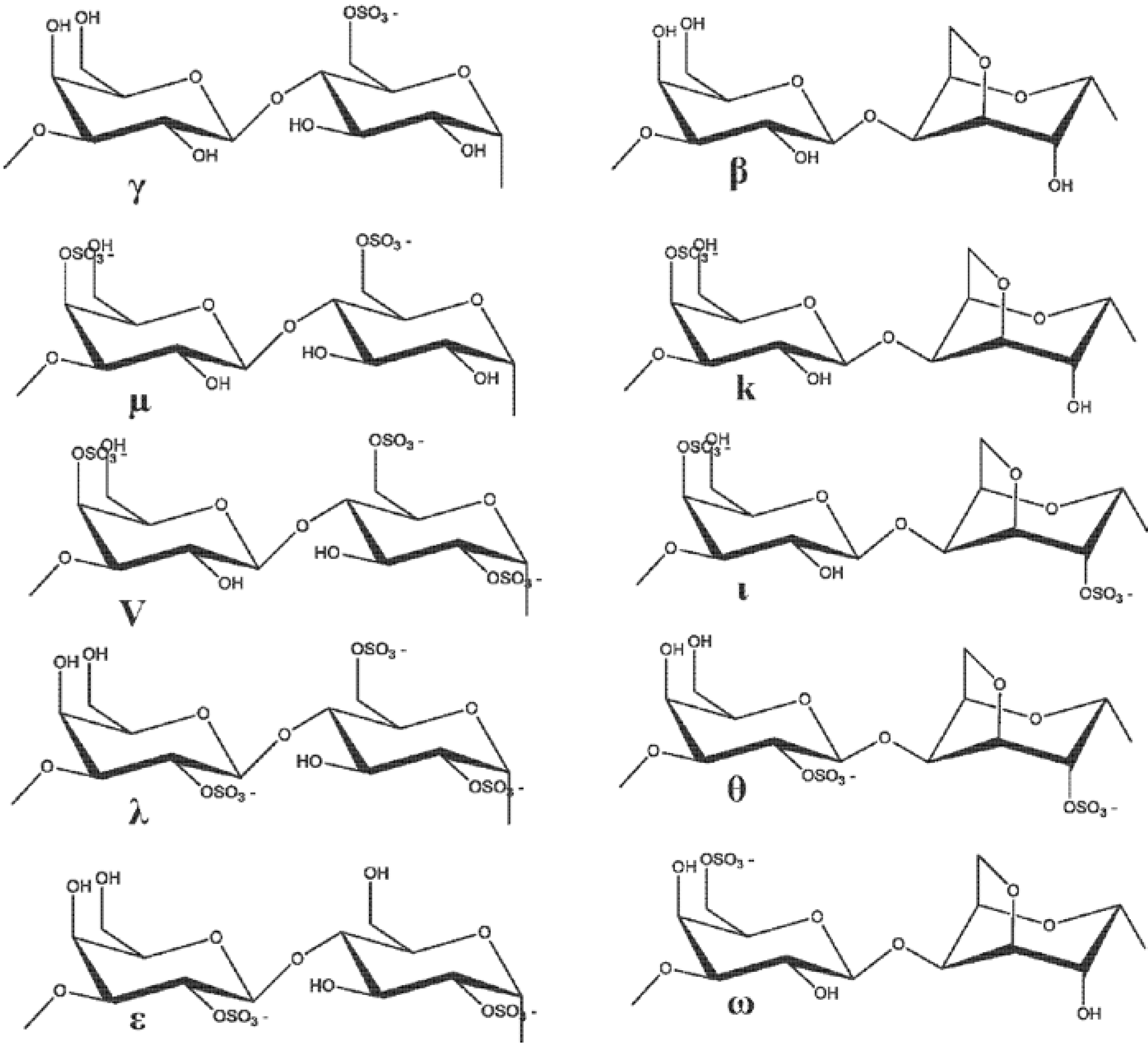
3. The Function of MMP Inhibitors in Skin-Related Diseases
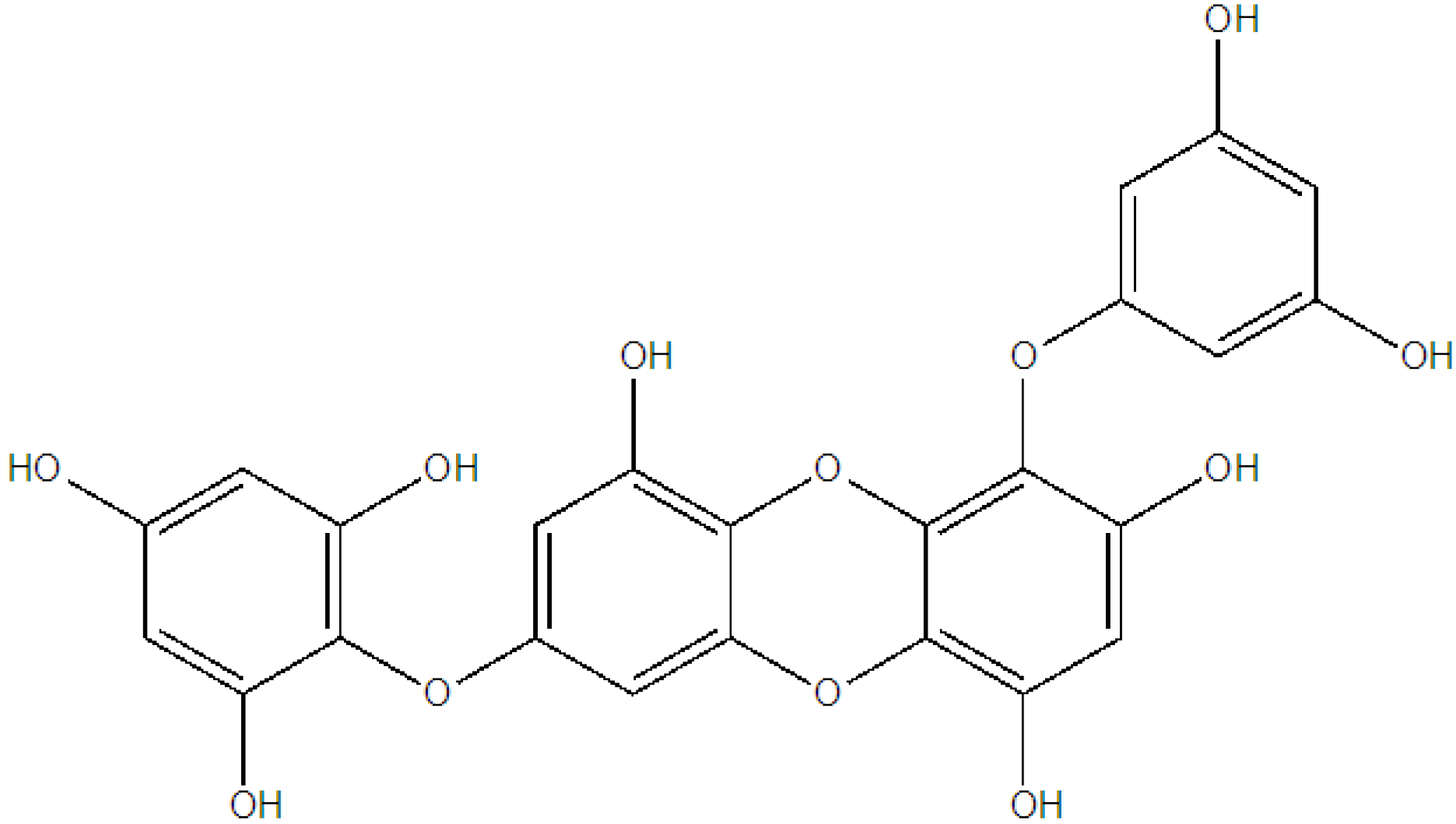
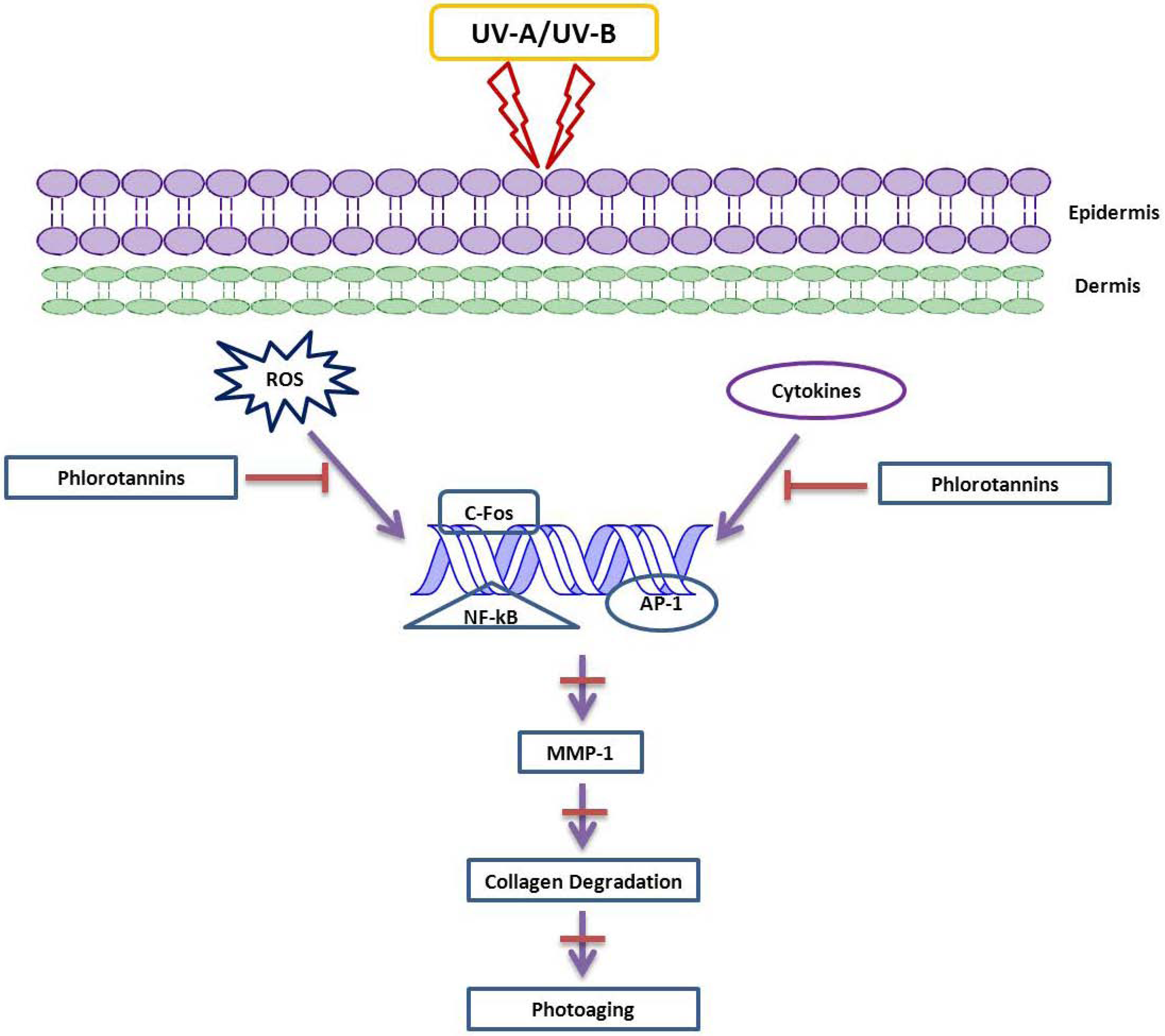
4. Future Prospects
5. Conclusions
Acknowledgements
References
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Metting, B.; Pyne, J.W. Biologically active compounds from microalgae. Enzyme Microb. Technol. 1986, 8, 386–394. [Google Scholar] [CrossRef]
- Moore, R.E. Volatile compounds from marine algae. Acc. Chem. Res. 1977, 10, 40–47. [Google Scholar] [CrossRef]
- Cannell, R.J.P. Algae as a source of biologically active products. Pestic. Sci. 2006, 39, 147–153. [Google Scholar] [CrossRef]
- Blunden, G. Marine algae as sources of biologically active compounds. Interdiscip. Sci. Rev. 1993, 18, 73–80. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011, 28, 186–195. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 47, 386–394. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and Characterization of Bioactive Compounds with Health Benefits from Marine Resources: Macro and Micro Algae, Cyanobacteria, and Invertebrates. In Marine Bioact Compounds; Haves, M., Ed.; Springer-Verlag New York Inc.: New York, NY, USA, 2012; pp. 55–98. [Google Scholar]
- Vo, T.S.; Kim, S.K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2012, in press. [Google Scholar]
- Venkatesan, J.; Kim, S.K. Osteoporosis treatment: Marine algal compounds. Adv. Food Nutr. Res. 2011, 64, 417–427. [Google Scholar]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel fucoidan extraction processes from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Gates, K.W. Marine polysaccharides—Food applications. Vazhiyil Venugopal. J. Aquat. Food Prod. Technol. 2012, 21, 181–186. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and Digestive Health Benefits of Seaweed. In Advances in Food and Nutrition Research; Kim, S.K., Ed.; Academic Press: San Diego, CA, USA, 2011; Volume 64, pp. 17–28. [Google Scholar]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Ali, M.; Jahangir, M.; Saleem, M.; Pervez, M.; Hameed, S.; Ahmad, V. Metabolites of marine algae collected from Karachi-coasts of Arabian sea. Nat. Prod. Sci. 2000, 6, 61–65. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Wijesekara, I.; Senevirathne, M.; Li, Y.X.; Kim, S.K. Functional Ingredients from Marine Algae as Potential Antioxidants in the Food Industry. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.K., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2012; pp. 398–402. [Google Scholar]
- Siqueira, R.C.L.; da Silva, M.S.J.; de Alencar, D.B.; Pires, A.F.; de Alencar, N.M.N.; Pereira, M.G.; Cavada, B.S.; Sampaio, A.H.; Farias, W.R.L.; Assreuy, A.M.S. In vivo anti-inflammatory effect of a sulfated polysaccharide isolated from the marine brown algae Lobophora variegata. Pharm. Biol. 2011, 49, 167–174. [Google Scholar]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: An overview. Inflamm. AllergyDrug Targets 2012, 11, 90–101. [Google Scholar]
- Dore, C.M.P.G.; Alves, M.G.C.F.; Costa, T.G.; Sabry, D.A.; Rêgo, L.A.S.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar]
- Xu, H.L.; Kitajima, C.; Ito, H.; Miyazaki, T.; Baba, M.; Okuyama, T.; Okada, Y. Antidiabetic effect of polyphenols from brown alga Ecklonia kurome in genetically diabetic KK-Ay mice. Pharm. Biol. 2012, 50, 393–400. [Google Scholar] [CrossRef]
- Grozdanic, N.; Stanojkovic, T.; Kljajic, Z.; Etahiri, S.; Assobhei, O.; Konic-Ristic, A.; Srdic-Rajic, T.; Kardum, N.; Backovic, S. In vitro evaluation of antioxidant and antitumoral activities of marine algae Gelidium sesquipedale and Fucus spiralis. Eur. J. Cancer 2012, 48, S26. [Google Scholar]
- Samarakoon, K.; Jeon, Y.J. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Nguyen, M.H.T.; Jung, W.K.; Kim, S.K. Marine algae possess therapeutic potential for Ca-mineralization via osteoblastic differentiation. Adv. Food Nutr. Res. 2011, 64, 429–441. [Google Scholar]
- Koyama, T. Extracts of marine algae show inhibitory activity against osteoclast differentiation. Adv. Food Nutr. Res. 2011, 64, 443–454. [Google Scholar] [CrossRef]
- O’Gorman, D.M.; Tierney, C.M.; Brennan, O.; O’Brien, F.J. The Marine-derived, multi-mineral formula, aquamin, enhances mineralisation of osteoblast cells in vitro. Phytother. Res. 2011, 26, 375–380. [Google Scholar]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovas. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Rios, A.O.; Antunes, L.M.G.; Bianchi, M.L.P. Bixin and lycopene modulation of free radical generation induced by cisplatin-DNA interaction. Food Chem. 2009, 113, 1113–1118. [Google Scholar] [CrossRef]
- Berson, D.S.; Cohen, J.L.; Rendon, M.I.; Roberts, W.E.; Starker, I.; Wang, B. Clinical role and application of superficial chemical peels in today’s practice. J. Drugs Dermatol. 2009, 8, 803–911. [Google Scholar]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar]
- Amornlerdpison, D.; Peerapornpisal, Y.; Rujjanawate, C.; Taesotikul, T.; Nualchareon, M.; Kanjanapothi, D. Antioxidant activity of Padina minor Yamada. KMITL Sci. Tech. J. 2007, 7, 1–7. [Google Scholar]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar]
- Babitha, S.; Kim, E.K. Effect of Marine Cosmeceuticals on the Pigmentation of Skin. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 63–66. [Google Scholar]
- Kim, S.K.; Bak, S.S. Hair Biology and Care Product Ingredients from Marine Organisms. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 201–210. [Google Scholar]
- Thomas, N.V.; Kim, S.K. Potential Cosmeceutical Applications of Phlorotannins and Fucoidans from Marine Algae in the Treatment of Atopic Dermatitis. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 257–264. [Google Scholar]
- Kim, S.K.; Karadeniz, F. Industrial Prospects of the Cosmeceuticals Derived from Marine Mucin. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 391–398. [Google Scholar]
- Zhang, S.; Hunter, D.J.; Forman, M.R.; Rosner, B.A.; Speizer, F.E.; Colditz, G.A.; Manson, J.E.; Hankinson, S.E.; Willett, W.C. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J. Natl. Cancer Inst. 1999, 91, 547–556. [Google Scholar]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar]
- Ryu, B.; Qian, Z.-J.; Kim, M.-M.; Nam, K.W.; Kim, S.-K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar]
- Pallela, R.; Na-Young, Y.; Kim, S.K. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar]
- Cha, S.H.; Ko, C.I.; Kim, D.; Jeon, Y.J. Protective effects of phlorotannins against ultraviolet B radiation in zebrafish (Danio rerio). Vet. Dermatol. 2012, 23, 51–56. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. Res. 2010, 62, 1137–1145. [Google Scholar]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar]
- Le, Q.T.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Sugiura, Y.; Takeuchi, Y.; Kakinuma, M.; Amano, H. Inhibitory effects of seaweeds on histamine release from rat basophile leukemia cells (RBL-2H3). Fish. Sci. 2006, 72, 1286–1291. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Anti-allergic phlorotannins from the edible brown alga, Eisenia arborea. Food Sci. Technol. Res. 2007, 13, 54–60. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Tech. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Shim, S.Y.; Quang-To, L.; Lee, S.H.; Kim, S.K. Ecklonia cava extract suppresses the high-affinity IgE receptor, FcεRI expression. Food Chem. Toxicol. 2009, 47, 555–560. [Google Scholar] [CrossRef]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef]
- Sorg, O.; Antille, C.; Kaya, G.; Saurat, J.H. Retinoids in cosmeceuticals. Dermatol. Ther. 2006, 19, 289–296. [Google Scholar] [CrossRef]
- Chávez-Crooker, P.; Obreque, J.; Vera, J.; Moya, K. Role of Astaxanthin in Cosmeceutical Applications. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 119–124. [Google Scholar]
- Draelos, Z.D. Cosmeceuticals. In Evidence Based Procedural Dermatology; Springer: New York, NY, USA, 2012; pp. 317–332. [Google Scholar]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2010, 84, 14–21. [Google Scholar]
- Yamashita, E. Biological activities of astaxanthin and its cosmeceutical application. Fragr. J. 2006, 34, 21–27. [Google Scholar]
- Yamashita, E. Astaxanthin: An effective anti-peroxidant in food: Cosmeceutical benefit for skin condition-Astaxanthin-1. Food Style 21 2007, 11, 31–34. [Google Scholar]
- Nene, Y. A glimpse at viral diseases in the ancient period 1. Asian Agri-Hist. 2007, 11, 33–46. [Google Scholar]
- Gopal, R.; Vijayakumaran, M.; Venkatesan, R.; Kathiroli, S. Marine organisms in Indian medicine and their future prospects. Nat. Prod. Radiance 2008, 7, 139–145. [Google Scholar]
- Kuniyoshi, M.; Wahome, P.G.; Miono, T.; Hashimoto, T.; Yokoyama, M.; Shrestha, K.L.; Higa, T. Terpenoids from Laurencia luzonensis. J. Nat. Prod. 2005, 68, 1314–1317. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Tuney, I.; Cadirci, B.H.; Unal, D.; Sukatar, A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey). Turk. J. Biol. 2006, 30, 171–175. [Google Scholar]
- Isolauri, E.; Turjanmaa, K. Combined skin prick and patch testing enhances identification of food allergy in infants with atopic dermatitis. J. Allergy Clin. Immunol. 1996, 97, 9–15. [Google Scholar] [CrossRef]
- Eigenmann, P.A.; Sicherer, S.H.; Borkowski, T.A.; Cohen, B.A.; Sampson, H.A. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics 1998, 101, E8. [Google Scholar]
- Niggemann, B.; Reibel, S.; Wahn, U. The atopy patch test (APT)—A useful tool for the diagnosis of food allergy in children with atopic dermatitis. Allergy 2008, 55, 281–285. [Google Scholar]
- Burks, A.W.; James, J.M.; Hiegel, A.; Wilson, G.; Wheeler, J.G.; Jones, S.M.; Zuerlein, N. Atopic dermatitis and food hypersensitivity reactions. J. Pediatr. 1998, 132, 132–136. [Google Scholar] [CrossRef]
- Hamid, Q.; Boguniewicz, M.; Leung, D. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J. Clin. Investig. 1994, 94, 870–876. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; He, R.; Kumar, L.; Yoon, J.; Geha, R.S. Cellular and molecular mechanisms in atopic dermatitis. Adv. Immunol. 2009, 102, 135–226. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003, 112, 252–262. [Google Scholar] [CrossRef]
- Kitaura, J.; Song, J.; Tsai, M.; Asai, K.; Maeda-Yamamoto, M.; Mocsai, A.; Kawakami, Y.; Liu, F.T.; Lowell, C.A.; Barisas, B.G.; et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the Fc RI. Proc. Natl. Acad. Sci. USA 2003, 100, 12911–12916. [Google Scholar]
- Sheinkopf, L.E.; Rafi, A.W.; Do, L.A.T.; Katz, R.M.; Klaustermeyer, W.B. Efficacy of omalizumab in the treatment of atopic dermatitis: A pilot study. Allergy Asthma Proc. 2008, 29, 530–537. [Google Scholar] [CrossRef]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Inhibitory effects of some natural products on the activation of hyaluronidase and their anti-allergic actions. Chem. Pharm. Bull. (Tokyo) 1992, 40, 1439–1442. [Google Scholar] [CrossRef]
- Maeda, Y.; Yamamoto, M.; Masui, T.; Sugiyama, K.; Yokota, M.; Nakagomi, K.; Tanaka, H.; Takahashi, I.; Kobayashi, T.; Kobayashi, E. Inhibitory effect of tea extracts on hyaluronidase. (Studies on anti-allergic activity in tea. 2). J. Food Hyg. Soc. Jpn. 1990, 31, 233–237. [Google Scholar] [CrossRef]
- Samee, H.; Li, Z.; Lin, H.; Khalid, J.; Guo, Y. Anti-allergic effects of ethanol extracts from brown seaweeds. J. Zhejiang Univ. Sci. B 2009, 10, 147–153. [Google Scholar] [CrossRef]
- Biotoxtech. Effects of Methanolic Extracts from Ecklonia cava on Dermatophagoides farinae Antigen Induced NC/Nga Mice; BTT Study No: B09517 Report. Biotoxtech: Cheongwon-Gun, Korea, 2010.
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Isolation of a new anti-allergic phlorotannin, phlorofucofuroeckol-B, from an edible brown alga, Eisenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef]
- Kusaykin, M.; Bakunina, I.; Sova, V.; Ermakova, S.; Kuznetsova, T.; Besednova, N.; Zaporozhets, T.; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008, 3, 904–915. [Google Scholar] [CrossRef]
- Fowler, J.E.; Quatrano, R.S. Plant cell morphogenesis: Plasma membrane interactions with the cytoskeleton and cell wall. Annu. Rev. Cell Dev. Biol. 1997, 13, 697–743. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifant’ev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Jeon, Y.J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonil, V.A.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef]
- Belford, D.A.; Hendry, I.A.; Parish, C.R. Investigation of the ability of several naturally occurring and synthetic polyanions to bind to and potentiate the biological activity of acidic fibroblast growth factor. J. Cell. Physiol. 1993, 157, 184–189. [Google Scholar] [CrossRef]
- Sellke, F.W.; Li, J.; Stamler, A.; Lopez, J.J.; Thomas, K.A.; Simons, M. Angiogenesis induced by acidic fibroblast growth factor as an alternative method of revascularization for chronic myocardial ischemia. Surgery 1996, 120, 182–188. [Google Scholar] [CrossRef]
- Salvucci, O.; Yao, L.; Villalba, S.; Sajewicz, A.; Pittaluga, S.; Tosato, G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood 2002, 99, 2703–2711. [Google Scholar] [CrossRef]
- Luyt, C.E.; Meddahi-Pellé, A.; Ho-Tin-Noe, B.; Colliec-Jouault, S.; Guezennec, J.; Louedec, L.; Prats, H.; Jacob, M.P.; Osborne-Pellegrin, M.; Letourneur, D.; et al. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J. Pharmacol. Exp. Ther. 2003, 305, 24–30. [Google Scholar] [CrossRef]
- Dong, X.; Song, Y.N.; Liu, W.G.; Guo, X.L. MMP-9, a potential target for cerebral ischemic treatment. Curr. Neuropharmacol. 2009, 7, 269–275. [Google Scholar] [CrossRef]
- Nelson, A.; Fingleton, B.; Rothenberg, M.; Matrisian, L. Matrix metalloproteinases: Biologic activity and clinical implications. J. Clin. Oncol. 2000, 18, 1135–1149. [Google Scholar]
- Ryu, B.M.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem. Biol. Interact. 2009, 179, 192–201. [Google Scholar] [CrossRef]
- Kim, S.K.; Thomas, N.V.; Li, X.F. Phlorotannins and Fucoidans From Marine Macroalgae as Matrix Metalloproteinase Inhibitory Substances and Their possible Application as Medicinal Foods. In Marine Medicinal Foods: Implications and Applications, Macro and Microalgae; Taylor, S., Ed.; Advances in Food and Nutrition Research Series 64; Academic Press Inc.: Waltham, MA, USA, 2011; pp. 129–139. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thomas, N.V.; Kim, S.-K. Beneficial Effects of Marine Algal Compounds in Cosmeceuticals. Mar. Drugs 2013, 11, 146-164. https://doi.org/10.3390/md11010146
Thomas NV, Kim S-K. Beneficial Effects of Marine Algal Compounds in Cosmeceuticals. Marine Drugs. 2013; 11(1):146-164. https://doi.org/10.3390/md11010146
Chicago/Turabian StyleThomas, Noel Vinay, and Se-Kwon Kim. 2013. "Beneficial Effects of Marine Algal Compounds in Cosmeceuticals" Marine Drugs 11, no. 1: 146-164. https://doi.org/10.3390/md11010146
APA StyleThomas, N. V., & Kim, S.-K. (2013). Beneficial Effects of Marine Algal Compounds in Cosmeceuticals. Marine Drugs, 11(1), 146-164. https://doi.org/10.3390/md11010146






