Targeted Heart Rate Control with Landiolol in Hemodynamically Unstable, Non-Surgical Intensive Care Unit Patients: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Landiolol and Standard Treatment
2.3. Primary Composite Endpoint
2.4. Secondary Endpoint
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Vital Parameters at ICU Admission
3.3. Conventional Therapy
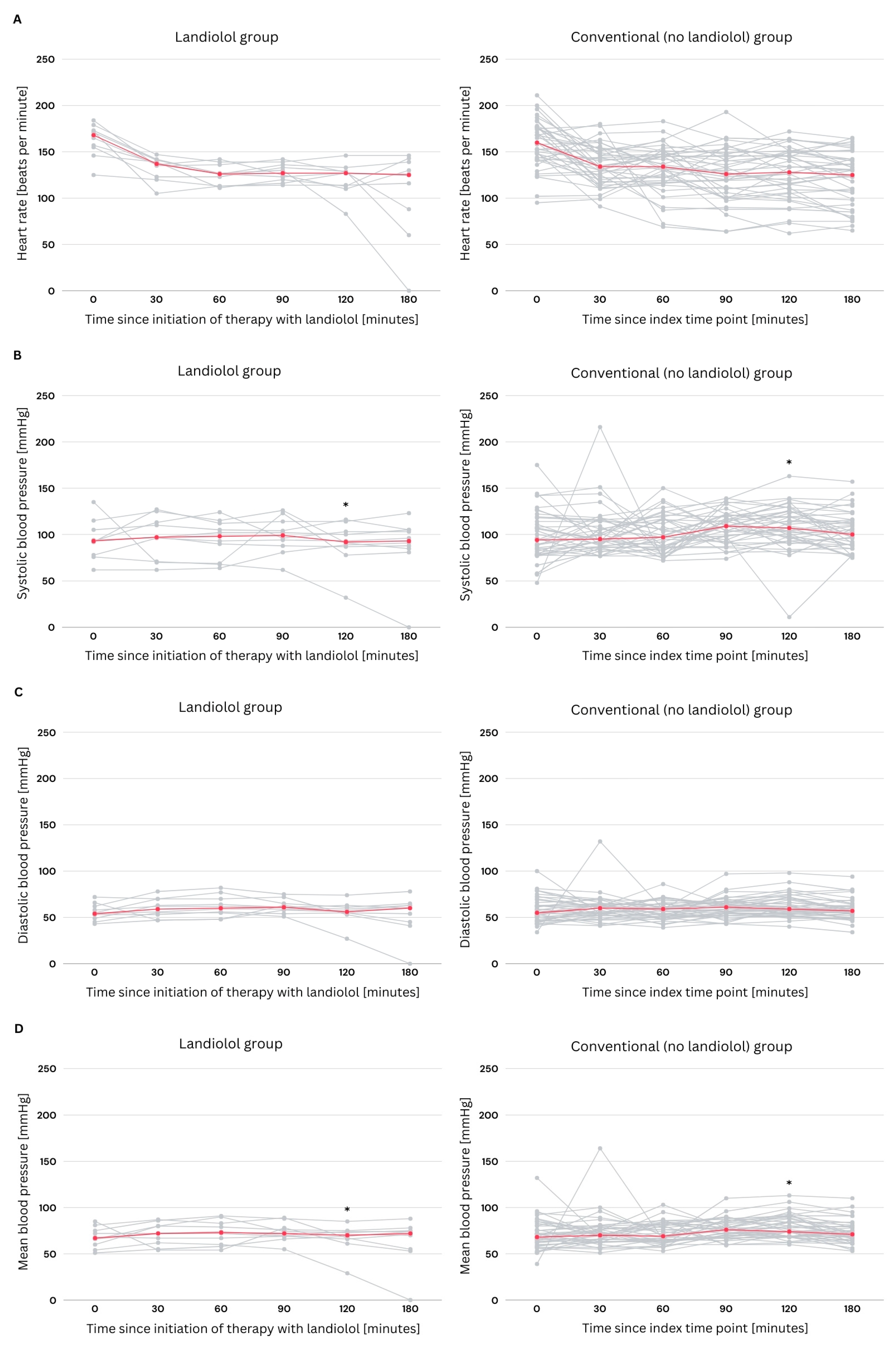
3.4. Hemodynamic Changes Within the First Three Hours of Treatment
3.5. Primary and Secondary Endpoints
3.6. Regression Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| Bpm | Beats per minute |
| CIP | Critically ill patients |
| e.g. | For example |
| HR | Heart rate |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| L-group | Landiolol group |
| LVEF | Left ventricular ejection fraction |
| Min. | Minutes |
| MmHg | Millimeters of mercury |
| NL-group | No landiolol group |
| NSTEMI | Non–ST-elevation myocardial infarction |
| OAC | Oral anticoagulation |
| PaO2 | Arterial oxygen pressure |
| pH | Potential of hydrogen |
| SpO2 | Oxygen saturation |
| SR | Sinus rhythm |
| STEMI | ST-elevation myocardial infarction |
References
- Chauhan, V.S.; Krahn, A.D.; Klein, G.J.; Skanes, A.C.; Yee, R. Supraventricular Tachycardia. Med. Clin. N. Am. 2001, 85, 193–223. [Google Scholar] [CrossRef]
- Duby, J.J.; Heintz, S.J.; Bajorek, S.A.; Heintz, B.H.; Durbin-Johnson, B.P.; Cocanour, C.S. Prevalence and Course of Atrial Fibrillation in Critically Ill Trauma Patients. J. Intensive Care Med. 2017, 32, 140–145. [Google Scholar] [CrossRef]
- Seguin, P.; Signouret, T.; Laviolle, B.; Branger, B.; Mallédant, Y. Incidence and Risk Factors of Atrial Fibrillation in a Surgical Intensive Care Unit. Crit. Care Med. 2004, 32, 722–726. [Google Scholar] [CrossRef]
- Maisel, W.H.; Rawn, J.D.; Stevenson, W.G. Atrial Fibrillation after Cardiac Surgery. Ann. Intern. Med. 2001, 135, 1061–1073. [Google Scholar] [CrossRef]
- Zakynthinos, G.E.; Tsolaki, V.; Xanthopoulos, A.; Karavidas, N.; Vazgiourakis, V.; Bardaka, F.; Giamouzis, G.; Pantazopoulos, I.; Makris, D. Prevalence, Risk Factors, and Mortality of New-Onset Atrial Fibrillation in Mechanically Ventilated Critically Ill Patients. J. Clin. Med. 2024, 13, 6750. [Google Scholar] [CrossRef]
- Bosch, N.A.; Cimini, J.; Walkey, A.J. Atrial Fibrillation in the ICU. Chest 2018, 154, 1424–1434. [Google Scholar] [CrossRef]
- Walkey, A.J.; Hogarth, D.K.; Lip, G.Y.H. Optimizing Atrial Fibrillation Management: From ICU and Beyond. Chest 2015, 148, 859–864. [Google Scholar] [CrossRef]
- Reyes, L.F.; Restrepo, M.I.; Hinojosa, C.A.; Soni, N.J.; Anzueto, A.; Babu, B.L.; Gonzalez-Juarbe, N.; Rodriguez, A.H.; Jimenez, A.; Chalmers, J.D.; et al. Severe Pneumococcal Pneumonia Causes Acute Cardiac Toxicity and Subsequent Cardiac Remodeling. Am. J. Respir. Crit. Care Med. 2017, 196, 609–620. [Google Scholar] [CrossRef]
- Brown, A.O.; Mann, B.; Gao, G.; Hankins, J.S.; Humann, J.; Giardina, J.; Faverio, P.; Restrepo, M.I.; Halade, G.V.; Mortensen, E.M.; et al. Streptococcus Pneumoniae Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function. PLoS Pathog. 2014, 10, e1004383. [Google Scholar] [CrossRef]
- Klouwenberg, P.M.C.K.; Frencken, J.F.; Kuipers, S.; Ong, D.S.Y.; Peelen, L.M.; van Vught, L.A.; Schultz, M.J.; van der Poll, T.; Bonten, M.J.; Cremer, O.L. Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am. J. Respir. Crit. Care Med. 2017, 195, 205–211. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC), with the Special Contribution of the European Heart Rhythm Association (EH-RA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef]
- Markman, T.M.; Jarrah, A.A.; Tian, Y.; Mustin, E.; Guandalini, G.S.; Lin, D.; Epstein, A.E.; Hyman, M.C.; Deo, R.; Supple, G.E.; et al. Safety of Pill-in-the-Pocket Class 1C Antiarrhythmic Drugs for Atrial Fibrillation. JACC Clin. Electrophysiol. 2022, 8, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.T.E.; Pamnani, P. Pill-in-the-Pocket for Paroxysmal Atrial Fibrillation: A Review and Case Study. J. Nurse Pract. 2024, 20, 105080. [Google Scholar] [CrossRef]
- Campbell, T.J.; Williams, K.M. Therapeutic Drug Monitoring: Antiarrhythmic Drugs. Br. J. Clin. Pharmacol. 1998, 46, 307–319. [Google Scholar] [CrossRef]
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Koniari, I.; Artopoulou, E.; Velissaris, D.; Mplani, V.; Anastasopoulou, M.; Kounis, N.; De Gregorio, C.; Tsigkas, G.; Karu-nakaran, A.; Plotas, P.; et al. Pharmacologic Rate versus Rhythm Control for Atrial Fibrillation in Heart Failure Patients. Medicina 2022, 58, 743. [Google Scholar] [CrossRef]
- Yoshida, Y.; Terajima, K.; Sato, C.; Akada, S.; Miyagi, Y.; Hongo, T.; Takeda, S.; Tanaka, K.; Sakamoto, A. Clinical Role and Efficacy of Landiolol in the Intensive Care Unit. J. Anesth. 2008, 22, 64–69. [Google Scholar] [CrossRef]
- Iguchi, S.; Iwamura, H.; Nishizaki, M.; Hayashi, A.; Senokuchi, K.; Kobayashi, K.; Sakaki, K.; Hachiya, K.; Ichioka, Y.; Kawamura, M. Development of a Highly Cardioselective Ultra Short-Acting β-Blocker, ONO-1101. Chem. Pharm. Bull. 1992, 40, 1462–1469. [Google Scholar] [CrossRef]
- Murakami, M.; Furuie, H.; Matsuguma, K.; Wanibuchi, A.; Kikawa, S.; Irie, S. Pharmacokinetics and Pharmacodynamics of Landiolol Hydrochloride, an Ultra Short-Acting β1-Selective Blocker, in a Dose Escalation Regimen in Healthy Male Volunteers. Drug Metab. Pharmacokinet. 2005, 20, 337–344. [Google Scholar] [CrossRef]
- Levy, B.; Slama, M.; Lakbar, I.; Maizel, J.; Kato, H.; Leone, M.; Okada, M. Landiolol for Treatment of New-Onset Atrial Fibrillation in Critical Care: A Systematic Review. J. Clin. Med. 2024, 13, 2951. [Google Scholar] [CrossRef]
- Si, X.; Yuan, H.; Shi, R.; Song, W.; Guo, J.; Jiang, J.; Yang, T.; Ma, X.; Wang, H.; Chen, M.; et al. Comparison of the Efficacy and Safety of Landiolol and Esmolol in Critically Ill Patients: A Propensity Score-Matched Study. Ann. Intensive Care 2025, 15, 5. [Google Scholar] [CrossRef]
- Arrigo, M.; Bettex, D.; Rudiger, A. Management of Atrial Fibrillation in Critically Ill Patients. Crit. Care Res. Pract. 2014, 2014, 840615. [Google Scholar] [CrossRef]
- Domanovits, H.; Wolzt, M.; Stix, G. Landiolol: Pharmacology and Its Use for Rate Control in Atrial Fibrillation in an Emergency Setting. Eur. Heart J. Suppl. 2018, 20, A1–A3. [Google Scholar] [CrossRef]
- Wells, G.L.; Morris, P.E. Incidence and Prognosis of Atrial Fibrillation in Patients with Sepsis. Cardiol. Res. 2011, 2, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, F.A.; Abraham, H.; Welte, T.; Westermann, L.; Bemtgen, X.; Gauchel, N.; Supady, A.; Wengenmayer, T.; Staudacher, D.L. Atrial Fibrillation and Survival on a Medical Intensive Care Unit. Int. J. Cardiol. 2024, 399, 131673. [Google Scholar] [CrossRef]
- Kuipers, S.; Klouwenberg, P.M.K.; Cremer, O.L. Incidence, Risk Factors and Outcomes of New-Onset Atrial Fibrillation in Patients with Sepsis: A Systematic Review. Crit. Care 2014, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Vignon, P.; Renault, A.; Bollaert, P.-E.; Charpentier, C.; Martin, C.; Troché, G.; Ricard, J.-D.; Nitenberg, G.; Papazian, L.; et al. Norepinephrine plus Dobutamine versus Epinephrine Alone for Management of Septic Shock: A Randomised Trial. Lancet 2007, 370, 676–684. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Backer, D.D. Circulatory Shock. N. Engl. J. Med. 2013, 369, 1726–1734. [Google Scholar] [CrossRef]
- Fuster, V.; Rydén, L.E.; Asinger, R.W.; Cannom, D.S.; Crijns, H.J.; Frye, R.L.; Halperin, J.L.; Kay, G.N.; Klein, W.W.; Lévy, S.; et al. ACC/AHA/ESC Guidelines for the Management of Patients with Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guide-lines for the Management of Patients With Atrial Fibrillation). J. Am. Coll. Cardiol. 2001, 38, 1231–1265. [Google Scholar]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063. [Google Scholar] [CrossRef]
- Kakihana, Y.; Nishida, O.; Taniguchi, T.; Okajima, M.; Morimatsu, H.; Ogura, H.; Yamada, Y.; Nagano, T.; Morishima, E.; Matsuda, N.; et al. Efficacy and Safety of Landiolol, an Ultra-Short-Acting Β1-Selective Antagonist, for Treatment of Sepsis-Related Tachyarrhythmia (J-Land 3S): A Multicentre, Open-Label, Randomised Controlled Trial. Lancet Respir. Med. 2020, 8, 863–872. [Google Scholar] [CrossRef]
- Sakamoto, A.; Kitakaze, M.; Takamoto, S.; Namiki, A.; Kasanuki, H.; Hosoda, S.; JL-KNIGHT Study Group. Landiolol, an Ultra-Short-Acting Β1-Blocker, More Effectively Terminates Atrial Fibrillation than Diltiazem after Open Heart Surgery: Prospective, Multicenter, Randomized, Open-Label Study (JL-KNIGHT Study). Circ. J. 2012, 76, 1097–1101. [Google Scholar] [CrossRef]
- Cafaro, T.; Allwood, M.; McIntyre, W.F.; Park, L.J.; Daza, J.; Ofori, S.N.; Ke Wang, M.; Borges, F.K.; Conen, D.; Marcucci, M.; et al. Landiolol for the Prevention of Postoperative Atrial Fibrillation after Cardiac Surgery: A Systematic Review and Meta-Analysis. Can. J. Anaesth. 2023, 70, 1828–1838. [Google Scholar] [CrossRef]
| Baseline Characteristics | Total (n = 51) | L-Group (n = 10) | NL-Group (n = 41) | p-Value |
|---|---|---|---|---|
| Age, median (IQR) | 69.0 (64.0–79.0) | 66.0 (57.5–79.2) | 70.0 (65.5–79.5) | 0.392 |
| Female, n (%) | 22.0 (43.1) | 5.0 (50.0) | 17.0 (41.5) | 0.728 |
| Male, n (%) | 29.0 (56.9) | 5.0 (50.0) | 24.0 (58.5) | |
| Invasive mechanical ventilation, n (%) | 38.0 (74.5) | 8.0 (80.0) | 30.0 (73.2) | 1.000 |
| APACHE II score, median (IQR) | 26.0 (18.5–30.0) | 25.0 (22.0–28.0) | 27.0 (17.7–30.0) | 0.930 |
| First diagnosis of AF, n (%) | 28.0 (54.9) | 5.0 (50.0) | 23.0 (56.1) | 0.728 |
| Paroxysmal AF, n (%) | 6.0 (11.8) | 3.0 (30.0) | 3.0 (7.3) | 0.050 |
| Persistent AF, n (%) | 12.0 (23.5) | 2.0 (20.0) | 10.0 (24.4) | 0.769 |
| Permanent AF, n (%) | 5.0 (9.8) | 0 | 5.0 (12.2) | 0.245 |
| Normal LVEF, n (%) | 23.0 (45.1) | 3.0 (30.0) | 20.0 (48.8) | 0.480 |
| Mildly reduced LVEF, n (%) | 7.0 (13.7) | 1.0 (10.0) | 6.0 (14.6) | 1.000 |
| Moderately reduced LVEF, n (%) | 5.0 (9.8) | 2.0 (20.0) | 3.0 (7.3) | 0.250 |
| Severely reduced LVEF, n (%) | 16.0 (31.4) | 4.0 (40.0) | 12.0 (29.3) | 0.705 |
| Cardiopulmonary resuscitation, n (%) | 10.0 (19.6) | 1.0 (10.0) | 9.0 (22.0) | 0.664 |
| Cardiogenic shock, n (%) | 17.0 (33.3) | 4.0 (40.0) | 13.0 (31.7) | 0.714 |
| STEMI, n (%) | 6.0 (11.8) | 1.0 (10.0) | 5.0 (12.2) | 1.000 |
| NSTEMI, n (%) | 1.0 (2.0) | 0 | 1.0 (2.4) | 1.000 |
| Primary rhythm disorder, n (%) | 5.0 (9.8) | 0 | 5.0 (12.2) | 0.569 |
| Myocarditis, n (%) | 1.0 (2.0) | 1.0 (10.0) | 0 | 0.196 |
| Pulmonary embolism, n (%) | 1.0 (2.0) | 1.0 (10.0) | 0 | 0.196 |
| Septic shock, n (%) | 35.0 (68.6) | 6.0 (60.0) | 29.0 (70.7) | 0.705 |
| Gastrointestinal bleeding, n (%) | 1.0 (2.0) | 1.0 (10.0) | 0 | 0.196 |
| Sodium [mmol/L], median (IQR) | 142.0 (138.0–144.0) | 143.0 (139.5–144.5) | 142.0 (137.5–143.5) | 0.424 |
| Potassium [mmol/L], median (IQR) | 4.1 (3.8–4.7) | 4.3 (4.1–4.9) | 4.1 (3.8–4.6) | 0.147 |
| Creatinine [µmol/L], median (IQR) | 142.0 (95.0–229.0) | 139.5 (122.5–233.0) | 142.0 (93.5–219.5) | 0.785 |
| Leukocytes [109/L], median (IQR) | 13.4 (7.7–21.1) | 11.7 (7.8–24.2) | 14.7 (6.8–20.6) | 0.849 |
| Vital Parameters at ICU Admission | Total (n = 51) | L-Group (n = 10) | NL-Group (n = 41) | p-Value |
|---|---|---|---|---|
| Heart rate [beats/min], median (IQR) | 160.0 (142.0–175.0) | 168.0 (152.7–174.5) | 158.0 (141.5–175.5) | 0.349 |
| Respiratory rate [breaths/min], median (IQR) | 24.0 (20.0–33.3) | 22.5 (20.7–25.0) | 24.5 (17.7–37.7) | 0.524 |
| Mean arterial pressure [mmHg], median (IQR) | 67.0 (60.0–75.0) | 70.0 (64.5–76.5) | 66.0 (59.5–78.0) | 0.462 |
| Temperature [°C], median (IQR) | 37.2 (36.2–37.8) | 37.5 (36.9–38.5) | 37.1 (36.1–37.7) | 0.092 |
| pH (arteriell), median (IQR) | 7.3 (7.2–7.4) | 7.3 (7.2–7.4) | 7.3 (7.2–7.4) | 0.868 |
| PaO2 [mmHg], median (IQR) | 86.0 (74.0–100.0) | 93.5 (84.0–111.2) | 83.0 (72.0–96.0) | 0.085 |
| SpO2 [%], median (IQR) | 95.0 (92.5–97.0) | 96.0 (93.2–97.2) | 95.0 (92.0–96.0) | 0.412 |
| Horovitz index, median (IQR) | 155.0 (113.7–215.0) | 164.0 (126.5–248.7) | 150.5 (106.0–213.2) | 0.235 |
| Conventional Treatment | Total (n = 51) | L-Group (n = 10) | NL-Group (n = 41) | p-Value |
|---|---|---|---|---|
| Electrical cardioversion, n (%) | 20.0 (39.2) | 6.0 (60.0) | 14.0 (34.1) | 0.163 |
| Amiodaron bolus therapy, n (%) | 23.0 (45.1) | 6.0 (60.0) | 17.0 (41.5) | 0.316 |
| Amiodaron continuous therapy, n (%) | 1.0 (2.0) | 0 | 1.0 (2.4) | 1.000 |
| Digoxin therapy, n (%) | 13.0 (25.5) | 2.0 (20.0) | 11.0 (26.8) | 1.000 |
| Metoprolol bolus therapy, n (%) | 3.0 (5.9) | 0 | 3.0 (7.3) | 1.000 |
| Metoprolol bolus and continuous therapy, n (%) | 2.0 (3.9) | 0 | 2.0 (4.9) | 1.000 |
| Combined Digoxin and Amiodaron therapy, n (%) | 6.0 (11.8) | 0 | 6.0 (14.6) | 0.331 |
| Intensified infusion therapy, n (%) | 22.0 (43.1) | 1.0 (10.0) | 21.0 (51.2) | 0.030 |
| Epinephrine therapy, n (%) | 4.0 (7.8) | 0 | 4.0 (9.8) | 0.573 |
| Norepinephrine therapy, n (%) | 32.0 (62.7) | 7.0 (70.0) | 25.0 (61.0) | 0.725 |
| Hemodynamic Parameters | Total (n = 51) | L-Group (n = 10) | NL-Group (n = 41) | p-Value |
|---|---|---|---|---|
| HR initial [bpm], median (IQR) | 160.0 (144.0–176.0) | 168.0 (152.7–174.5) | 160.0 (142.5–176.5) | 0.610 |
| HR 30 min [bpm], median (IQR) | 135.0 (120.0–147.0) | 137.0 (122.2–141.2) | 134.0 (119.0–151.5) | 0.794 |
| HR 60 min [bpm], median (IQR) | 133.0 (117.0–145.0) | 126.0 (113.0–136.7) | 134.0 (118.0–148.0) | 0.141 |
| HR 90 min [bpm], median (IQR) | 126.0 (110.0–142.0) | 127.5 (119.0–136.7) | 126.0 (103.5–146.5) | 0.906 |
| HR 120 min [bpm], median (IQR) | 127.0 (110.0–146.0) | 127.0 (112.2–130.0) | 128.0 (107.5–149.5) | 0.393 |
| HR 180 min [bpm], median (IQR) | 125.0 (98.0–142.0) | 125.5 (81.0–140.0) | 125.0 (98.5–142.0) | 0.569 |
| Sys ABP initial [mmHg], median (IQR) | 94.0 (78.0–114.0) | 93.0 (77.5–107.5) | 94.0 (78.5–116.0) | 0.669 |
| Sys ABP 30 min [mmHg], median (IQR) | 97.0 (87.0–114.0) | 97.0 (70.7–116.0) | 95.0 (87.0–114.5) | 0.849 |
| Sys ABP 60 min [mmHg], median (IQR) | 97.0 (84.0–116.0) | 97.5 (68.7–112.7) | 97.0 (85.0–117.0) | 0.491 |
| Sys ABP 90 min [mmHg], median (IQR) | 109.0 (94.0–119.0) | 99.0 (86.2–116.2) | 109.0 (96.0–120.0) | 0.217 |
| Sys ABP 120 min [mmHg], median (IQR) | 104.0 (93.0–119.0) | 92.0 (84.7–105.7) | 107.0 (96.0–125.0) | 0.021 |
| Sys ABP 180 min [mmHg], median (IQR) | 97.0 (88.0–113.0) | 93.5 (84.0–105.0) | 100.0 (88.5–115,5) | 0.217 |
| Dia ABP initial [mmHg], median (IQR) | 55.0 (47.0–63.0) | 54.5 (48.0–63.0) | 55.0 (46.5–64.5) | 0.868 |
| Dia ABP 30 min [mmHg], median (IQR) | 60.0 (53.0–64.0) | 59.0 (51.5–70.0) | 60.0 (53.0–64.0) | 0.669 |
| Dia ABP 60 min [mmHg], median (IQR) | 59.0 (51.0–64.0) | 60.5 (53.2–71.7) | 59.0 (50.5–63.0) | 0.318 |
| Dia ABP 90 min [mmHg], median (IQR) | 61.0 (54.0–66.0) | 61.0 (57.0–66.7) | 61.0 (54.0–66.5) | 0.660 |
| Dia ABP 120 min [mmHg], median (IQR) | 59.0 (55.0–69.0) | 56.5 (54.5–61.5) | 59.0 (54.5–70.0) | 0.235 |
| Dia ABP 180 min [mmHg], median (IQR) | 57.0 (52.0–63.0) | 60.5 (44.0–63.5) | 57.0 (52.5–62.5) | 0.972 |
| Mean ABP initial [mmHg], median (IQR) | 68.0 (60.0–80.0) | 67.0 (58.5–76.5) | 68.0 (59.5–80.5) | 0.749 |
| Mean ABP 30 min [mmHg], median (IQR) | 71.0 (63.0–80.0) | 72.0 (60.2–81.5) | 70.0 (63.0–78.5) | 0.953 |
| Mean ABP 60 min [mmHg], median (IQR) | 70.0 (64.0–82.0) | 73.0 (59.5–84.7) | 69.0 (64.0–82.0) | 0.896 |
| Mean ABP 90 min [mmHg], median (IQR) | 75.0 (68.0–83.0) | 72.0 (68.2–80.5) | 76.0 (67.5–83.5) | 0.521 |
| Mean ABP 120 min [mmHg], median (IQR) | 74.0 (69.0–85.0) | 70.0 (64.7–74.2) | 74.0 (69.0–88.5) | 0.027 |
| Mean ABP 180 min [mmHg], median (IQR) | 71.0 (64.0–78.0) | 72.5 (54.5–75.7) | 71.0 (65.0–79.5) | 0.569 |
| Regression Coefficient B | Standard Error | p-Value | OR (95% CI) | |
|---|---|---|---|---|
| Age [years] | 0 | 0 | 0.650 | 0.99 (0.94–1.04) |
| Female gender | 0.5 | 0.6 | 0.400 | 1.66 (0.51–5.38) |
| Landiolol treatment | −0.7 | 0.8 | 0.330 | 0.47 (0.10–2.14) |
| Reduced LVEF | 0.8 | 0.6 | 0.180 | 2.22 (0.69–7.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katov, L.; Gierak, J.; Teumer, Y.; Diofano, F.; Bothner, C.; Rottbauer, W.; Weinmann-Emhardt, K. Targeted Heart Rate Control with Landiolol in Hemodynamically Unstable, Non-Surgical Intensive Care Unit Patients: A Comparative Study. Medicina 2025, 61, 1703. https://doi.org/10.3390/medicina61091703
Katov L, Gierak J, Teumer Y, Diofano F, Bothner C, Rottbauer W, Weinmann-Emhardt K. Targeted Heart Rate Control with Landiolol in Hemodynamically Unstable, Non-Surgical Intensive Care Unit Patients: A Comparative Study. Medicina. 2025; 61(9):1703. https://doi.org/10.3390/medicina61091703
Chicago/Turabian StyleKatov, Lyuboslav, Jessica Gierak, Yannick Teumer, Federica Diofano, Carlo Bothner, Wolfgang Rottbauer, and Karolina Weinmann-Emhardt. 2025. "Targeted Heart Rate Control with Landiolol in Hemodynamically Unstable, Non-Surgical Intensive Care Unit Patients: A Comparative Study" Medicina 61, no. 9: 1703. https://doi.org/10.3390/medicina61091703
APA StyleKatov, L., Gierak, J., Teumer, Y., Diofano, F., Bothner, C., Rottbauer, W., & Weinmann-Emhardt, K. (2025). Targeted Heart Rate Control with Landiolol in Hemodynamically Unstable, Non-Surgical Intensive Care Unit Patients: A Comparative Study. Medicina, 61(9), 1703. https://doi.org/10.3390/medicina61091703








