Improving MDS Risk Assessment: The Role of Monocytopenia and Lymphocytopenia Beyond IPSS-R
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Parameters
2.3. Assessment of Blood Counts and Absolute Lymphocyte/Monocyte Counts
2.4. Treatment
2.5. Statistical Methods
3. Results
3.1. Baseline and Disease-Related Characteristics
3.2. Comparison of Patients Stratified by the AMC and ALC
3.3. The Prognostic Relevance of Monocytopenia and Lymphocytopenia for Overall Survival
3.3.1. Univariate Analysis
3.3.2. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALC | Absolute Lymphocyte Count |
| allo-HSCT | Allogeneic Hematopoietic Stem Cell |
| AML | Acute Myeloid Leukemia |
| ANC | Absolute Neutrophil Count |
| ASCT | Allogeneic Stem Cell Transplantation |
| BM | Bone Marrow |
| CMML | Chronic Myelomonocytic Leukemia |
| ICC | International Consensus Classification |
| IPSS | International Prognostic Scoring System |
| IPSS-M | Molecular International Prognostic Scoring System |
| IPSS-R | Revised International Prognostic Scoring System |
| KW | Kruskal–Wallis test |
| MDS | Myelodysplastic Syndrome(s) |
| MPN | Myeloproliferative Neoplasm |
| OS | Overall Survival |
| MWU | Mann–Whitney U test |
| PB | Peripheral Blood |
| PFS | Progression-Free Survival |
| WBC | White Blood Cell Count |
| WHO | World Health Organization |
References
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Haase, D.; Santini, V.; Sanz, G.; Platzbecker, U.; Mey, U.; ESMO Guidelines Committee. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Ossa, J.E.A.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef]
- Winter, S.; Shoaie, S.; Kordasti, S.; Platzbecker, U. Integrating the “Immunome” in the Stratification of Myelodysplastic Syndromes and Future Clinical Trial Design. J. Clin. Oncol. 2020, 38, 1723–1735. [Google Scholar] [CrossRef]
- Trowbridge, J.J.; Starczynowski, D.T. Innate immune pathways and inflammation in hematopoietic aging, clonal hematopoiesis, and MDS. J. Exp. Med. 2021, 218, e20201544. [Google Scholar] [CrossRef]
- Simoni, Y.; Chapuis, N. Diagnosis of Myelodysplastic Syndromes: From Immunological Observations to Clinical Applications. Diagnostics 2022, 12, 1659. [Google Scholar] [CrossRef]
- Kouroukli, O.; Symeonidis, A.; Foukas, P.; Maragkou, M.-K.; Kourea, E.P. Bone Marrow Immune Microenvironment in Myelodysplastic Syndromes. Cancers 2022, 14, 5656. [Google Scholar] [CrossRef]
- Tentori, C.A.; Gregorio, C.; Robin, M.; Gagelmann, N.; Gurnari, C.; Ball, S.; Berrocal, J.C.C.; Lanino, L.; D’AMico, S.; Spreafico, M.; et al. Clinical and Genomic-Based Decision Support System to Define the Optimal Timing of Allogeneic Hematopoietic Stem-Cell Transplantation in Patients with Myelodysplastic Syndromes. J. Clin. Oncol. 2024, 42, 2873–2886. [Google Scholar] [CrossRef]
- Sauta, E.; Robin, M.; Bersanelli, M.; Travaglino, E.; Meggendorfer, M.; Zhao, L.-P.; Berrocal, J.C.C.; Sala, C.; Maggioni, G.; Bernardi, M.; et al. Real-World Validation of Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. J. Clin. Oncol. 2023, 41, 2827–2842. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T. Does monocyte count have prognostic significance in cancer? Leuk. Res. 2013, 37, 1193–1194. [Google Scholar] [CrossRef]
- Jakovic, L.R.; Mihaljevic, B.S.; Andjelic, B.M.; Bogdanovic, A.D.; Perunicic Jovanovic, M.D.; Babic, D.D.; Bumbasirevic, V.Z. Prognostic value of lymphocyte/monocyte ratio in advanced Hodgkin lymphoma: Correlation with International Prognostic Score and tumor associated macrophages. Leuk. Lymphoma 2016, 57, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, J.; Izura, J.; Manrique, A.; Sala, F.; Gaminde, I. Early prognosis in severe sepsis via analyzing the monocyte immunophenotype. Intensive Care Med. 2001, 27, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.A.; Verstovsek, S.; Dingli, D.; Schwager, S.M.; Mesa, R.A.; Li, C.Y.; Tefferi, A. Monocytosis is an adverse prognostic factor for survival in younger patients with primary myelofibrosis. Leuk. Res. 2007, 31, 1503–1509. [Google Scholar] [CrossRef]
- Silzle, T.; Blum, S.; Schuler, E.; Kaivers, J.; Rudelius, M.; Hildebrandt, B.; Gattermann, N.; Haas, R.; Germing, U. Lymphopenia at diagnosis is highly prevalent in myelodysplastic syndromes and has an independent negative prognostic value in IPSS-R-low-risk patients. Blood Cancer J. 2019, 9, 63. [Google Scholar] [CrossRef]
- Saeed, L.; Patnaik, M.M.; Begna, K.H.; Al-Kali, A.; Litzow, M.R.; Hanson, C.A.; Ketterling, R.P.; Porrata, L.F.; Pardanani, A.; Gangat, N.; et al. Prognostic relevance of lymphocytopenia, monocytopenia and lymphocyte-to-monocyte ratio in primary myelodysplastic syndromes: A single center experience in 889 patients. Blood Cancer J. 2017, 7, e550. [Google Scholar] [CrossRef]
- Silzle, T.; Blum, S.; Kasprzak, A.; Nachtkamp, K.; Rudelius, M.; Hildebrandt, B.; Götze, K.S.; Gattermann, N.; Lauseker, M.; Germing, U. The Absolute Monocyte Count at Diagnosis Affects Prognosis in Myelodysplastic Syndromes Independently of the IPSS-R Risk Score. Cancers 2023, 15, 3572. [Google Scholar] [CrossRef]
- Diamantopoulos, P.T.; Charakopoulos, E.; Symeonidis, A.; Kotsianidis, I.; Viniou, N.-A.; Pappa, V.; Pontikoglou, C.; Tsokanas, D.; Drakos, G.; Kourakli, A.; et al. Real world data on the prognostic significance of monocytopenia in myelodysplastic syndrome. Sci. Rep. 2022, 12, 17914. [Google Scholar] [CrossRef]
- Nordic MDS Group. Guidelines for the Diagnosis and Treatment of Myelodysplastic Syndrome and Chronic Myelomonocytic Leukemia [Internet]. 2021. Available online: https://www.nmds.org/attachments/article/121/NMDSG_guidelines_dec2021.pdf (accessed on 25 August 2022).
- Clinical and Laboratory Standards Institute. Reference Leukocyte Differential Count (Proportional) and Evaluation of Instrument Methods; Approved standard. CLSI document H20-A2; CLSI: Wayne, PA, USA, 2007. [Google Scholar]
- Lee, S.; Erber, W.N.; Porwit, A.; Tomonaga, M.; Peterson, L.C.; International Council for Standardization In Hematology. ICSH guidelines for the standardization of bone marrow specimens and reports. Int. J. Lab. Hematol. 2008, 30, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Itzykson, R.; Fenaux, P.; Bowen, D.; Cross, N.C.; Cortes, J.; De Witte, T.; Germing, U.; Onida, F.; Padron, E.; Platzbecker, U.; et al. Diagnosis and Treatment of Chronic Myelomonocytic Leukemias in Adults: Recommendations From the European Hematology Association and the European LeukemiaNet. Hemasphere 2018, 2, e150. [Google Scholar] [CrossRef]
- Bain, B.J. Ethnic and sex differences in the total and differential white cell count and platelet count. J. Clin. Pathol. 1996, 49, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Merz, A.M.A.; Platzbecker, U. Treatment of lower-risk myelodysplastic syndromes. Haematologica 2025, 110, 330–338. [Google Scholar] [CrossRef]
- Velegraki, M.; Papakonstanti, E.; Mavroudi, I.; Psyllaki, M.; Tsatsanis, C.; Oulas, A.; Iliopoulos, I.; Katonis, P.; Papadaki, H.A. Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica 2013, 98, 1206–1215. [Google Scholar] [CrossRef]
- Pang, W.W.; Pluvinage, J.V.; Price, E.A.; Sridhar, K.; Arber, D.A.; Greenberg, P.L.; Schrier, S.L.; Park, C.Y.; Weissman, I.L. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc. Natl. Acad. Sci. USA 2013, 110, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, F.; Shayanmanesh, M.; Safari, M.; Alaei, A.; Pouriafar, Y.; Rasti, Z.; Zaker, F.; Rostami, S.; Damerchiloo, F.; Safa, M. Bone marrow microenvironment in myelodysplastic neoplasms: Insights into pathogenesis, biomarkers, and therapeutic targets. Cancer Cell Int. 2025, 25, 175. [Google Scholar] [CrossRef]
- Kordasti, S.Y.; Afzali, B.; Lim, Z.; Ingram, W.; Hayden, J.; Barber, L.; Matthews, K.; Chelliah, R.; Guinn, B.; Lombardi, G.; et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br. J. Haematol. 2009, 145, 64–72. [Google Scholar] [CrossRef]
- Kasprzak, A.; Assadi, C.; Nachtkamp, K.; Rudelius, M.; Haas, R.; Giagounidis, A.; Götze, K.; Gattermann, N.; Germing, U. Monocytosis at the time of diagnosis has a negative prognostic impact in myelodysplastic syndromes with less than 5% bone marrow blasts. Ann. Hematol. 2023, 102, 99–106. [Google Scholar] [CrossRef]
- Qu, H.; Chu, J.; Wang, L.; Zhang, J.; Han, J.; Li, Z.; Hou, H.; Wang, Y.; Liu, Y.; Wu, H. Platelet-to-lymphocyte ratio and absolute monocyte count have prognostic potential in primary myelodysplastic neoplasms. Int. J. Lab. Hematol. 2024, 46, 275–285. [Google Scholar] [CrossRef]
- Holtan, S.G.; Santana-Davila, R.; DeWald, G.W.; Khetterling, R.P.; Knudson, R.A.; Hoyer, J.D.; Chen, D.; Hanson, C.A.; Porrata, L.; Tefferi, A.; et al. Myelodysplastic syndromes associated with interstitial deletion of chromosome 5q: Clinicopathologic correlations and new insights from the pre-lenalidomide era. Am. J. Hematol. 2008, 83, 708–713. [Google Scholar] [CrossRef]
- Jacobs, N.L.; Holtan, S.G.; Porrata, L.F.; Markovic, S.N.; Tefferi, A.; Steensma, D.P. Host immunity affects survival in myelodysplastic syndromes: Independent prognostic value of the absolute lymphocyte count. Am. J. Hematol. 2010, 85, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Goksu, S.Y.; Ozer, M.; Goksu, B.B.; Wang, R.; Khatib, J.; Patel, P.A.; Vusirikala, M.; Cole, S.; Seyhanli, A.; Collins, R.H.; et al. The impact of race and ethnicity on outcomes of patients with myelodysplastic syndromes: A population-based analysis. Leuk. Lymphoma 2022, 63, 1651–1659. [Google Scholar] [CrossRef]
- Mir, M.A.; Kochuparambil, S.T.; Abraham, R.S.; Rodriguez, V.; Howard, M.; Hsu, A.P.; Jackson, A.E.; Holland, S.M.; Patnaik, M.M. Spectrum of myeloid neoplasms and immune deficiency associated with germline GATA2 mutations. Cancer Med. 2015, 4, 490–499. [Google Scholar] [CrossRef]
- Platzbecker, U.; Della Porta, M.G.; Santini, V.; Zeidan, A.M.; Komrokji, R.S.; Shortt, J.; Valcarcel, D.; Jonasova, A.; Dimicoli-Salazar, S.; Tiong, I.S.; et al. Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): Interim analysis of a phase 3, open-label, randomised controlled trial. Lancet 2023, 402, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Santini, V.; Fenaux, P.; Sekeres, M.A.; Savona, M.R.; Madanat, Y.F.; Díez-Campelo, M.; Valcárcel, D.; Illmer, T.; Jonášová, A.; et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): A multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 403, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; List, A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood 2019, 133, 1039–1048. [Google Scholar] [CrossRef]
- Schneider, M.; Rolfs, C.; Trumpp, M.; Winter, S.; Fischer, L.; Richter, M.; Menger, V.; Nenoff, K.; Grieb, N.; Metzeler, K.H.; et al. Activation of distinct inflammatory pathways in subgroups of LR-MDS. Leukemia 2023, 37, 1709–1718. [Google Scholar] [CrossRef]
- Mina, A.; McGraw, K.L.; Cunningham, L.; Kim, N.; Jen, E.Y.; Calvo, K.R.; Ehrlich, L.A.; Aplan, P.D.; Garcia-Manero, G.; Foran, J.M.; et al. Advancing drug development in myelodysplastic syndromes. Blood Adv. 2025, 9, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.A.; Kim, N.; DeZern, A.E.; Norsworthy, K.J.; Garcia, J.S.; de Claro, R.A.; Theoret, M.R.; Jen, E.Y.; Ehrlich, L.A.; Zeidan, A.M.; et al. Considerations for Drug Development in Myelodysplastic Syndromes. Clin Cancer Res. 2023, 29, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
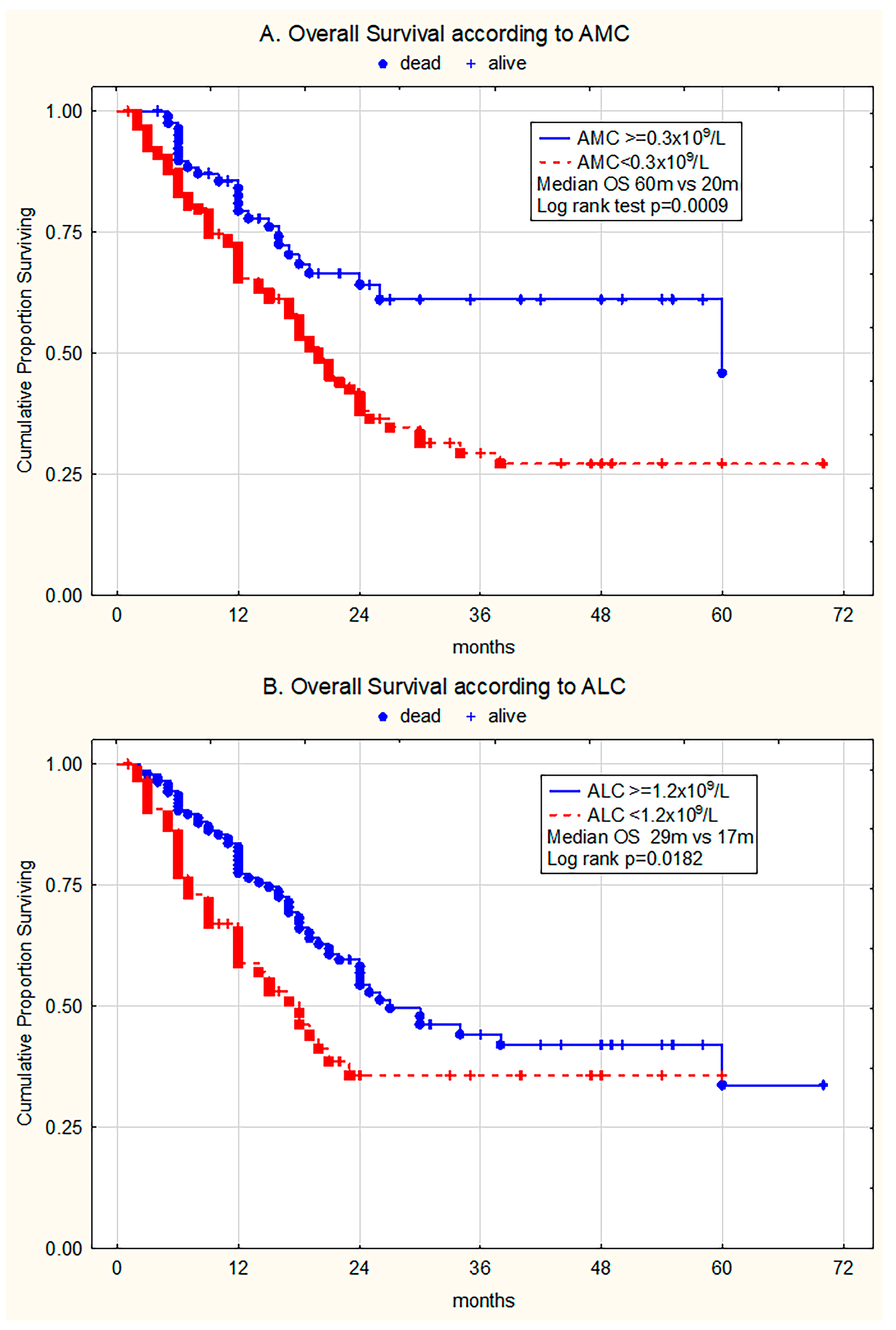
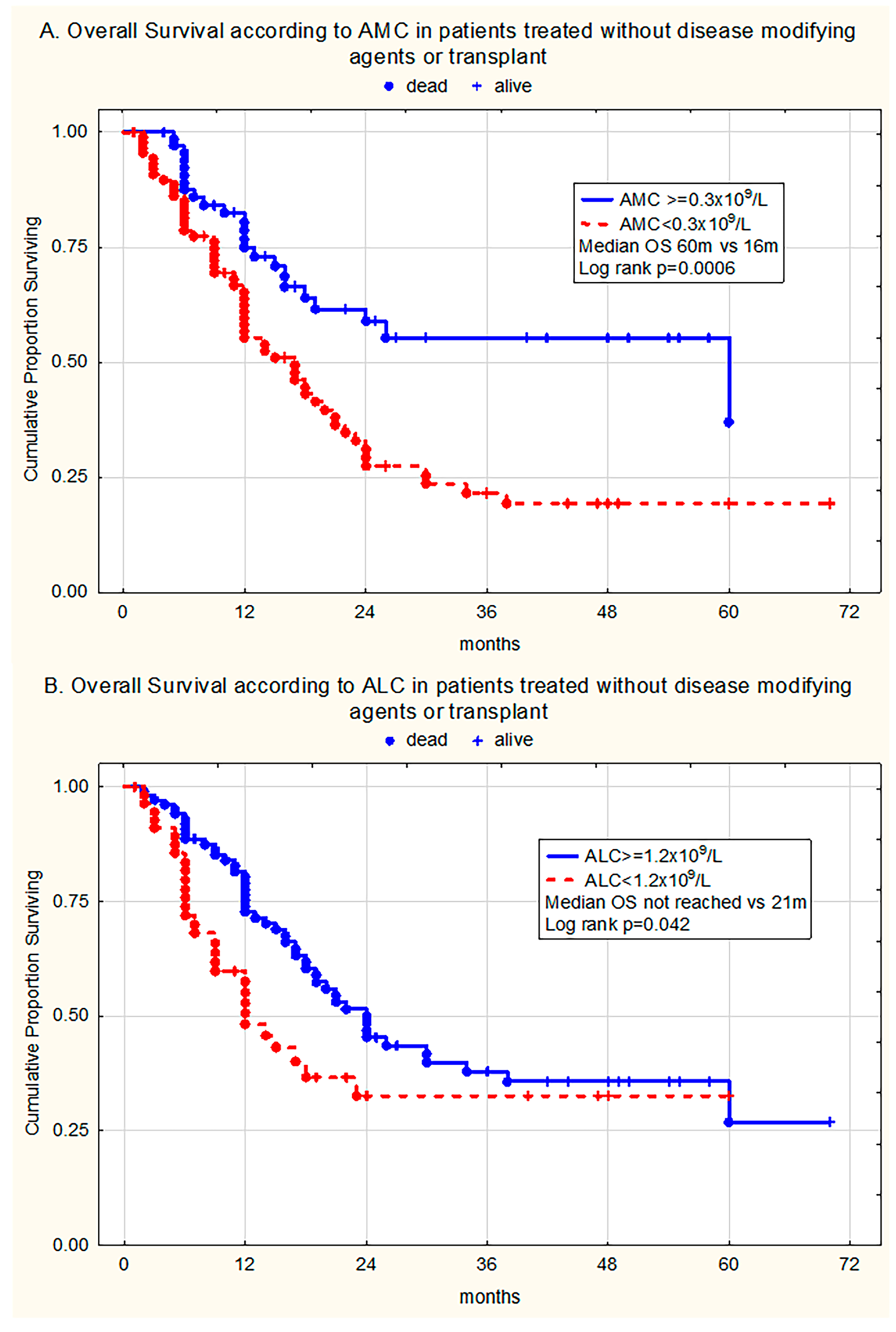
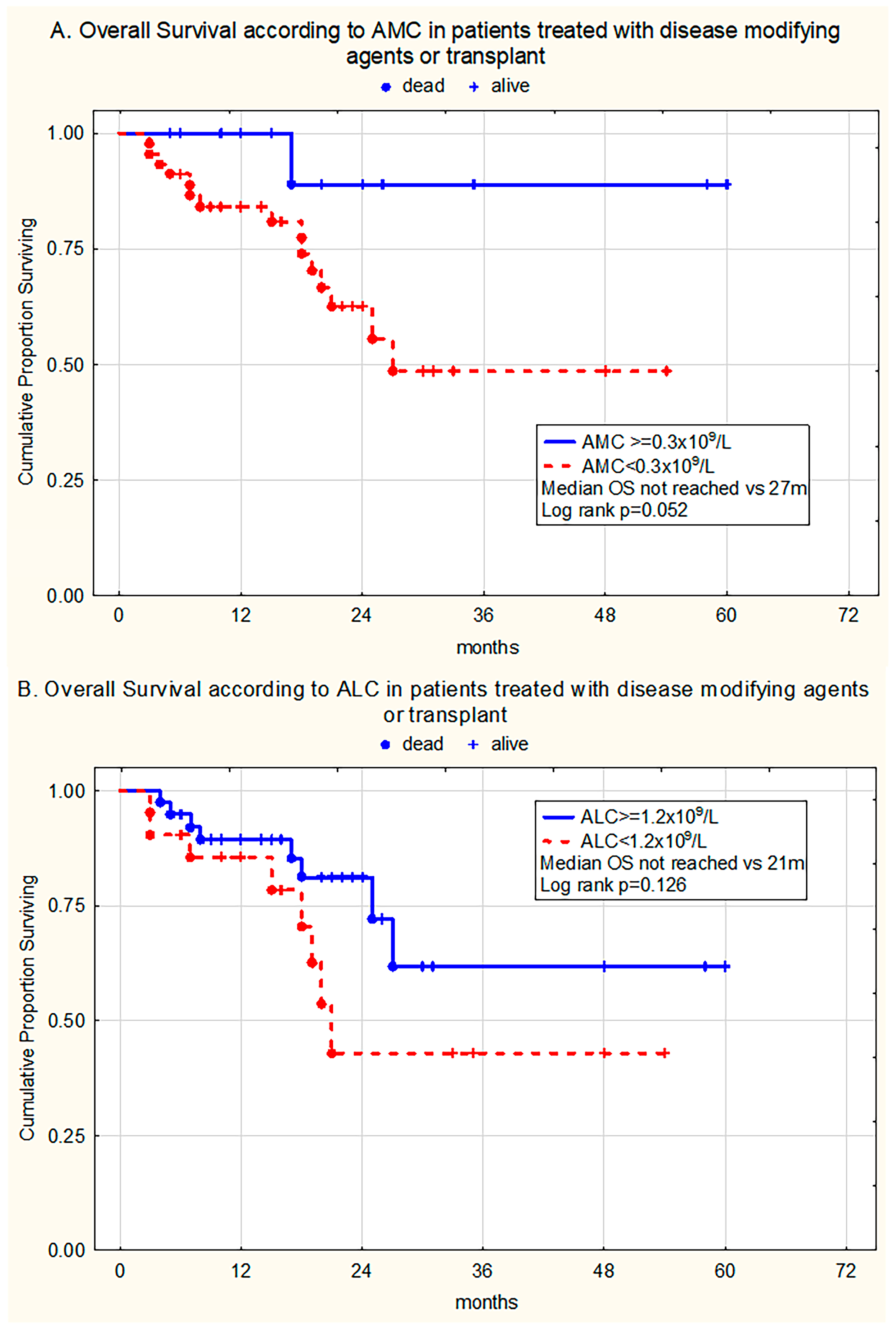
| Characteristics | Value; % n = 217 | AMC < 0.3 × 109/L (n = 133/61.3; %) | AMC ≥ 0.3 × 109/L (n = 84/38.7; %) | p Value | ALC < 1.2 × 109/L (n = 77/35.5; %) | ALC ≥ 1.2 × 109/L (n = 140/64.5; %) | p Value |
|---|---|---|---|---|---|---|---|
| Age (median yrs, range) | 70 (28–92) | 70 (28–92) | 71 (33–89) | 0.754 # | 74 (31–91) | 69 (28–92) | 0.003 # |
| Sex: (M/F) | 117/100 | 69/64 | 48/36 | 0.548 | 44/33 | 73/67 | 0.513 |
| Hgb (g/L), median (range) | 91 (50–147) | 92 (50–147) | 88 (52–147) | 0.797 # | 94 (50–147) | 90 (52–147) | 0.321 # |
| Hgb < 100 g/L, n (%) | 139 (64) | 87 | 52 | 0.546 | 45 | 94 | 0.201 |
| WBC ×109/L (median; range) | 3.4 (0.8–11.5) | 2.7 (0.8–10) | 5.0 (2.2–11.5) | 0.0001 # | 2.8 (0.8–9.2) | 3.6 (1.3–11.5) | 0.0001 # |
| WBC < 4 × 109/L, n (%) | 141 (65) | 110 | 31 | 0.0001 # | 63 | 78 | 0.001 # |
| Plt (×109/L), median (range) | 112 (2–583) | 104 (2–583) | 125 (4–574) | 0.438 # | 93 (8–455) | 125 (2–583) | 0.084 # |
| Plt < 100 × 109/L, n (%) | 100 (46.1) | 66 | 34 | 0.312 | 40 | 60 | 0.126 |
| Plt < 50 × 109/L, n (%) | 50 (23) | 30 | 20 | 0.472 | 16 | 34 | 0.341 |
| Absolute neutrophil count (×109/L), median (range) | 1.4 (0.1–7.68) | 1.02 (0.08–7.68) | 2.19 (0.26–6.72) | 0.0001 # | 1.44 (0.08–7.68) | 1.44 (0.15–6.72) | 0.621 # |
| Absolute lymphocyte count (×109/L), median (range) | 1.4 (0.09–3.65) | 1.29 (0.09–3.42) | 1.62 (0.25–3.65) | 0.0001 # | 0.82 (0.09–1.19) | 1.65 (1.0–3.65) | / |
| Absolute monocyte count (×109/L), median (range) | 0.22 (0–0.99) | 0.13 (0–0.29) | 0.55 (0.3–0.99) | / | 0.18 (0–0.98) | 0.23 (0–0.99) | 0.081 # |
| PB blasts (% median, range) | 0 (0–13) | 0 (0–13) | 0 (0–7) | 0.597 # | 0 (0–13) | 0 (0–9) | 0.585 # |
| BM blasts (% median, range) | 6 (1–18) | 6 (1–18) | 3.5 (1–18) | 0.005 # | 6 (1–18) | 5.5 (1–18) | 0.489 # |
| Characteristics | Value; % n = 217 | AMC < 0.3 × 109/L (n = 133/61.3; %) | AMC ≥ 0.3 × 109/L (n = 84/38.7; %) | p Value | ALC < 1.2 × 109/L (n = 77/35.5; %) | ALC ≥ 1.2 × 109/L (n = 140/64.5; %) | p Value | |
|---|---|---|---|---|---|---|---|---|
| WHO 2016, n (%)) | MDS-SLD | 28 (12.9) | 16 (57.1) | 12 (42.9) | 0.223 ♦ 0.414 # | 13 (46.4) | 15 (53.6) | 0.069 ♦ 0.805 # |
| MDS-MLD | 64 (29.5) | 38 (59.3) | 26 (40.7) | 24 (37.5) | 40 (62.5) | |||
| MDS-SLD/MLD-RS | 12 (5.5) | 4 (33.3) | 8 (66.7) | 0 (0) | 12 (100) | |||
| MDS(del5q) | 8 (3.7) | 4 (50) | 4 (50) | 3 (37.5) | 5 (62.5) | |||
| MDS-EB-1 | 44 (20.3) | 27 (61.4) | 17 (38.6) | 11 (25) | 33 (75) | |||
| MDS-EB-2 | 61 (28.1) | 44 (72.1) | 17 (27.9) | 26 (42.6) | 35 (57.4) | |||
| MDS-U | 0 | 0 | 0 | 0 | 0 | |||
| IPSS-R, n (%) | Very low | 22 (10.1) | 11 (50) | 11 (50) | 0.007 ♦ 0.001 # | 12 (54.5) | 10 (45.5) | 0.336 ♦ 0.515 # |
| Low | 60 (27.7) | 28 (46.7) | 32 (53.3) | 19 (31.7) | 41 (68.3) | |||
| Intermediate | 69 (31.8) | 43 (62.3) | 26 (37.7) | 23 (33.3) | 46 (66.7) | |||
| High | 44 (20.3) | 35 (79.5) | 9 (20.5) | 14 (31.8) | 30 (68.2) | |||
| Very high | 22 (10.1) | 16 (72.7) | 6 (27.3) | 9 (40.9) | 13 (59.1) | |||
| Cytogenetics | Very good | 4 (1.8) | 2 (50) | 2 (50) | 0.006 ♦ 0.002 # | 4 (100) | 0 | 0.107 ♦ 0.555 # |
| Good | 172 (79.3) | 96 (55.8) | 76 (44.2) | 58 (33.7) | 114 (66.3) | |||
| Intermediate | 24 (11) | 22 (91.7) | 2 (8.3) | 9 (37.5) | 15 (62.5) | |||
| Poor | 5 (2.3) | 3 (60) | 2 (40) | 2 (40) | 3 (60) | |||
| Very poor | 12 (5.6) | 10 (83.3) | 2 (16.7) | 4 (33.3) | 8 (66.7) | |||
| Transfusion dependent, n (%) | 54 (24.9) | 39 (72.2) | 15 (27.8) | 0.049 ♦ | 22 (40.7) | 32 (59.3) | 0.382 ♦ | |
| Progression of disease and leukemic transformation (%) | 40 (18.4) | 28 (70) | 12 (30) | 0.210 ♦ | 8 (20) | 32 (80) | 0.016 ♦ | |
| Deceased (%) | 96 (44.2) | 71 (73.9) | 25 (26.1) | 0.0001 ♦ | 39 (40.6) | 57 (59.4) | 0.158 ♦ |
| (A) | ||||
| n = 217 | Beta | p Value | HR | CI 95% |
| IPSS-R (risk groups) | 0.4795 | 0.0000 | 1.6153 | 1.3396–1.9478 |
| AMC < 0.3 × 109/L | 0.5472 | 0.0237 | 1.7284 | 1.0758–2.7768 |
| ALC < 1.2 × 109/L | 0.5903 | 0.0060 | 1.8045 | 1.1845–2.7492 |
| DMAs/HSCT | 1.3399 | 0.0000 | 3.8190 | 2.1716–6.7160 |
| (B) | ||||
| n = 157 | Beta | p Value | HR | CI 95% |
| IPSS-R > 3 | 0.5612 | 0.0000 | 2.9273 | 1.7528–4.8886 |
| AMC < 0.3 × 109/L | 0.0434 | 0.0329 | 1.7065 | 1.0444–2.7885 |
| ALC < 1.2 × 109/L | 0.0945 | 0.0184 | 1.7525 | 1.0993–2.7937 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virijevic, M.; Jakovic, L.; Trajkovic, L.; Cvetkovic, M.; Pravdic, Z.; Mitrovic, M.; Suvajdzic-Vukovic, N.; Bogdanovic, A. Improving MDS Risk Assessment: The Role of Monocytopenia and Lymphocytopenia Beyond IPSS-R. Medicina 2025, 61, 1689. https://doi.org/10.3390/medicina61091689
Virijevic M, Jakovic L, Trajkovic L, Cvetkovic M, Pravdic Z, Mitrovic M, Suvajdzic-Vukovic N, Bogdanovic A. Improving MDS Risk Assessment: The Role of Monocytopenia and Lymphocytopenia Beyond IPSS-R. Medicina. 2025; 61(9):1689. https://doi.org/10.3390/medicina61091689
Chicago/Turabian StyleVirijevic, Marijana, Ljubomir Jakovic, Lazar Trajkovic, Mirjana Cvetkovic, Zlatko Pravdic, Mirjana Mitrovic, Nada Suvajdzic-Vukovic, and Andrija Bogdanovic. 2025. "Improving MDS Risk Assessment: The Role of Monocytopenia and Lymphocytopenia Beyond IPSS-R" Medicina 61, no. 9: 1689. https://doi.org/10.3390/medicina61091689
APA StyleVirijevic, M., Jakovic, L., Trajkovic, L., Cvetkovic, M., Pravdic, Z., Mitrovic, M., Suvajdzic-Vukovic, N., & Bogdanovic, A. (2025). Improving MDS Risk Assessment: The Role of Monocytopenia and Lymphocytopenia Beyond IPSS-R. Medicina, 61(9), 1689. https://doi.org/10.3390/medicina61091689






