A Multivalent Vaccine Based on Ferritin Nanocage Elicits Potent Protective Immune Responses against SARS-CoV-2 Mutations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production and Characterization of SARS-CoV-2 Multivalent Ferritin Nanocage
2.2. Activation of Immune Response of SARS-CoV-2 Multivalent Ferritin Nanocage
2.3. SARS-CoV-2 Variant-Specific Immune Response of Multivalent Ferritin Nanocage
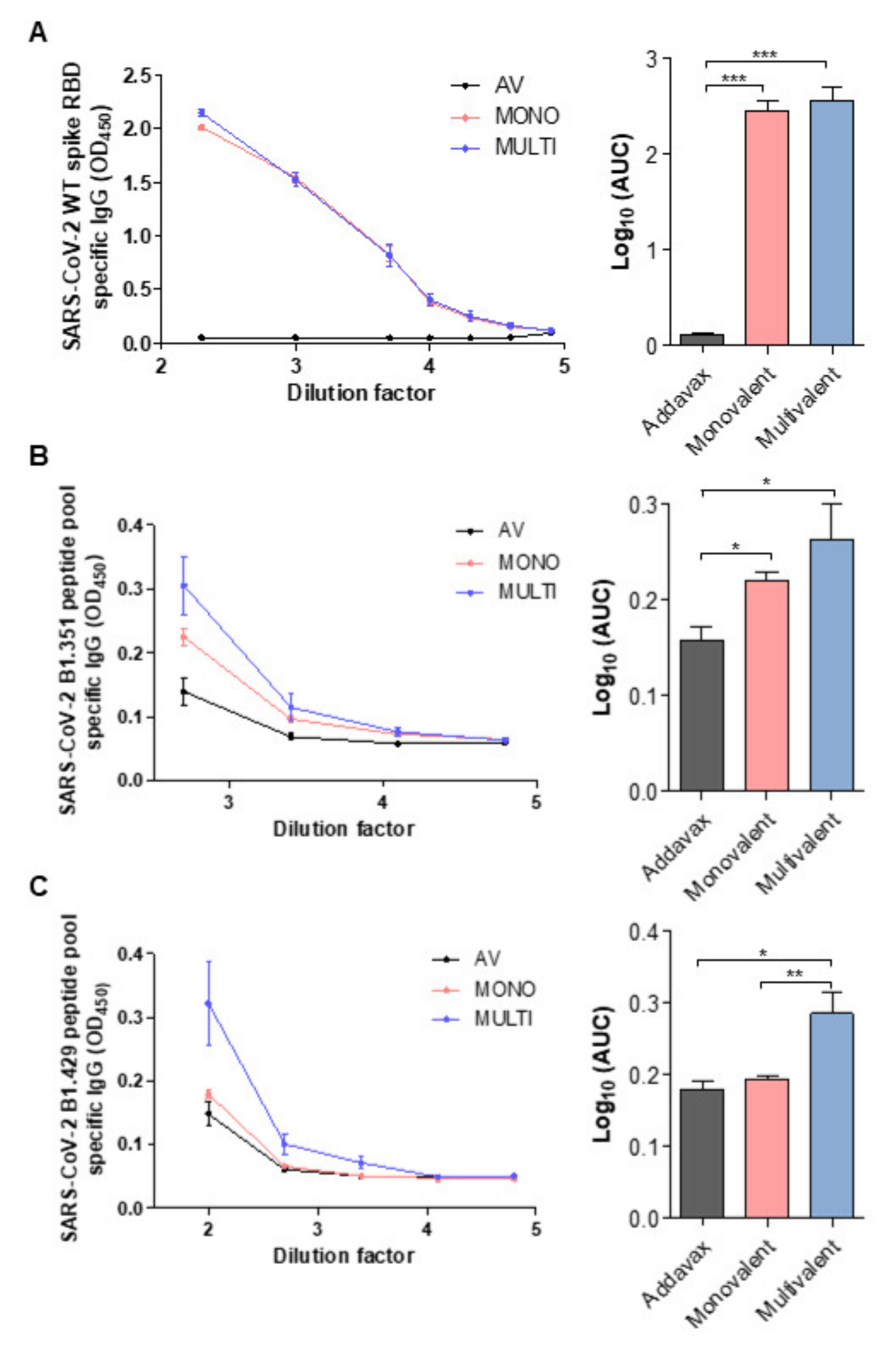
2.4. SARS-CoV-2 Variant-Specific Antibody Formation of Multivalent Ferritin Nanocage
3. Materials and Methods
3.1. Animals
3.2. DNA Construct (Cloning)
3.3. Cell Culture, Protein Expression, and Purification
3.4. Characterization
3.5. Immune Profile Analysis
3.6. Neutralizing Antibody ELISA
3.7. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 14 April 2022).
- Zhao, Y.; Huang, J.; Zhang, L.; Chen, S.; Gao, J.; Jiao, H. The Global Transmission of New Coronavirus Variants. Environ. Res. 2022, 206, 112240. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-Y.; Wang, W.-B.; Gao, R.-D.; Zhou, A.-M. Omicron Variant (B.1.1.529) of SARS-CoV-2: Mutation, Infectivity, Transmission, and Vaccine Resistance. World J. Clin. Cases 2022, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Loes, A.N.; Crawford, K.H.D.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. Comprehensive Mapping of Mutations in the SARS-CoV-2 Receptor-Binding Domain That Affect Recognition by Polyclonal Human Plasma Antibodies. Cell Host Microbe. 2021, 29, 463–476.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Catherine, R.; Roanne, K.; Thandeka, M.-G.; Tandile, H.; Prudence, K.; Richard, B.; Ziyaad, V.-O.; Mikhail, S.; Houriiyah, T.; Deelan, D.; et al. Escape from Recognition of SARS-CoV-2 Variant Spike Epitopes but Overall Preservation of T Cell Immunity. Sci. Transl. Med. 2022, 14, eabj6824. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Becker, M.; Dulovic, A.; Junker, D.; Ruetalo, N.; Kaiser, P.D.; Pinilla, Y.T.; Heinzel, C.; Haering, J.; Traenkle, B.; Wagner, T.R.; et al. Immune Response to SARS-CoV-2 Variants of Concern in Vaccinated Individuals. Nat. Commun. 2021, 12, 3109. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 Vaccine Effort: Viruses, Vaccines and Variants versus Efficacy, Effectiveness and Escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Pambudi, N.A.; Sarifudin, A.; Gandidi, I.M.; Romadhon, R. Vaccine Cold Chain Management and Cold Storage Technology to Address the Challenges of Vaccination Programs. Energy Rep. 2022, 8, 955–972. [Google Scholar] [CrossRef]
- Yan, Y.; Pang, Y.; Lyu, Z.; Wang, R.; Wu, X.; You, C.; Zhao, H.; Manickam, S.; Lester, E.; Wu, T.; et al. The COVID-19 Vaccines: Recent Development, Challenges and Prospects. Vaccines 2021, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kumru, O.S.; Joshi, S.B.; Smith, D.E.; Middaugh, C.R.; Prusik, T.; Volkin, D.B. Vaccine Instability in the Cold Chain: Mechanisms, Analysis and Formulation Strategies. Biologicals 2014, 42, 237–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snapper, C.M. Distinct Immunologic Properties of Soluble Versus Particulate Antigens. Front. Immunol. 2018, 9, 598. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [Green Version]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-Based Vaccines Against Respiratory Viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- King, H.A.D.; Joyce, M.G.; Lakhal-Naouar, I.; Ahmed, A.; Cincotta, C.M.; Subra, C.; Peachman, K.K.; Hack, H.R.; Chen, R.E.; Thomas, P.V.; et al. Efficacy and Breadth of Adjuvanted SARS-CoV-2 Receptor-Binding Domain Nanoparticle Vaccine in Macaques. Proc. Natl. Acad. Sci. USA 2021, 118, e2106433118. [Google Scholar] [CrossRef]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Lee, E.J.; Nam, G.-H.; Lee, N.K.; Kih, M.; Koh, E.; Kim, Y.K.; Hong, Y.; Kim, S.; Park, S.-Y.; Jeong, C.; et al. Nanocage-Therapeutics Prevailing Phagocytosis and Immunogenic Cell Death Awakens Immunity against Cancer. Adv. Mater. 2018, 30, 1705581. [Google Scholar] [CrossRef]
- Lee, B.-R.; Ko, H.K.; Ryu, J.H.; Ahn, K.Y.; Lee, Y.-H.; Oh, S.J.; Na, J.H.; Kim, T.W.; Byun, Y.; Kwon, I.C.; et al. Engineered Human Ferritin Nanoparticles for Direct Delivery of Tumor Antigens to Lymph Node and Cancer Immunotherapy. Sci. Rep. 2016, 6, 35182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.-A.; Kang, Y.J.; Shin, C.; Ra, J.-S.; Shin, H.-H.; Hong, S.Y.; Do, Y.; Kang, S. Ferritin Protein Cage Nanoparticles as Versatile Antigen Delivery Nanoplatforms for Dendritic Cell (DC)-Based Vaccine Development. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.E.; Zhang, K.; Sanyal, M.; Tang, S.; Weidenbacher, P.A.; Li, S.; Pham, T.D.; Pak, J.E.; Chiu, W.; Kim, P.S. A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Mice. ACS Cent. Sci. 2021, 7, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Son, A.; Kim, J.; Kwon, S.B.; Kim, M.H.; Kim, P.; Kim, J.; Byun, Y.H.; Sung, J.; Lee, J.; et al. Chaperna-Mediated Assembly of Ferritin-Based Middle East Respiratory Syndrome-Coronavirus Nanoparticles. Front. Immunol. 2018, 9, 1093. [Google Scholar] [CrossRef] [Green Version]
- Corbett, K.S.; Moin, S.M.; Yassine, H.M.; Cagigi, A.; Kanekiyo, M.; Boyoglu-Barnum, S.; Myers, S.I.; Tsybovsky, Y.; Wheatley, A.K.; Schramm, C.A.; et al. Design of Nanoparticulate Group 2 Influenza Virus Hemagglutinin Stem Antigens That Activate Unmutated Ancestor B Cell Receptors of Broadly Neutralizing Antibody Lineages. mBio 2022, 10, e02810-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-Assembling Protein Nanoparticles in the Design of Vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.B.; Sung, H.-D.; Nam, G.-H.; Kim, W.; Kim, S.; Kang, D.; Lee, E.J.; Kim, I.-S. Design of PD-1-Decorated Nanocages Targeting Tumor-Draining Lymph Node for Promoting T Cell Activation. J. Control. Release 2021, 333, 328–338. [Google Scholar] [CrossRef]
- Houser, K.V.; Chen, G.L.; Carter, C.; Crank, M.C.; Nguyen, T.A.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Gordon, I.J.; et al. Safety and Immunogenicity of a Ferritin Nanoparticle H2 Influenza Vaccine in Healthy Adults: A Phase 1 Trial. Nat. Med. 2022, 28, 383–391. [Google Scholar] [CrossRef]
- Mohanty, A.; Parida, A.; Raut, R.K.; Behera, R.K. Ferritin: A Promising Nanoreactor and Nanocarrier for Bionanotechnology. ACS Bio. Med. Chem. Au. 2022. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, C.; Chen, L.; Bai, G.; Zhao, G.; Xu, C. Encapsulation of Anthocyanin Molecules within a Ferritin Nanocage Increases Their Stability and Cell Uptake Efficiency. Food Res. Int. 2014, 62, 183–192. [Google Scholar] [CrossRef]
- Kalathiya, U.; Padariya, M.; Fahraeus, R.; Chakraborti, S.; Hupp, T.R. Multivalent Display of SARS-CoV-2 Spike (RBD Domain) of COVID-19 to Nanomaterial, Protein Ferritin Nanocages. Biomolecules 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral Targets for Vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Huang, L.; Zhang, G.; Yao, Y.; Zhou, H.; Shen, S.; Shen, B.; Li, B.; Li, X.; Zhang, Q.; et al. A SARS-CoV-2 Neutralizing Antibody with Extensive Spike Binding Coverage and Modified for Optimal Therapeutic Outcomes. Nat. Commun. 2021, 12, 2623. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Nie, J.; Zhang, L.; Hao, H.; Liu, S.; Zhao, C.; Zhang, Q.; Liu, H.; Nie, L.; et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell 2020, 182, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Gong, W. Will Mutations in the Spike Protein of SARS-CoV-2 Lead to the Failure of COVID-19 Vaccines? J. Korean Med. Sci. 2021, 36, e124. [Google Scholar] [CrossRef]
- Nguyen, B.; Tolia, N.H. Protein-Based Antigen Presentation Platforms for Nanoparticle Vaccines. npj Vaccines 2021, 6, 70. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Wei, C.J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.P.; Wang, L.; Nabel, G.J. Self-Assembling Influenza Nanoparticle Vaccines Elicit Broadly Neutralizing H1N1 Antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [Green Version]
- Rha, M.-S.; Shin, E.-C. Activation or Exhaustion of CD8+ T Cells in Patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 2325–2333. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding Roles for CD4+ T Cells in Immunity to Viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Gordon Joyce, M.; King, H.A.D.; Elakhal-Naouar, I.; Ahmed, A.; Peachman, K.K.; Macedo Cincotta, C.; Subra, C.; Chen, R.E.; Thomas, P.V.; Chen, W.-H.; et al. A SARS-CoV-2 Ferritin Nanoparticle Vaccine Elicits Protective Immune Responses in Nonhuman Primates. Sci. Transl. Med. 2022, 14, eabi5735. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Aleebrahim-Dehkordi, E.; Molavi, B.; Mokhtari, M.; Deravi, N.; Fathi, M.; Fazel, T.; Mohebalizadeh, M.; Koochaki, P.; Shobeiri, P.; Hasanpour-Dehkordi, A. T Helper Type (Th1/Th2) Responses to SARS-CoV-2 and Influenza A (H1N1) Virus: From Cytokines Produced to Immune Responses. Transpl. Immunol. 2022, 70, 101495. [Google Scholar] [CrossRef]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Sodeifian, F.; Nikfarjam, M.; Kian, N.; Mohamed, K.; Rezaei, N. The Role of Type I Interferon in the Treatment of COVID-19. J. Med. Virol. 2022, 94, 63–81. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, N.K.; Kim, I.S. Bioengineered Protein-Based Nanocage for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 157–171. [Google Scholar] [CrossRef]
- Je, H.; Nam, G.-H.; Kim, G.B.; Kim, W.; Kim, S.R.; Kim, I.-S.; Lee, E.J. Overcoming therapeutic efficiency limitations against TRAIL-resistant tumors using re-sensitizing agent-loaded trimeric TRAIL-presenting nanocages. J. Control. Release 2021, 331, 7–18. [Google Scholar] [CrossRef]
- Sung, H.-D.; Kim, N.; Lee, Y.; Lee, E.J. Protein-Based Nanoparticle Vaccines for SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 13445. [Google Scholar] [CrossRef]
- Bhaskar, S.; Lim, S. Engineering Protein Nanocages as Carriers for Biomedical Applications. NPG Asia. Mater. 2017, 9, e371. [Google Scholar] [CrossRef] [PubMed]
- Tokatlian, T.; Read, B.J.; Jones, C.A.; Kulp, D.W.; Menis, S.; Chang, J.Y.H.; Steichen, J.M.; Kumari, S.; Allen, J.D.; Dane, E.L.; et al. Innate Immune Recognition of Glycans Targets HIV Nanoparticle Immunogens to Germinal Centers. Science 2019, 363, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Immunological Basis for Enhanced Immunity of Nanoparticle Vaccines. Expert Rev. Vaccines 2019, 18, 269–280. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Marles-Wright, J. Ferritin family proteins and their use in bionanotechnology. New Biotechnol. 2015, 32, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Huang, B.; Zhu, Y.; Tan, W.; Zhu, M. Ferritin Nanoparticle-Based SARS-CoV-2 RBD Vaccine Induces a Persistent Antibody Response and Long-Term Memory in Mice. Cell. Mol. Immunol. 2021, 18, 749–751. [Google Scholar] [CrossRef]
- Joyce, M. SARS-CoV-2 Ferritin Nanoparticle Vaccines Elicit Broad SARS Coronavirus Immunogenicity. Acta Crystallogr. Sect. A Found. Adv. 2021, 77, a163. [Google Scholar] [CrossRef]
- Lainšček, D.; Fink, T.; Forstnerič, V.; Hafner-Bratkovič, I.; Orehek, S.; Strmšek, Ž.; Manček-Keber, M.; Pečan, P.; Esih, H.; Malenšek, Š.; et al. A Nanoscaffolded Spike-Rbd Vaccine Provides Protection against SARS-CoV-2 with Minimal Anti-Scaffold Response. Vaccines 2021, 9, 431. [Google Scholar] [CrossRef]



| Template | Primer Sequence |
|---|---|
| Ferritin vector | Forward 5′-AGCTCGGATCCGATGTTATCAAAAGACA Reverse 5′-CAAGCTTCGTACGGCGCGC |
| Spike WT vector Spike V3 vector | Forward 5′-GCCGTACGAAGCTTGCTGGTCAGTTCCCA Reverse 5′-CATCGGATCCGAGCTTCCAAGCTCCTCCTTGAA |
| Spike V4 vector | Forward 5′-GCCGTACGAAGCTTGCTGGTCAGTATCCAATG Reverse 5′-CATCGGATCCGAGCTTCCAAGCTCCTCCTTGAA |
| Spike WT vector | Forward 5′-AAAAAGGATCCACTGGTCAGTTCCCAA Reverse 5′-TTTTTCTCGAGAAAGCTCCTCCTTGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.A.; Kim, S.; Kim, G.B.; Goo, J.; Kim, N.; Lee, Y.; Nam, G.-H.; Lim, S.; Kim, T.; Chang, K.H.; et al. A Multivalent Vaccine Based on Ferritin Nanocage Elicits Potent Protective Immune Responses against SARS-CoV-2 Mutations. Int. J. Mol. Sci. 2022, 23, 6123. https://doi.org/10.3390/ijms23116123
Kim SA, Kim S, Kim GB, Goo J, Kim N, Lee Y, Nam G-H, Lim S, Kim T, Chang KH, et al. A Multivalent Vaccine Based on Ferritin Nanocage Elicits Potent Protective Immune Responses against SARS-CoV-2 Mutations. International Journal of Molecular Sciences. 2022; 23(11):6123. https://doi.org/10.3390/ijms23116123
Chicago/Turabian StyleKim, Seong A., Seohyun Kim, Gi Beom Kim, Jiyoung Goo, Nayeon Kim, Yeram Lee, Gi-Hoon Nam, Seungho Lim, Taeerk Kim, Ki Hwan Chang, and et al. 2022. "A Multivalent Vaccine Based on Ferritin Nanocage Elicits Potent Protective Immune Responses against SARS-CoV-2 Mutations" International Journal of Molecular Sciences 23, no. 11: 6123. https://doi.org/10.3390/ijms23116123






