Three New Cembranoids from the Taiwanese Soft Coral Sarcophyton ehrenbergi
Abstract
:1. Introduction


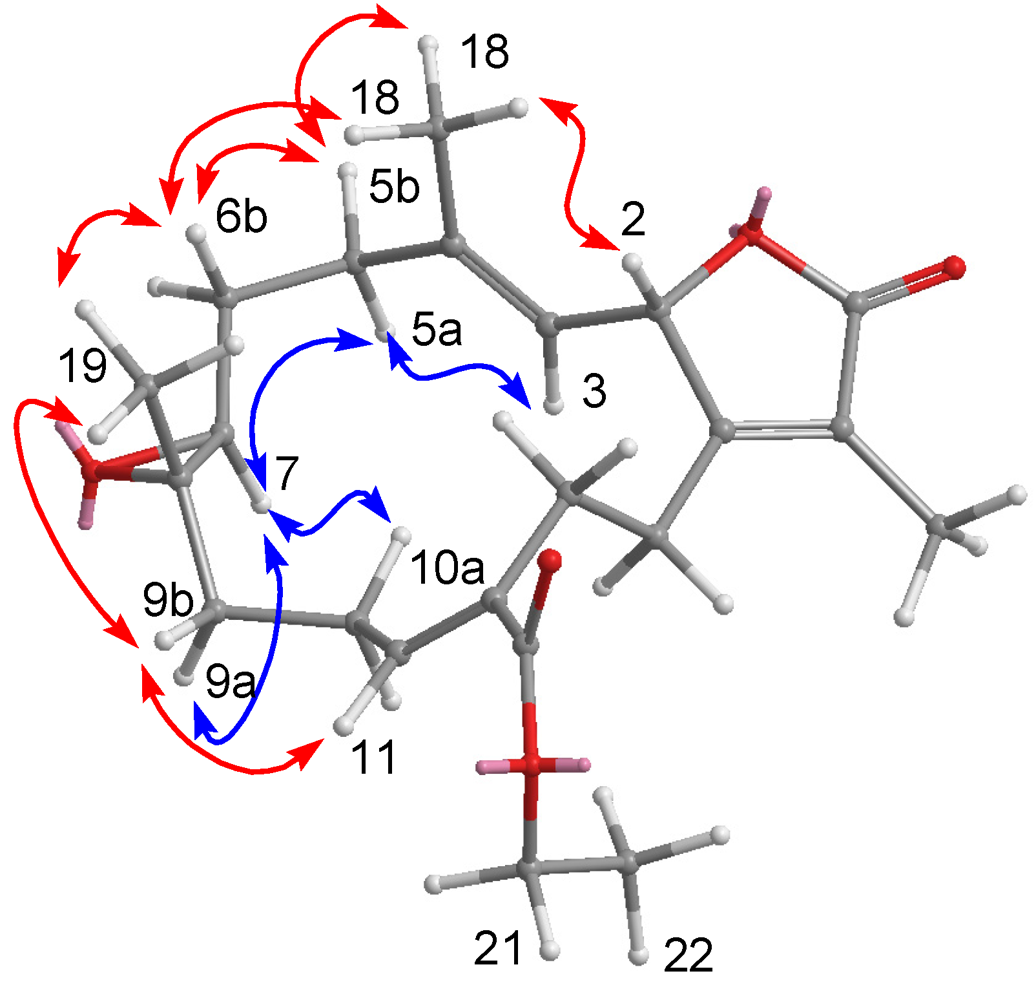
2. Results and Discussion
| Position | δH a (J in Hz) | δC b, Type | HMBC | COSY | NOESY |
|---|---|---|---|---|---|
| 1 | 160.7, C | ||||
| 2 | 5.57, dd (10, 2.0) | 78.2, CH | 3, 17 | 18 | |
| 3 | 5.08, d (10.4) | 121.1, CH | 5, 18 | 2, 18 | 5a |
| 4 | 144.5, C | ||||
| 5a | 2.39, m | 37.7, CH2 | 3, 4, 6, 18 | 5b, 6a | 3, 5b, 7 |
| 5b | 2.41, m | 3, 4, 6, 18 | 5a, 6a | 5a,18 | |
| 6a | 1.92, m | 22.9, CH2 | 6b, 5a, 5b | 6b | |
| 6b | 1.72, m | 6a, 7 | 6a,18, 19 | ||
| 7 | 2.56, br d (6.4) | 62.4, CH | 6 | 6b | 9a, 10a |
| 8 | 61.3, C | ||||
| 9a | 0.97, m | 38.2, CH2 | 10 | 9b | 9b, 11 |
| 9b | 2.22, m | 11 | 9a, 10a, 10b | 7, 9a, 19 | |
| 10a | 2.11, m | 25.9, CH2 | 12 | 9a, 10b | 7, 10b |
| 10b | 2.19, m | 9a, 10a, 11 | 10a | ||
| 11 | 6.80, dd (10.4, 4.8) | 142.0, CH | 10, 13, 20 | 10a | 9b |
| 12 | 131.1, C | ||||
| 13 | 2.36, m | 25.2, CH2 | 12, 20 | 14b | |
| 14a | 2.50, m | 27.0, CH2 | 1, 2, 15 | 14b | 14b |
| 14b | 2.11, m | 13a, 14a | 14a | ||
| 15 | 124.2, C | ||||
| 16 | 174.4, C | ||||
| 17 | 1.90, s | 8.6, CH3 | 1, 15, 16 | 2 | |
| 18 | 1.87, s | 15.3, CH3 | 3, 4, 5 | 3 | 2, 5b |
| 19 | 1.30, s | 16.8, CH3 | 7, 8, 9 | 6b, 9b | |
| 20 | 166.9. C | ||||
| 21 | 4.23, m | 60.8, CH2 | 20 | 22 | 22 |
| 22 | 1.33, t (7.2) | 14.3, CH3 | 21 | 21 | 21 |

| Position | δH a (J in Hz) | δC b, Type | HMBC | COSY | NOESY |
|---|---|---|---|---|---|
| 1 | 147.1, C | ||||
| 2 | 6.53, d (11.0) | 118.4, CH | 4, 14, 15 | 3 | 16, 17, 18 |
| 3 | 6.08, d (11.0) | 123.8, CH | 5, 18 | 2, 18 | 5a, 7, 13a |
| 4 | 135.8, C | ||||
| 5a | 2.12, m | 37.8, CH2 | 3 | 5b, 18 | 3, 5b |
| 5b | 1.97, m | 3 | 5a, 6b, 18 | 5a, 6b, 18 | |
| 6a | 1.94, m | 25.5, CH2 | 7 | 6b, 7 | 6b |
| 6b | 1.17, m | 5b, 6a, 7 | 5b, 6a,19 | ||
| 7 | 2.67, dd (10.8, 2.8) | 62.1, CH | 6a, 6b | 3, 9a | |
| 8 | 60.6, C | ||||
| 9a | 0.85, td (12.4, 4.0) | 39.7, CH2 | 8, 19 | 9b, 10a, 10b | 9b, 7 |
| 9b | 1.95, m | 8, 10, 19 | 9a, 10a, 10b | 9a, 19 | |
| 10a | 2.16, m | 26.7, CH2 | 9, 10b, 11 | 10b | |
| 10b | 1.75,m | 9, 11, 12 | 9, 10a, 11 | 10a, 19 | |
| 11 | 6.89, dd (10.4, 6.8) | 141.1, CH | 20 | 10a, 10b | 9 |
| 12 | 133.9, C | ||||
| 13a | 2.26,m | 28.4, CH2 | 3, 13b, 14b | ||
| 13b | 2.38, m | 11, 12, 20 | 13a | ||
| 14a | 2.52, m | 28.6, CH2 | 12 | 14b, 17 | |
| 14b | 2.24, m | 12 | 14a | ||
| 15 | 73.6, C | ||||
| 16 | 1.33, s | 29.5, CH3 | 1, 15, 17 | 2 | |
| 17 | 1.44, s | 29.3, CH3 | 1, 16, 17 | 2 | |
| 18 | 1.58, s | 15.6, CH3 | 3, 4, 5 | 3, 5a, 5b | 2, 5b |
| 19 | 1.04, s | 15.6, CH3 | 7, 8, 9 | 6b, 9b, 10b | |
| 20 | 167.6, C | ||||
| 21 | 3.42, s | 51.2, CH3 | 20 |

| Position | δH a (J in Hz) | δC b, Type | HMBC | COSY | NOESY |
|---|---|---|---|---|---|
| 1 | 150.2, qC | ||||
| 2 | 5.89, br d (5.0) | 119.6, CH | 4, 14, 15 | 3 | 3, 15, 16, 18 |
| 3 | 5.99, br d (5.0) | 122.3, CH | 1, 5, 18 | 2, 18 | 2, 5a |
| 4 | 137.1, qC | ||||
| 5 | 2.16, m | 39.6, CH2 | 3, 4, 6, 7 | 6a, 6b | 3, 7, 6b, 18 |
| 6a | 1.42, m | 26.5, CH2 | 8 | 5a, 5b, 6b | 5b, 19 |
| 6b | 1.69, m | 4, 5, 7 | 5a, 5b, 6a | ||
| 7 | 3.40, d (7.6) | 85.1, CH | 5, 6, 8, 9, 11, 19 | 5a, 9a | |
| 8 | 80.7, qC | ||||
| 9a | 1.73, m | 35.4, CH2 | 9b, 10a, 10b | 7, 9b, 11 | |
| 9b | 2.83, dt (12.4, 4.0) | 19 | 9a, 10a, 10b | 9a, 10b, 19 | |
| 10a | 1.56, m | 23.0, CH2 | 10b | 10b, 11 | |
| 10b | 1.39, m | 10a | 10b, 20 | ||
| 11 | 3.32, dd (11.2, 2.0) | 80.1, CH | 7, 12, 20 | 10a, 10b | 9a, 10a, 14a |
| 12 | 73.0, qC | ||||
| 13a | 1.46, m | 41.0, CH2 | 20 | 13b, 14a | |
| 13b | 1.80, m | 12, 20 | 13a | 17, 20 | |
| 14a | 2.22, m | 24.0, CH2 | 1, 2 | 13a, 13b, 14b | 3, 11 |
| 14b | 1.77, m | 13 | 14a | ||
| 15 | 2.28, m | 35.2, CH | 16, 17 | 16, 17 | 2, 16, 17 |
| 16 | 1.08, d (7.2) | 22.0, CH3 | 1, 15, 17 | 15 | 2, 15 |
| 17 | 1.10, d (6.4) | 22.6, CH3 | 1, 15, 16 | 15 | 2, 13b, 15 |
| 18 | 1.64, s | 17.2, CH3 | 3, 4, 5 | 3 | 2, 5b |
| 19 | 1.50, s | 17.1, CH3 | 7, 8, 9 | 6b, 9b | |
| 20 | 1.01, s | 23.8, CH3 | 11, 12, 13 | 10b, 13b | |
| OAc | 1.67, s | 169.2, qC | |||
| 21.9, CH3 |

| Compounds | ED50 (μg/mL) | ||||
|---|---|---|---|---|---|
| A549 | HT-29 | P-388 | HEL | Anti-HCMV | |
| 1 | 20.8 | >50 | 5.8 | >50 | 60 |
| 2 | >50 | >50 | 7.4 | >50 | 46 |
| 3 | 10.2 | >50 | 4.7 | >50 | 5.0 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
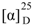 +77 (c 0.2, CHCl3); UV (MeOH) λmax (log ε) 228 (3.72) nm; IR (neat) νmax 3481, 2933, 1754, 1705, 1455, 1387, 1242, 1096, 991, 760 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 1; HRESIMS m/z 397.1993 [M + Na]+ (calcd for C22H30O5Na, 397.1991).
+77 (c 0.2, CHCl3); UV (MeOH) λmax (log ε) 228 (3.72) nm; IR (neat) νmax 3481, 2933, 1754, 1705, 1455, 1387, 1242, 1096, 991, 760 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 1; HRESIMS m/z 397.1993 [M + Na]+ (calcd for C22H30O5Na, 397.1991).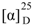 −184 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 221 (3.72), 242 (3.32) nm; IR (neat) νmax 3447, 2961, 2925, 2851, 1715, 1458, 1260, 1101, 1026, 799, 759 cm−1; 1H NMR (C6D6, 400 MHz) and 13C NMR (C6D6, 100 MHz) data in Table 1; HRESIMS m/z 371.2195 [M + Na]+ (calcd for C21H32O4Na, 371.2198).
−184 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 221 (3.72), 242 (3.32) nm; IR (neat) νmax 3447, 2961, 2925, 2851, 1715, 1458, 1260, 1101, 1026, 799, 759 cm−1; 1H NMR (C6D6, 400 MHz) and 13C NMR (C6D6, 100 MHz) data in Table 1; HRESIMS m/z 371.2195 [M + Na]+ (calcd for C21H32O4Na, 371.2198).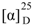 −84.0 (c 0.1, CHCl3); IR (neat) νmax 3461, 2959, 1737, 1634, 1456, 1378, 1259, 1089, 1026, 801 cm−1; 1H NMR (C6D6, 400 MHz) and 13C NMR (C6D6, 100 MHz) data in Table 2; HRESIMS m/z 387.2509 [M + Na]+ (calcd for C22H36O4Na, 387.2511).
−84.0 (c 0.1, CHCl3); IR (neat) νmax 3461, 2959, 1737, 1634, 1456, 1378, 1259, 1089, 1026, 801 cm−1; 1H NMR (C6D6, 400 MHz) and 13C NMR (C6D6, 100 MHz) data in Table 2; HRESIMS m/z 387.2509 [M + Na]+ (calcd for C22H36O4Na, 387.2511).3.4. Cytotoxicity Assay
3.5. Anti-HCMV Assay
4. Conclusion
Acknowledgments
References
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Coll, J.C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92, 613–631. [Google Scholar]
- Gross, H.; Wright, A.D.; Beil, W.; König, G.M. Two new bicyclic cembranolides from a new Sarcophyton species and determination of the absolute configuration of sarcoglaucol-16-one. Org. Biomol. Chem. 2004, 2, 1133–1138. [Google Scholar] [CrossRef]
- Huang, H.-C.; Ahmed, A.F.; Su, J.-H.; Chao, C.-H.; Wu, Y.-C.; Chiang, M.Y.; Sheu, J.-H. Crassocolides A–F, new cembranoids with a trans-fused lactone from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1554–1559. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Su, J.; Liang, Y.; Yang, X.; Zheng, K.; Zeng, L. Cytotoxic diterpenoids from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1476–1480. [Google Scholar] [CrossRef]
- Feller, M.; Rudi, A.; Berer, N.; Goldberg, I.; Stein, Z.; Benayahu, Y.; Schleyer, M.; Kashman, Y. Isoprenoids of the soft coral Sarcophyton glaucum: Nyalolide, a new biscembranoid, and other terpenoids. J. Nat. Prod. 2004, 67, 1303–1308. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Hamann, M.T.; Waddling, C.A.; Jensen, C.; Lee, S.K.; Dunstan, C.A.; Pezzuto, J.M. Structurally novel bioconversion products of the marine natural product sarcophine effectively inhibit JB6 cell transformation. J. Org. Chem. 1998, 63, 7449–7455. [Google Scholar]
- Yan, X.-H.; Gavagnin, M.; Cimino, G.; Guo, Y.-W. Two new biscembranes with unprecedented carbon skeleton and their probable biogenetic precursor from the Hainan soft coral Sarcophyton latum. Tetrahedron Lett. 2007, 48, 5313–5316. [Google Scholar]
- Cheng, Y.-B.; Shen, Y.-C.; Kuo, Y.-H.; Khalil, A.T. Cembrane diterpenoids from the Taiwanese soft coral Sarcophyton stolidotum. J. Nat. Prod. 2008, 71, 1141–1145. [Google Scholar] [CrossRef]
- Iwagawa, T.; Nakamura, S.; Masuda, T.; Okamura, H.; Nakatani, M.; Siro, M. Irregular cembranoids containing a 13-membered carbocyclic skeleton isolated from a soft coral, Sarcophyton species. Tetrahedron 1995, 51, 5291–5298. [Google Scholar]
- Sawant, S.; Youssef, D.; Mayer, A.; Sylvester, P.; Wali, V.; Arant, M.; El Sayed, K. Anticancer and anti-inflammatory sulfur-containing semisynthetic derivatives of sarcophine. Chem. Pharm. Bull. 2006, 54, 1119–1123. [Google Scholar] [CrossRef]
- Badria, F.A.; Guirguis, A.N.; Perovic, S.; Steffen, R.; Müller, W.E.G.; Schröder, H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology 1998, 131, 133–143. [Google Scholar] [CrossRef]
- Bishara, A.; Rudi, A.; Benayahu, Y.; Kashman, Y. Three biscembranoids and their monomeric counterpart cembranoid, a biogenetic Diels-Alder precursor, from the soft coral Sarcophyton elegans. J. Nat. Prod. 2007, 70, 1951–1954. [Google Scholar] [CrossRef]
- Sawant, S.; Youssef, D.; Reiland, J.; Ferniz, M.; Marchtti, D.; El Sayed, K.A. Biocatalytic and antimetastatic studies of the marine cembranoids sarcophine and 2-epi-16-deoxysarcophine. J. Nat. Prod. 2006, 69, 1010–1013. [Google Scholar] [CrossRef]
- Cuong, N.X.; Tuan, T.A.; Kiem, P.V.; Minh, C.V.; Choi, E.M.; Kim, Y.H. New cembranoid diterpenes from the Vietnamese soft coral Sarcophyton mililatensis stimulate osteoblastic differentiation in MC3T3-E1 cells. Chem. Pharm. Bull. 2008, 56, 988–992. [Google Scholar] [CrossRef]
- Wang, S.-K.; Duh, C.-Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Mar. Drugs 2012, 10, 306–318. [Google Scholar]
- Lin, S.-T.; Wang, S.-K.; Duh, C.-Y. Cembranoids from the Dongsha atoll soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 2705–2716. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chen, P.-W.; Chen, H.P.; Wang, S.-K.; Duh, C.-Y. New cembranolides from the Dongsha atoll soft coral Lobophytum durum. Mar. Drugs 2011, 9, 1307–1318. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Cembranoids from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2010, 73, 197–203. [Google Scholar]
- Cheng, S.-Y.; Chiou, S.-F.; Tsai, C.-W.; Wang, S.-K.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Wang, W.-H.; Duh, C.-Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009, 72, 465–468. [Google Scholar]
- Roengsumran, S.; Achayindee, S.; Petsom, A.; Pudhom, K.; Singtothong, P.; Surachetapan, C.; Vilaivan, T. Two new cembranoids from Croton oblongifolius. J. Nat. Prod. 1998, 61, 652–654. [Google Scholar] [CrossRef]
- Kashman, Y.; Zadock, E.; Néeman, I. Some new cembrane derivatives of marine origin. Tetrahedron 1974, 30, 3615–3620. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972, 3, 1–91. [Google Scholar]
- Hou, R.-S.; Duh, C.-Y.; Chiang, M.Y.; Lin, C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995, 58, 1126–1130. [Google Scholar] [CrossRef]
- Chen, W.-H.; Wang, S.-K.; Duh, C.-Y. Polyhydroxylated Steroids from the Bamboo Coral Isis hippuris. Mar. Drugs 2011, 9, 1829–1839. [Google Scholar] [CrossRef]
- Stevens, M.; Balzarini, J.; Tabarrini, O.; Andrei, G.; Snoeck, R.; Cecchetti, V.; Fravolini, A.; de Clercq, E.; Pannecouque, C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005, 56, 847–855. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Huang, K.-J.; Wang, S.-K.; Duh, C.-Y. Capilloquinol: A novel farnesyl quinol from the Dongsha atoll soft coral Sinularia capillosa. Mar. Drugs 2011, 9, 1469–1476. [Google Scholar] [CrossRef]
- Samples Availability: Not available.
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, S.-K.; Hsieh, M.-K.; Duh, C.-Y. Three New Cembranoids from the Taiwanese Soft Coral Sarcophyton ehrenbergi. Mar. Drugs 2012, 10, 1433-1444. https://doi.org/10.3390/md10071433
Wang S-K, Hsieh M-K, Duh C-Y. Three New Cembranoids from the Taiwanese Soft Coral Sarcophyton ehrenbergi. Marine Drugs. 2012; 10(7):1433-1444. https://doi.org/10.3390/md10071433
Chicago/Turabian StyleWang, Shang-Kwei, Mu-Keng Hsieh, and Chang-Yih Duh. 2012. "Three New Cembranoids from the Taiwanese Soft Coral Sarcophyton ehrenbergi" Marine Drugs 10, no. 7: 1433-1444. https://doi.org/10.3390/md10071433
APA StyleWang, S.-K., Hsieh, M.-K., & Duh, C.-Y. (2012). Three New Cembranoids from the Taiwanese Soft Coral Sarcophyton ehrenbergi. Marine Drugs, 10(7), 1433-1444. https://doi.org/10.3390/md10071433




