The Predictive Role of Thiol/Disulfide Homeostasis as an Oxidative Stress Parameter in Sarcopenic Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical and Functional Assessments
2.3. Anthropometric and Body Composition Measurements
2.4. Muscle Strength
2.5. Measurement of Oxidative Stress Parameters
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Comprehensive Geriatric Assessment
3.3. Oxidative Stress Parameters
3.4. Predictors of Sarcopenic Obesity
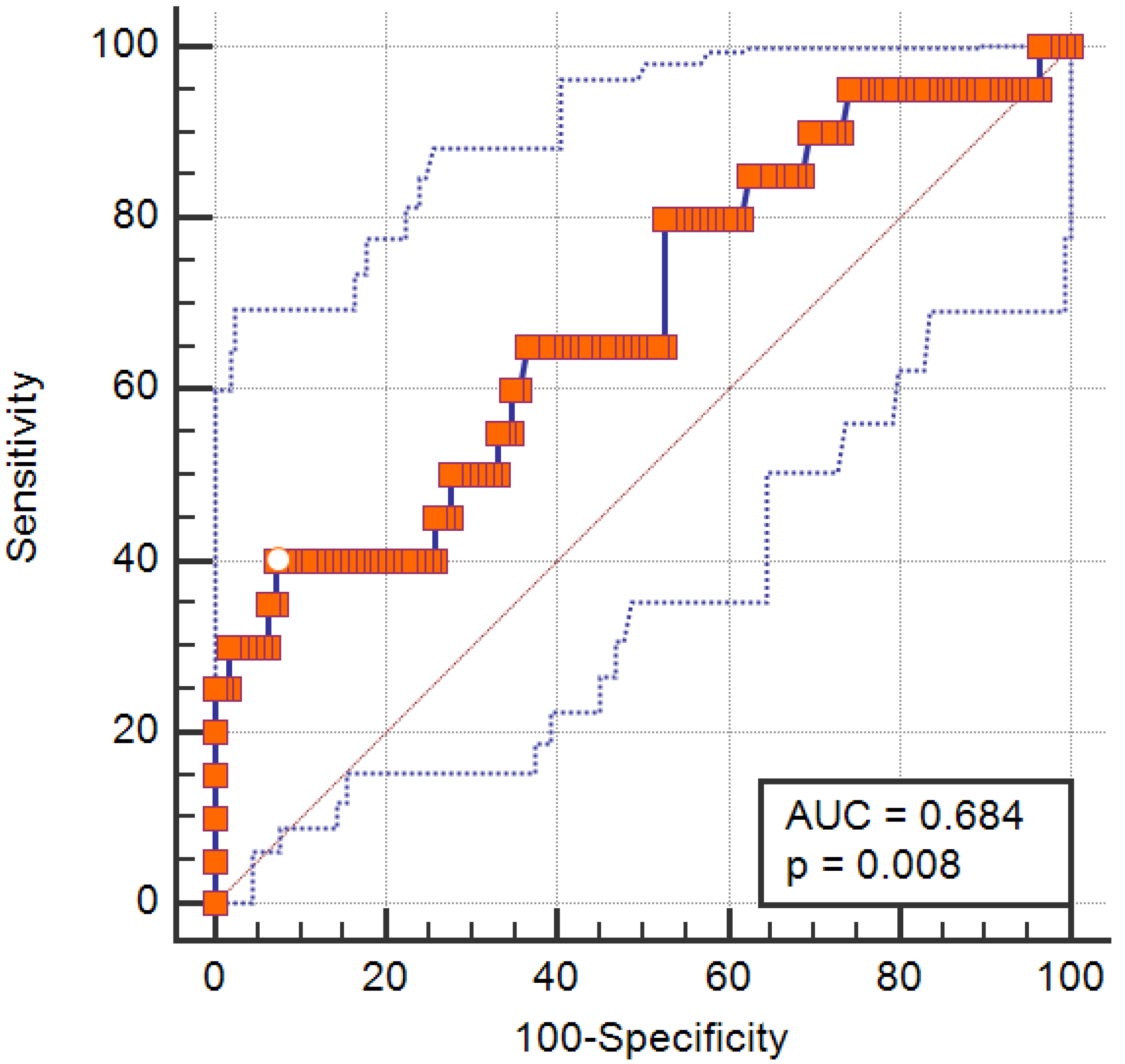
3.5. Predictive Accuracy of Disulfide/Native Thiol Ratio for Sarcopenic Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benz, E.; Pinel, A.; Guillet, C.; Capel, F.; Pereira, B.; De Antonio, M.; Pouget, M.; Cruz-Jentoft, A.J.; Eglseer, D.; Topinkova, E.; et al. Sarcopenia and Sarcopenic Obesity and Mortality Among Older People. JAMA Netw. Open 2024, 7, e243604. [Google Scholar] [CrossRef]
- Yao, G.; Ma, X.; Wan, X.; Yang, Y.; Xu, Y.; Zheng, L.; Qiu, Y.; Li, G.; Chen, L. Association between sarcopenic obesity and risk of frailty in older adults: A systematic review and meta-analysis. Age Ageing 2025, 54, afae286. [Google Scholar] [CrossRef]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef]
- Jiang, M.; Ren, X.; Han, L.; Zheng, X. Associations between sarcopenic obesity and risk of cardiovascular disease: A population-based cohort study among middle-aged and older adults using the CHARLS. Clin. Nutr. 2024, 43, 796–802. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic obesity: Emerging mechanisms and therapeutic potential. Metab. Clin. Exp. 2023, 146, 155639. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Simon, F.; Achiardi, O.; Vilos, C.; Cabrera, D.; Cabello-Verrugio, C. The Critical Role of Oxidative Stress in Sarcopenic Obesity. Oxidative Med. Cell. Longev. 2021, 2021, 4493817. [Google Scholar] [CrossRef]
- Jung, U.J. Sarcopenic Obesity: Involvement of Oxidative Stress and Beneficial Role of Antioxidant Flavonoids. Antioxidants 2023, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Erel, Ö.; Erdoğan, S. Thiol-disulfide homeostasis: An integrated approach with biochemical and clinical aspects. Turk. J. Med. Sci. 2020, 50, 1728–1738. [Google Scholar] [CrossRef]
- Erenler, A.K.; Yardan, T. Clinical Utility of Thiol/Disulfide Homeostasis. Clin. Lab. 2017, 63, 867–870. [Google Scholar] [CrossRef]
- Özsürekci, C.; Şengül Ayçiçek, G.; Çalışkan, H.; Tuna Doğrul, R.; Neşelioğlu, S.; Özcan, M.; Doğu, B.B.; Cankurtaran, M.; Erel, Ö.; Halil, M.G. Thiol-disulfide homeostasis and ischemia-modified albumin as a marker of oxidative stress in patients with sarcopenia. Geriatr. Gerontol. Int. 2021, 21, 584–589. [Google Scholar] [CrossRef]
- Ileri, I.; Eren, F.; Neselioglu, S.; Hafızoglu, M.; Karaduman, D.; Atbas, C.; Sahiner, Z.; Dikmeer, A.; Balcı, C.; Dogu, B.B.; et al. The role of thiol-disulfide homeostasis and ischemia-modified albumin in osteosarcopenia. Ir. J. Med. Sci. 2024, 193, 2109–2114. [Google Scholar] [CrossRef] [PubMed]
- Ates, E.; Set, T.; Karahan, S.C.; Biçer, C.; Erel, Ö. Thiol/Disulphide Homeostasis, Ischemia Modified Albumin, and Ferroxidase as Oxidative Stress Markers in Women with Obesity with Insulin Resistance. J. Med. Biochem. 2019, 38, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.; Kilic, T.; Kaplan, D.S.; Eren, M.A.; Erel, O.; Karakilcik, A.Z.; Bagci, C. The effect of newly initiated exercise training on dynamic thiol / disulphide homeostasis in sedentary obese adults. Anais da Academia Brasileira de Ciências 2019, 91, e20180930. [Google Scholar] [CrossRef] [PubMed]
- Gol, M.; Özkaya, B.; Yildirim, C.; Bal, R. Regular exercise, overweight/obesity and sedentary lifestyle cause adaptive changes in thiol-disulfide homeostasis. Anais da Academia Brasileira de Ciências 2019, 91, e20180547. [Google Scholar] [CrossRef]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef]
- NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report; National Institutes of Health: Bethesda, MD, USA, 1998; Volume 6, pp. 51s–209s. [Google Scholar]
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization Technical Report Series; WHO: Geneva, Sitzerland, 2000; Volume 894, pp. 1–253. [Google Scholar]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging And Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Arik, G.; Varan, H.D.; Yavuz, B.B.; Karabulut, E.; Kara, O.; Kilic, M.K.; Kizilarslanoglu, M.C.; Sumer, F.; Kuyumcu, M.E.; Yesil, Y.; et al. Validation of Katz index of independence in activities of daily living in Turkish older adults. Arch. Gerontol. Geriatr. 2015, 61, 344–350. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. the index of adl: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Isik, E.I.; Yilmaz, S.; Uysal, I.; Basar, S. Adaptation of the Lawton Instrumental Activities of Daily Living Scale to Turkish: Validity and Reliability Study. Ann. Geriatr. Med. Res. 2020, 24, 35–40. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Özsürekci, C.; Balcı, C.; Kızılarslanoğlu, M.C.; Çalışkan, H.; Tuna Doğrul, R.; Ayçiçek, G.; Sümer, F.; Karabulut, E.; Yavuz, B.B.; Cankurtaran, M.; et al. An important problem in an aging country: Identifying the frailty via 9 Point Clinical Frailty Scale. Acta Clin. Belg. 2020, 75, 200–204. [Google Scholar] [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Sarikaya, D.; Halil, M.; Kuyumcu, M.E.; Kilic, M.K.; Yesil, Y.; Kara, O.; Ozturk, S.; Gungor, E.; Karabulut, E.; Balam Yavuz, B.; et al. Mini nutritional assessment test long and short form are valid screening tools in Turkish older adults. Arch. Gerontol. Geriatr. 2015, 61, 56–60. [Google Scholar] [CrossRef]
- Durmaz, B.; Soysal, P.; Ellidokuz, H.; Isik, A.T. Validity and reliability of geriatric depression scale-15 (short form) in Turkish older adults. N. Clin. Istanb. 2018, 5, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Molloy, D.W.; Alemayehu, E.; Roberts, R. Reliability of a Standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am. J. Psychiatry 1991, 148, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lean, M.E.; Han, T.S.; Morrison, C.E. Waist circumference as a measure for indicating need for weight management. BMJ Clin. Res. Ed. 1995, 311, 158–161. [Google Scholar] [CrossRef]
- Kotler, D.P.; Burastero, S.; Wang, J.; Pierson, R.N., Jr. Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: Effects of race, sex, and disease. Am. J. Clin. Nutr. 1996, 64, 489s–497s. [Google Scholar] [CrossRef] [PubMed]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef]
- Hafızoğlu, M.; Eren, F.; Neşelioğlu, S.; Şahiner, Z.; Karaduman, D.; Atbaş, C.; Dikmeer, A.; İleri, İ.; Balcı, C.; Doğu, B.B.; et al. Physical frailty is related to oxidative stress through thiol/disulfide homeostasis parameters. Eur. Geriatr. Med. 2024, 15, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Şahiner, Z.; Eren, F.; Neşelioğlu, S.; Ceylan, S.; Güner, M.; Hafızoğlu, M.; Karaduman, D.; Atbas, C.; Ileri, I.; Dikmeer, A.; et al. Evaluation of oxidative stress parameters in older patients with urinary incontinence. Turk. J. Biochem. 2025, 50, 283–289. [Google Scholar] [CrossRef]
- Coverdale, J.P.C.; Katundu, K.G.H.; Sobczak, A.I.S.; Arya, S.; Blindauer, C.A.; Stewart, A.J. Ischemia-modified albumin: Crosstalk between fatty acid and cobalt binding. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 147–157. [Google Scholar] [CrossRef]
- Zoico, E.; Roubenoff, R. The role of cytokines in regulating protein metabolism and muscle function. Nutr. Rev. 2002, 60, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bergamini, C.M. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clin. Chem. Lab. Med. 2016, 54, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.B.; Agarwal, A. The role of oxidative stress in menopause. J. Mid-Life Health 2013, 4, 140–146. [Google Scholar] [CrossRef]
- Di Florio, D.N.; Sin, J.; Coronado, M.J.; Atwal, P.S.; Fairweather, D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020, 31, 101482. [Google Scholar] [CrossRef]
| SO (n = 20) | Non-SO (n = 112) | p | |
|---|---|---|---|
| Age, years, IQR | 78 (71–87) | 73 (68–76) | 0.007 |
| Sex, female, n, % | 10 (50.0%) | 75 (67.0%) | 0.14 |
| Height, cm, ±SD | 160.30 ± 10.45 | 160.12 ± 8.82 | 0.93 |
| Weight, kg, ±SD | 85.35 ± 11.82 | 77.93 ± 12.94 | 0.018 |
| BMI, kg/m2, IQR | 33.2 (28.35–38.77) | 29.40 (27.02–32.87) | 0.026 |
| Waist circumference, cm, ±SD | |||
| Female | 112.60 ± 11.04 | 99.97 ± 8.78 | <0.001 |
| Male | 103.90 ± 9.80 | 102.16 ± 8.99 | 0.59 |
| Handgrip strength, kg, IQR | |||
| Female | 13.9 (10.6–15.4) | 20.0 (15.6–23.1) | 0.001 |
| Male | 21.1 (18.7–25.8) | (29.9 (25.3–34.6) | 0.007 |
| Chair stand, s, IQR | 17.26 (12.52–18.44) | 14.25 (11.98–16.99) | 0.029 |
| SMM, kg, IQR | |||
| Female | 22.85 (18.91–24.72) | 23.59 (21.29–25.97) | 0.29 |
| Male | 30.19 (26.31–34.74) | 31.89 (29.94–36.40) | 0.094 |
| SMM/W, IQR | |||
| Female | 0.262 (0.258–0.269) | 0.308 (0.290–0.332) | <0.001 |
| Male | 0.360 (0.337–0.370) | 0.400 (0.382–0.418) | <0.001 |
| Fat mass, %, IQR | |||
| Female | 53.55 (52.32–54.50) | 45.60 (41.40–49.00) | <0.001 |
| Male | 36.70 (34.52–40.50) | 28.90 (26.15–32.25) | <0.001 |
| Comprehensive Geriatric Assessment | |||
| Katz ADL, IQR | 5 (4–6) | 6 (5–6) | 0.059 |
| Lawton–Brody IADL, IQR | 6 (4–8) | 8 (8–8) | 0.002 |
| CFS, IQR | 5 (3–5) | 3 (3–4) | 0.007 |
| GDS, IQR | 3 (0–6) | 3 (0–6) | 0.90 |
| SMMSE, IQR | 24 (21–28) | 27 (24–29) | 0.092 |
| MNA-SF, IQR | 13 (11–14) | 14 (12–14) | 0.56 |
| Charlson comorbidity index, IQR | 5 (3–6) | 4 (3–5) | 0.053 |
| Oxidative Stress Parameters | |||
| Native thiol, μmol/L ±SD | 263.55 ± 74.51 | 307.55 ± 57.77 | 0.003 |
| Total thiol, μmol/L ±SD | 283.71 ± 69.59 | 339.21 ± 60.59 | <0.001 |
| Disulfide, μmol/L, IQR | 15.05 (14.3–17.55) | 15.67 (14.26–17.0) | 0.74 |
| Disulfide/Nativethiol, IQR | 5.62 (5.09–7.99) | 5.15 (4.61–5.88) | 0.009 |
| Disulfide/Totalthiol, IQR | 5.05 (4.62–6.88) | 4.67 (4.22–5.26) | 0.009 |
| IMA, mg/dL, IQR | 0.88 (0.72–0.98) | 0.84 (0.68–0.92) | 0.13 |
| SO | ||||
|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | ||
| Model 8 | Age | 1.09 | 1.006–1.189 | 0.035 |
| Native thiol | 1.35 | 0.94–1.93 | 0.095 | |
| Total thiol | 0.75 | 0.54–1.04 | 0.093 | |
| Disulfide/Nativethiol | 5.71 | 1.07–30.32 | 0.041 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikmeer, A.; Eren, F.; Neselioglu, S.; Sahiner, Z.; Hafizoglu, M.; Karaduman, D.; Atbas, C.; Ileri, I.; Dogu, B.B.; Cankurtaran, M.; et al. The Predictive Role of Thiol/Disulfide Homeostasis as an Oxidative Stress Parameter in Sarcopenic Obesity. Medicina 2025, 61, 1708. https://doi.org/10.3390/medicina61091708
Dikmeer A, Eren F, Neselioglu S, Sahiner Z, Hafizoglu M, Karaduman D, Atbas C, Ileri I, Dogu BB, Cankurtaran M, et al. The Predictive Role of Thiol/Disulfide Homeostasis as an Oxidative Stress Parameter in Sarcopenic Obesity. Medicina. 2025; 61(9):1708. https://doi.org/10.3390/medicina61091708
Chicago/Turabian StyleDikmeer, Ayse, Funda Eren, Salim Neselioglu, Zeynep Sahiner, Merve Hafizoglu, Didem Karaduman, Cansu Atbas, Ibrahim Ileri, Burcu Balam Dogu, Mustafa Cankurtaran, and et al. 2025. "The Predictive Role of Thiol/Disulfide Homeostasis as an Oxidative Stress Parameter in Sarcopenic Obesity" Medicina 61, no. 9: 1708. https://doi.org/10.3390/medicina61091708
APA StyleDikmeer, A., Eren, F., Neselioglu, S., Sahiner, Z., Hafizoglu, M., Karaduman, D., Atbas, C., Ileri, I., Dogu, B. B., Cankurtaran, M., Akbiyik, F., Erel, O., & Halil, M. G. (2025). The Predictive Role of Thiol/Disulfide Homeostasis as an Oxidative Stress Parameter in Sarcopenic Obesity. Medicina, 61(9), 1708. https://doi.org/10.3390/medicina61091708





