Upadacitinib in Biologic-Experienced Inflammatory Bowel Disease: Real-World Efficacy, Safety, and Laboratory Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Upadacitinib and Efficacy
3.3. Upadacitinib and Laboratory Parameters and Side Effects
4. Discussion
Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| FCAL | Fecal calprotectin |
| GIS | Gastrointestinal system |
| HBI | Harvey–Bradshaw Index |
| IBD | Inflammatory bowel disease |
| JAK | Janus kinase |
| MACE | Major adverse cardiac events |
| PMS | Partial Mayo score |
| PSC | Primary sclerosing cholangitis |
| TC | Total cholesterol |
| UC | Ulcerative colitis |
| UPA | Upadacitinib |
References
- McInnes, I.B.; Gravallese, E.M. Immune-mediated inflammatory disease therapeutics: Past, present and future. Nat. Rev. Immunol. 2021, 21, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hussain, F.; D’Haens, G.; Feagan, B.G.; Loftus, E.V.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomized trial. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Elford, A.T.; Bishara, M.; Plevris, N.; Gros, B.; Constantine-Cooke, N.; Goodhand, J.; Kennedy, N.A.; Ahmad, T.; Lees, C.W. Real-world effectiveness of upadacitinib in Crohn’s disease: A UK multicentre retrospective cohort study. Frontline Gastroenterol. 2024, 15, 297–304. [Google Scholar] [CrossRef]
- Panés, J.; Dubinsky, M.C.; Ishiguro, Y.; Shukla, N.; Dubcenco, E.; Remple, V.; Sharma, D.; Panaccione, R. Achievement of long-term treatment goals in upadacitinib-treated patients with moderately to severely active ulcerative colitis: A post hoc analysis of phase 3 trial data. J. Crohns Colitis 2025, 19, jjaf095. [Google Scholar] [CrossRef]
- Drugs.com. FDA Approves Xeljanz for Rheumatoid Arthritis. Available online: https://www.drugs.com/newdrugs/fda-approves-xeljanz-rheumatoid-arthritis-3558.html (accessed on 6 November 2012).
- Karpouzas, G.A.; Szekanecz, Z.; Baecklund, E.; Mikuls, T.R.; Bhatt, D.L.; Wang, C.; Sawyerr, G.A.; Chen, Y.; Menon, S.; Connell, C.A.; et al. Rheumatoid arthritis disease activity and adverse events in patients receiving tofacitinib or tumor necrosis factor inhibitors: A post hoc analysis of ORAL Surveillance. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231201047. [Google Scholar] [CrossRef]
- Citera, G.; Mysler, E.; Kakehasi, A.M.; Pascual-Ramos, V.; Masson, W.; Cadatal, M.J.; Rivas, J.L.; Sheibanie, F.; Helling, C.; Ponce de Leon, D. Cardiovascular Events, Malignancies, and Efficacy Outcomes in Latin American Patients with Rheumatoid Arthritis Receiving Tofacitinib or Tumor Necrosis Factor Inhibitors: A Post Hoc Analysis of the ORAL Surveillance Study. J. Clin. Rheumatol. 2024, 30, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Tanaka, Y.; Mariette, X.; Curtis, J.R.; Lee, E.B.; Nash, P.; Winthrop, K.L.; Charles-Schoeman, C.; Wang, L.; Chen, C.; et al. Long-term safety of tofacitinib up to 9.5 years: A comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open 2020, 6, e001395. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef]
- Falcon, B.T.Q.; de Mello Guimaraes, T.; Halpern, G.A.; Mota, J.; Goulart, C.; Dourado, A. Insights into adverse events and safety profile of upadacitinib in the management of inflammatory bowel diseases—A meta-analysis of randomized controlled trials. Indian. J. Gastroenterol. 2025, 44, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhang, J.; Napel, M.; Xu, F.; Liu, S.; Cao, X.; Yu, Y.; Niu, W.; Cui, Y. Systematic Review with Meta-analysis: Efficacy and Safety of Upadacitinib in Managing Moderate-to-Severe Crohn’s Disease and Ulcerative Colitis. Clin. Drug Investig. 2024, 44, 371–385. [Google Scholar] [CrossRef]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular, and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. SA), 5A–36A. [Google Scholar] [CrossRef]
- Friedberg, S.; Choi, D.; Hunold, T.; Choi, N.K.; Garcia, N.M.; Picker, E.A.; Cohen, N.A.; Cohen, R.D.; Dalal, S.R.; Pekow, J.; et al. Upadacitinib Is Effective and Safe in Both Ulcerative Colitis and Crohn’s Disease: Prospective Real-World Experience. Clin. Gastroenterol. Hepatol. 2023, 21, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Osty, M.; Altwegg, R.; Serrero, M.; Benezech, A.; Lecomte, A.; Cadiot, G.; Vuitton, L.; Wampach, A.; Nancey, S.; Buisson, A.; et al. Effectiveness and Safety of a Second JAK Inhibitor in Ulcerative Colitis: The J2J Multicentre Study. Aliment. Pharmacol. Ther. 2025, 62, 430–439. [Google Scholar] [CrossRef]
- Agouridis, A.; Elisaf, M.; Milionis, H. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann. Gastroenterol. 2011, 24, 181–187. [Google Scholar]
- Lopez-Sanroman, A.; Esplugues, J.; Domenech, E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol. Hepatol. 2021, 44, 39–48. [Google Scholar] [CrossRef]
- Sands, B.; Taub, P.; Armuzzi, A.; Friedman, G.; Moscariello, M.; Lawendy, N.; Pangan, A.L.; Chen, C.; Biswas, P.; Shapiro, A.B.; et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 123–132.e3. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.G.; Giles, J.T.; Lane, N.E.; Choy, E.; Camp, H.; Song, Y.; Anyanwu, S.; McInnes, I. Relationship between changes in lipid levels and improvement in disease activity outcomes in patients with rheumatoid arthritis receiving upadacitinib treatment: Pooled analysis of data from two Phase 3 studies [Abstract]. Arthritis Rheumatol. 2020, 72 (Suppl. S10). Available online: https://acrabstracts.org/abstract/relationship-between-changes-in-lipid-levels-and-improvement-in-disease-activity-outcomes-in-patients-with-rheumatoid-arthritis-receiving-upadacitinib-treatment-pooled-analysis-of-data-from-two-phase/ (accessed on 17 September 2025). [CrossRef]
- Makris, A.; Barkas, F.; Sfikakis, P.P.; Liberopoulos, E.; Agouridis, A.P. The Effect of Upadacitinib on Lipid Profile and Cardiovascular Events: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2022, 11, 6894. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Molina, C.; Diaz-Torne, C.; Park, H.S.; Feliu, A.; Vidal, S.; Corominas, H. Tofacitinib and Baricitinib in Type 2 Diabetic Patients with Rheumatoid Arthritis. Medicina 2024, 60, 360. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, T.; Yu, Q.; Su, T.; Zhang, M.; Wu, L.; Wang, X.; Peng, X.; Zhi, M.; Yao, J. An Analysis of the Effectiveness and Safety of Upadacitinib in the Treatment of Inflammatory Bowel Disease: A Multicenter Real-World Study. Biomedicines 2025, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Wollenhaupt, J.; Lee, E.B.; Curtis, J.; Silverfield, J.; Terry, K.; Soma, K.; Takiya, L.; Golembesky, A.; Zwillich, S. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: Final results of a global, open-label, long-term extension study. Arthritis Res. Ther. 2019, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Boneschansker, L.; Ananthakrishnan, A.N. Comparative Effectiveness of Upadacitinib and Tofacitinib in Inducing Remission in Ulcerative Colitis: Real-World Data. Clin. Gastroenterol. Hepatol. 2023, 21, 2427–2429.e1. [Google Scholar] [CrossRef] [PubMed]


| Total Group (n = 41) | UC (n = 22) | CD (n = 19) | ||
|---|---|---|---|---|
| Gender (female) | 36.6% (n = 15) | 40.1% (n = 9) | 31.6% (n = 6) | |
| Age (years) | 43.6 ± 14.3 | 48 ± 15.4 | 38.1 ± 10.7 | |
| Disease duration (years) | 8.1 ± 5.1 | 7.8 ± 4.4 | 8.5 ± 5.9 | |
| Smoking | 41.5% (n = 17) | 36.4% (n = 8) | 47.4% (n = 9) | |
| Prior surgery | 12.2% (n = 5) | 0% (n = 0) | 26.3% (n = 5) | |
| 1 Prior biologics | 53.7% (n = 22) | 59.1% (n = 13) | 47.4% (n = 9) | |
| >1 Prior biologics | 46.3% (n = 19) | 40.9% (n = 9) | 53.7% (n = 10) | |
| Location (CD) | L1(ileal) | - | - | 53.7% (n = 10) |
| (Montreal classification) | L2 (colonic) | - | - | 15.8% (n = 3) |
| L3 (ileocolonic) | - | - | 26.3% (n = 5) | |
| L4 (upper GIS) | - | - | 5.3% (n = 1) | |
| Behavior (CD) | B1 | - | - | 63.2% (n = 12) |
| (Montreal classification) | B2 | - | - | 26.3% (n = 5) |
| B3 | - | - | 21.1% (n = 4) | |
| Perianal disease | 14.6% (n = 4) | 0% (n = 0) | 31.6% (n = 6) | |
| Location (UC) | Left-sided | - | 31.8% (n = 7) | - |
| Extensive | - | 40.9% (n = 9) | - | |
| Pancolitis | - | 27.3% (n = 6) | - | |
| EIM | Enteropathic arthritis | 36.6% (n = 15) | 22.7% (n = 5) | 53.7% (n = 10) |
| Dermatological | 9.8% (n = 4) | 13.6% (n = 3) | 5.3% (n = 1) | |
| Ocular | 4.9% (n = 2) | 4.5% (n = 1) | 5.3% (n = 1) | |
| PSC | 2.4% (n = 1) | 4.5% (n = 1) | 0% (n = 0) | |
| Ankylosing spondylitis | 19.5% (n = 8) | 4.5% (n = 1) | 36.9% (n = 7) | |
| Comorbidities | Diabetes | 2.4% (n = 1) | 4.5% (n = 1) | 0% (n = 0) |
| Hypertension | 14.6% (n = 6) | 13.6% (n = 3) | 15.8% (n = 3) | |
| Respiratory | 7.3% (n = 3) | 9.1% (n = 2) | 5.3% (n = 1) | |
| Renal disease | 2.4% (n = 1) | 4.5% (n = 1) | 0% (n = 0) | |
| Other | 12.2% (n = 5) | 59.1% (n = 2) | 15.8 (n = 3) | |
| Total | 39% (n = 16) | 40.9% (n = 9) | 36.9% (n = 7) | |
| Fecal calprotectin (μg/g) | 405 ± 260 | 521 ± 246 | 257 ± 198 | |
| CRP (mg/L) | 52 ± 49 | 74 ± 55 | 23 ± 16 | |
| Albumin (g/dL) | 4 ± 0.6 | 3.9 ± 0.52 | 4.1 ± 0.36 |
| Total Group (n = 41) n (%) | UC (n = 22) n (%) | CD (n = 19) n (%) | |
|---|---|---|---|
| Infliximab | 26 (63.4%) | 13 (59.1%) | 13 (68.4%) |
| Adalimumab | 22 (53.7%) | 11 (50%) | 11 (57.9%) |
| Vedolizumab | 10 (24.4%) | 5 (22.7%) | 5 (26.3%) |
| Ustekinumab | 9 (22%) | 5 (22.7%) | 4 (21.1%) |
| Two biological molecules | 14 (34.1%) | 7 (31.8%) | 7 (36.9%) |
| Three biological molecules | 3 (7.3%) | 1 (4.5%) | 2 (10.5%) |
| Four biological molecules | 2 (4.9%) | 1 (4.5%) | 1 (5.3%) |
| Azathioprine | 36 (87.8%) | 19 (86.4%) | 17 (89.5%) |
| Methotrexate | 2 (4.9%) | 0 (0%) | 2 (10.5%) |
| Steroid + UPA | 11 (26.8%) | 5 (22.7%) | 6 (31.6%) |
| Ulcerative Colitis (n = 22) | ||||
|---|---|---|---|---|
| Baseline n (%) | 3rd Month of UPA n (%) | 6th Month of UPA n (%) | ||
| PMS | <2 (remission) | 0 (0%) | 10 (45.5%) | 14 (63.6%) |
| 2–4 (mild) | 2 (9.1%) | 9 (40.1%) | 6 (27.3%) | |
| 5–7 (moderate) | 8 (36.4%) | 3 (13.6%) | 1 (4.5%) | |
| >7 (severe) | 12 (54.5%) | 0 (0%) | 1 (4.5%) | |
| FCAL (µg/g) | >250 | 18 (81.8%) | 5 (22.7%) | 4 (18.2%) |
| 100–250 | 4 (18.2%) | 8 (36.4%) | 9 (40.1%) | |
| <100 | 0 (0%) | 9 (40.1%) | 9 (40.1%) | |
| CRP (mg/L) | >5 | 22 (100%) | 7 (31.8%) | 4 (18.2%) |
| ≤5 | 0 (0%) | 15 (68.2%) | 18 (81.8%) | |
| Crohn Disease (n = 19) | ||||
|---|---|---|---|---|
| Baseline n (%) | 3rd Month of UPA n (%) | 6th Month of UPA n (%) | ||
| HBI | <5 (remission) | 0 (0%) | 9 (47.4%) | 11 (57.9%) |
| 5–7 (mild) | 5 (26.3%) | 4 (21.1%) | 3 (15.8%) | |
| 8–16 (moderate) | 11 (57.9%) | 6 (31.6%) | 5 (26.3%) | |
| >16 (severe) | 3 (15.8%) | 0 (0%) | 0 (0%) | |
| FCAL (µg/g) | >250 | 8 (42.1%) | 4 (21.1%) | 2 (10.5%) |
| 100–250 | 8 (42.1%) | 4 (21.1%) | 6 (31.6%) | |
| <100 | 3 (15.8%) | 11 (57.9%) | 11 (57.9%) | |
| CRP (mg/L) | >5 | 18 (94.7%) | 11 (57.9%) | 10 (52.6%) |
| ≤5 | 1 (5.3%) | 8 (42.1%) | 9 (47.4%) | |
| All Patients (n = 41) | Baseline (Mean ± Std) | Month 3 of UPA (Mean ± Std) | Month 6 of UPA (Mean ± Std) | p-Value |
|---|---|---|---|---|
| Glucose (mg/dL) | 93.3 ± 18.9 | 92.4 ± 19.2 | 90.8 ± 11.7 | 0.289 |
| HbA1c (%) | 5.27 ± 0.75 | 5.28 ± 0.86 | 5.26 ± 0.79 | 0.914 |
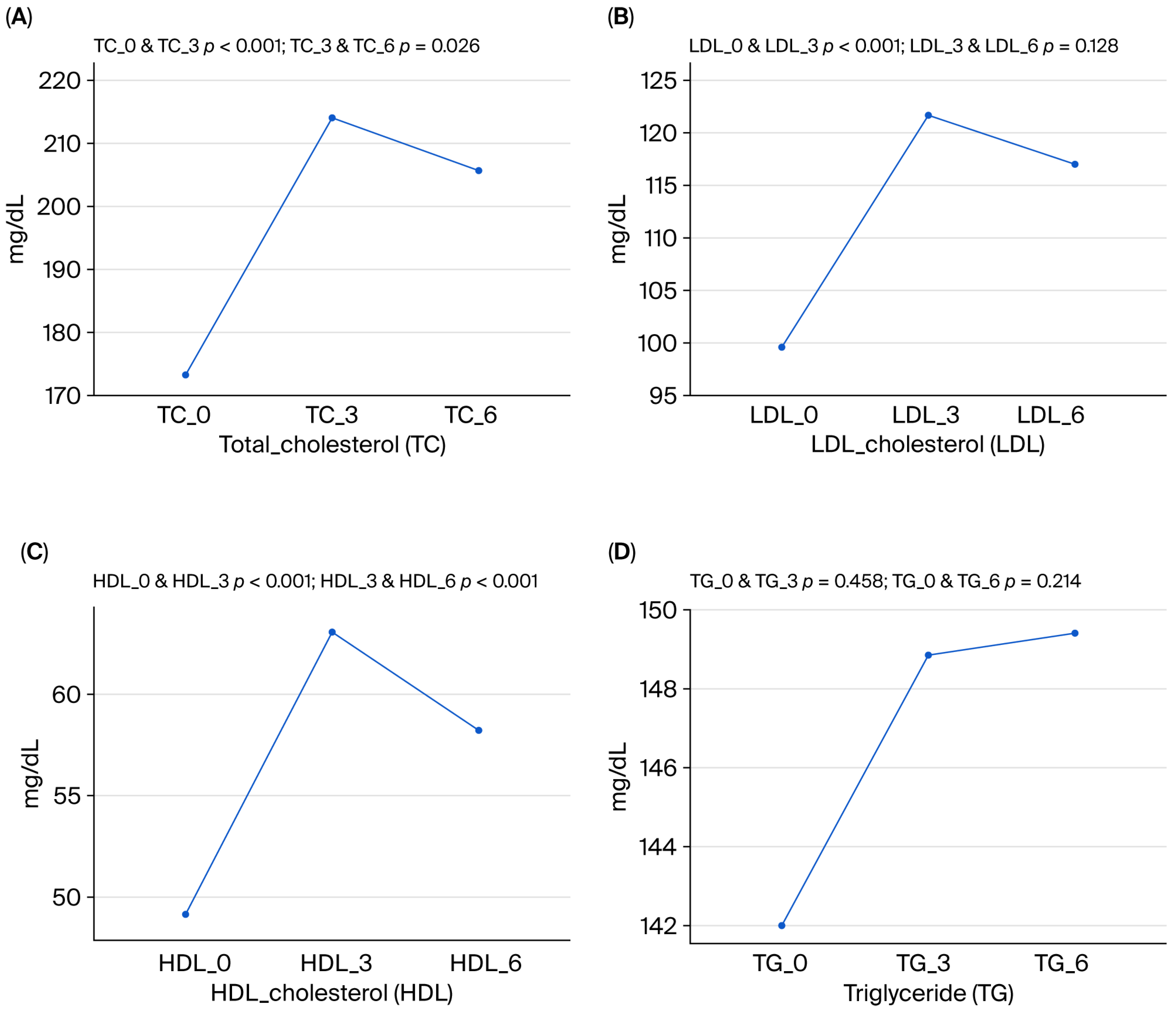
| Total-C (mg/dL) | 173.3 ± 41.8 | 214.1 ± 50.1 | 205.6 ± 52.7 | 0.001 |
| LDL-C (mg/dL) | 99.6 ± 35.0 | 121.7 ± 40.8 | 117.0 ± 42.5 | 0.000 |
| HDL-C (mg/dL) | 49.1 ± 13.9 | 63.1 ± 17.1 | 58.2 ± 15.0 | 0.000 |
| LDL-C/HDL-C (ratio) | 2.09 ± 0.68 | 2.00 ± 0.69 | 2.08 ± 0.74 | 0.233 |
| Triglyceride(mg/dL) | 142.0 ± 75.0 | 148.9 ± 86.6 | 149.4 ± 79.4 | 0.542 |
| AST (U/L) | 20.5 ± 7.9 | 23.4 ± 11.0 | 21.3 ± 7.1 | 0.429 |
| ALT (U/L) | 21.1 ± 14.6 | 29 ± 29.6 | 22.5 ± 14.3 | 0.076 |
| ALP (U/L) | 76.5 ± 19.9 | 76.3 ± 20.9 | 74.2 ± 21.1 | 0.433 |
| GGT (U/L) | 22.4 ± 10.9 | 21.9 ± 13.2 | 19.3 ± 9.3 | 0.076 |
| Total bilirubin (mg/dL) | 0.55 ± 0.29 | 0.58 ± 0.30 | 0.56 ± 0.30 | 0.614 |
| Albumin (g/dl) | 4.01 ± 0.46 | 4.34 ± 0.38 | 4.37 ± 0.36 | 0.000 |
| Calcium (mg/dL) | 9.21 ± 0.43 | 9.46 ± 0.55 | 9.43 ± 0.52 | 0.002 |
| Creatinine (mg/dL) | 0.78 ± 0.22 | 0.78 ± 0.22 | 0.81 ± 0.26 | 0.502 |
| TSH (µIU/mL) | 1.74 ± 0.85 | 1.70 ± 0.77 | 1.55 ± 0.73 | 0.059 |
| FT4 (pmol/L) | 11.2 ± 2.1 | 10.8 ± 1.43 | 10.8 ± 1.24 | 0.072 |
| Vit_B12 (pg/mL) | 267 ± 112 | 230 ± 81 | 218 ± 72 | 0.000 |
| Folic acid (ng/mL) | 8.18 ± 3.08 | 7.57 ± 3.29 | 7.28 ± 3.27 | 0.016 |
| Ferritin (ng/mL) | 80.8 ± 118 | 53.4 ± 77.1 | 50.7 ± 73.6 | 0.001 |
| Hgb (g/dL) | 13.0 ± 1.40 | 13.2 ± 1.24 | 13.1 ± 2.51 | 0.689 |
| Plt (×103/µL) | 348 ± 93.2 | 320 ± 88.4 | 302 ± 86.6 | 0.000 |
| WBC (×/µL) | 9747 ± 2820 | 8161 ± 2699 | 7818 ± 2451 | 0.000 |
| Neutrophil (×/µL) | 7199 ± 5577 | 4934 ± 2260 | 4975 ± 2166 | 0.012 |
| Lymphocyte (×/µL) | 2436 ± 1358 | 2314 ± 944 | 2122 ± 719 | 0.118 |
| CRP (mg/L) | 29.8 ± 31.5 | 8.56 ± 13.3 | 7.49 ± 11.23 | 0.000 |
| FCAL (µg/g) | 405 ± 259 | 158 ± 158 | 120 ± 112 | 0.000 |
| Adverse Events | All Patients (n = 41) n (%) | |
|---|---|---|
| Infection | Upper respiratory tract infection | 3 (7.3%) |
| Pneumonia | 1 (2.4%) | |
| Perianal abscess | 1 (2.4%) | |
| Other | 2 (4.9%) | |
| Dermatological | Herpes zoster reactivation | 1 (2.4%) |
| Acne | 3 (7.3%) | |
| Urticaria | 1 (2.4%) | |
| Symptoms | Nausea and vomiting | 4 (9.8%) |
| Headache | 2 (4.9%) | |
| Myalgia | 2 (4.9%) | |
| Total | 20 (48.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdoğan, O.; Yaraş, S. Upadacitinib in Biologic-Experienced Inflammatory Bowel Disease: Real-World Efficacy, Safety, and Laboratory Outcomes. Medicina 2025, 61, 1692. https://doi.org/10.3390/medicina61091692
Özdoğan O, Yaraş S. Upadacitinib in Biologic-Experienced Inflammatory Bowel Disease: Real-World Efficacy, Safety, and Laboratory Outcomes. Medicina. 2025; 61(9):1692. https://doi.org/10.3390/medicina61091692
Chicago/Turabian StyleÖzdoğan, Osman, and Serkan Yaraş. 2025. "Upadacitinib in Biologic-Experienced Inflammatory Bowel Disease: Real-World Efficacy, Safety, and Laboratory Outcomes" Medicina 61, no. 9: 1692. https://doi.org/10.3390/medicina61091692
APA StyleÖzdoğan, O., & Yaraş, S. (2025). Upadacitinib in Biologic-Experienced Inflammatory Bowel Disease: Real-World Efficacy, Safety, and Laboratory Outcomes. Medicina, 61(9), 1692. https://doi.org/10.3390/medicina61091692






