Impact of Pancreatic Stump Wrapping with Mesh on Post-Operative Pancreatic Fistula in Patients Undergoing Distal/Left Pancreatectomy for Malignant or Benign Diseases: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Outcomes
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
2.7. Summary of Findings and Assessment of the Certainty of the Evidence
2.7.1. Summary of Findings Tables
- Overall Post-Operative Pancreatic Fistula
- Clinically Relevant Post-Operative Pancreatic Fistula
2.7.2. Assessment of the Certainty of the Evidence
- Study limitations: moderate (−1) or serious (−2) risk of bias; in addition, for non-randomized studies (−3) for critical risk of bias
- Inconsistency: serious (−1) or very serious (−2) inconsistency
- Indirectness: serious (−1) or very serious (−2) uncertainty about directness
- Imprecision: serious (−1) or very serious (−2) imprecise or sparse data
- Publication bias: serious (−1) or very serious (−2) probability of reporting bias
3. Results
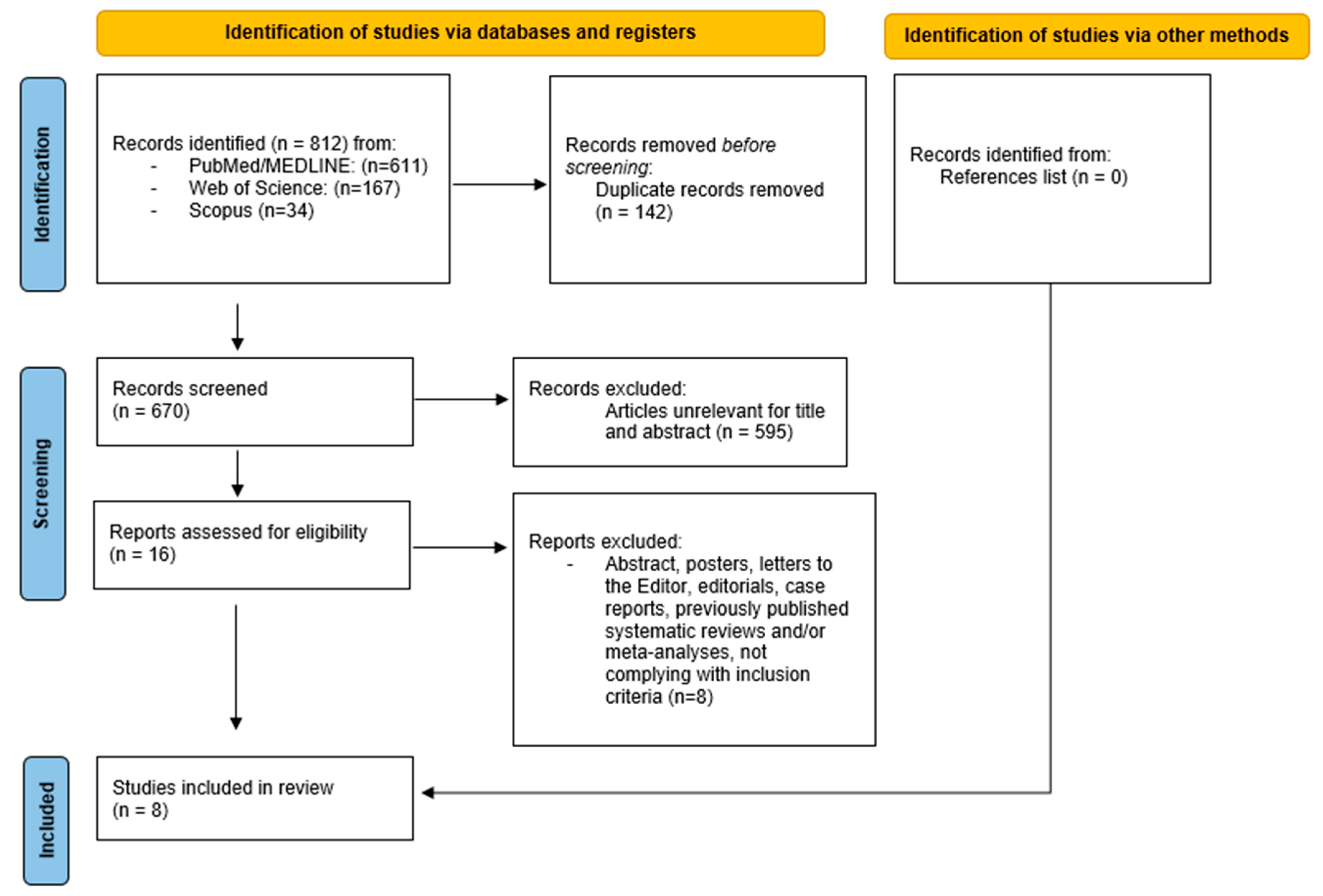
3.1. Search Results
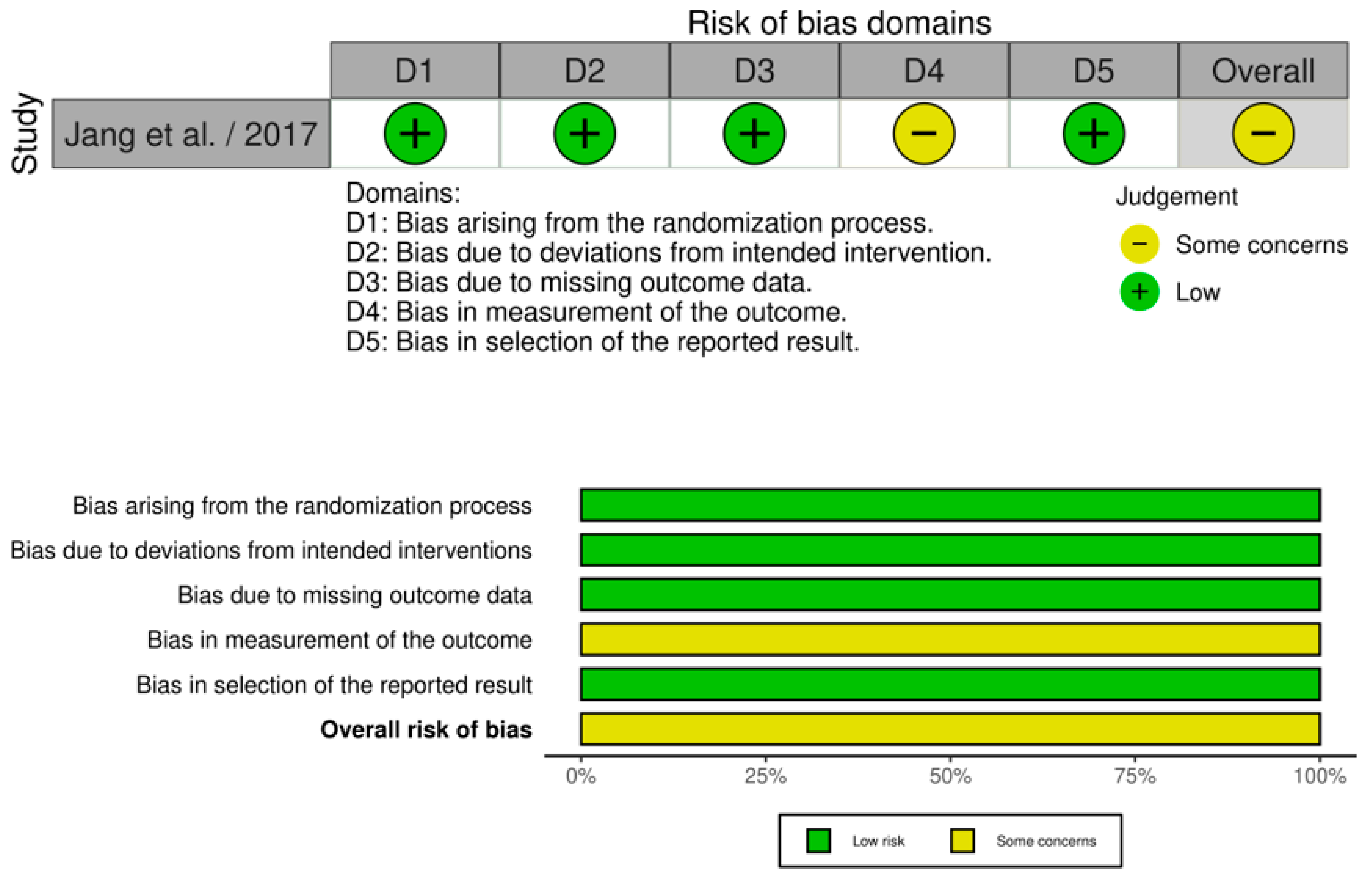
3.2. Quality of Studies
3.3. Study and Population Characteristics
3.4. Meta-Analyses Results
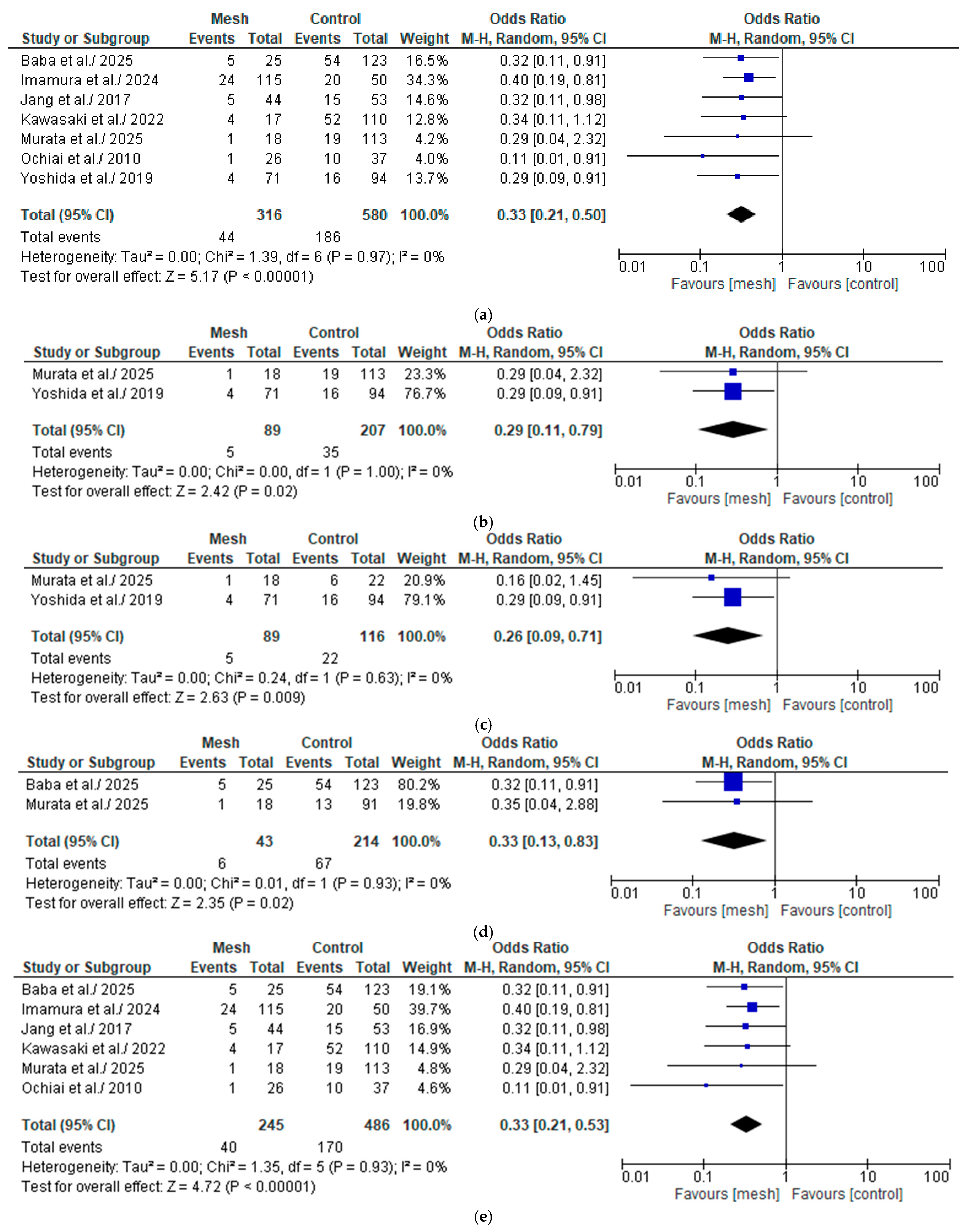
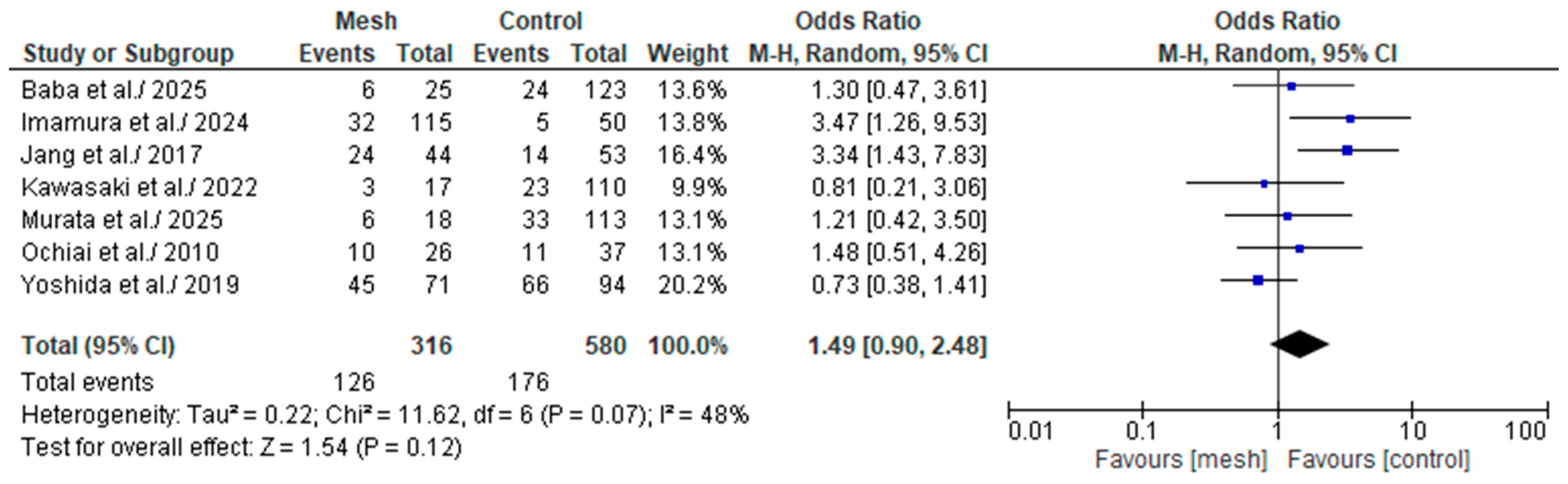
3.4.1. Overall Post-Operative Pancreatic Fistula
Wrapping Mesh Group vs. Control Group
Wrapping Mesh Group (Only with Transpancreatic Mattress Suture) vs. Control Group
Wrapping Mesh Group vs. Control Group (Only with Hand-Sewn Closure Technique)
Wrapping Mesh Group vs. Control Group (Only with Reinforced Stapler Suture Closure)
Wrapping Mesh Group (Only with PGA Mesh) vs. Control Group
Wrapping Mesh Group (Only with PGA Mesh and Without Reinforced Stapler Suture Closure) vs. Control Group
3.4.2. Clinically Relevant Post-Operative Pancreatic Fistula
Wrapping Mesh Group vs. Control Group
Wrapping Mesh Group (Only with Transpancreatic Mattress Suture) vs. Control Group
Wrapping Mesh Group vs. Control Group (Only with Hand-Sewn Closure Technique)
Wrapping Mesh Group vs. Control Group (Only with Reinforced Stapler Suture Closure)
Wrapping Mesh Group (Only with PGA Mesh) vs. Control Group
Wrapping Mesh Group (Only with PGA Mesh and Without Reinforced Stapler Suture Closure) vs. Control Group
3.4.3. Overall Operative Time
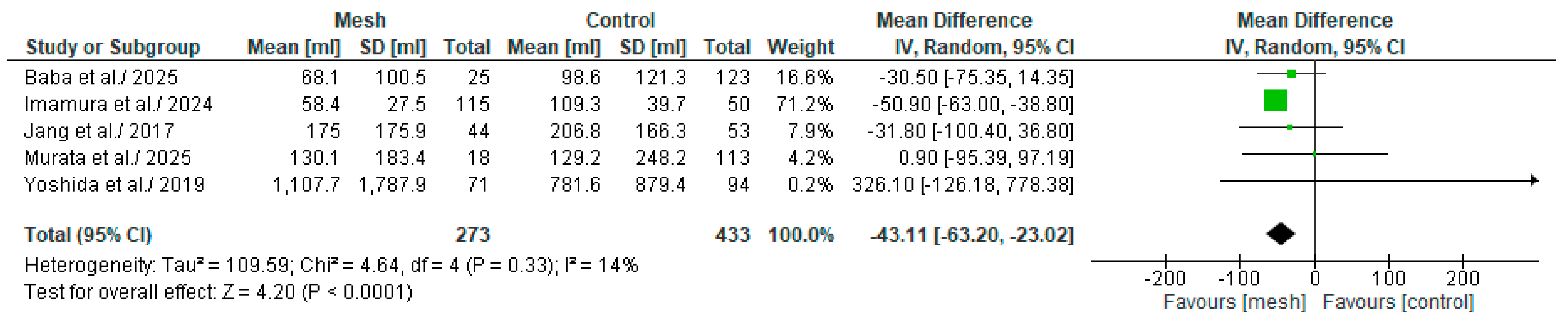
3.4.4. Estimated Blood Loss
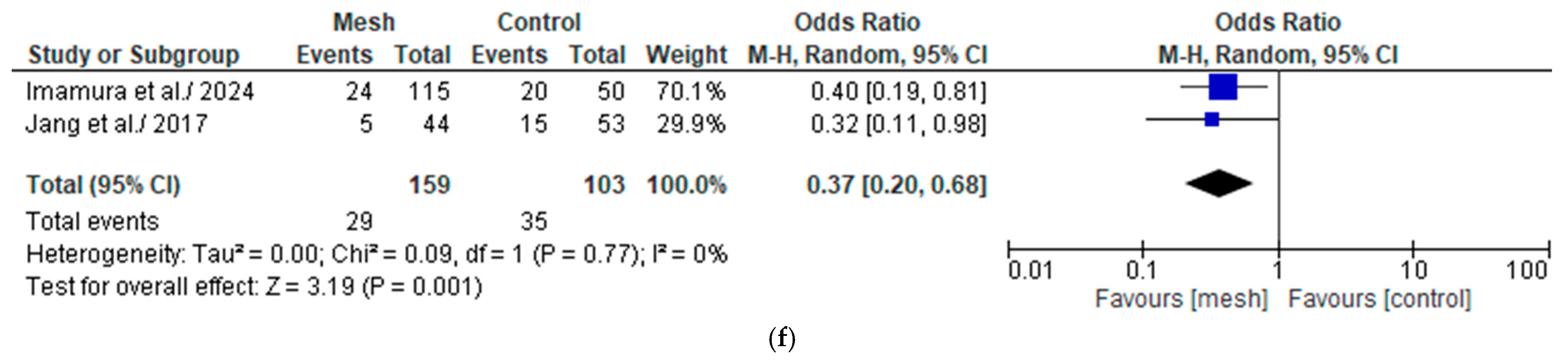
3.4.5. Biochemical Leak/Grade A Post-Operative Pancreatic Fistula
3.4.6. Grade B Post-Operative Pancreatic Fistula
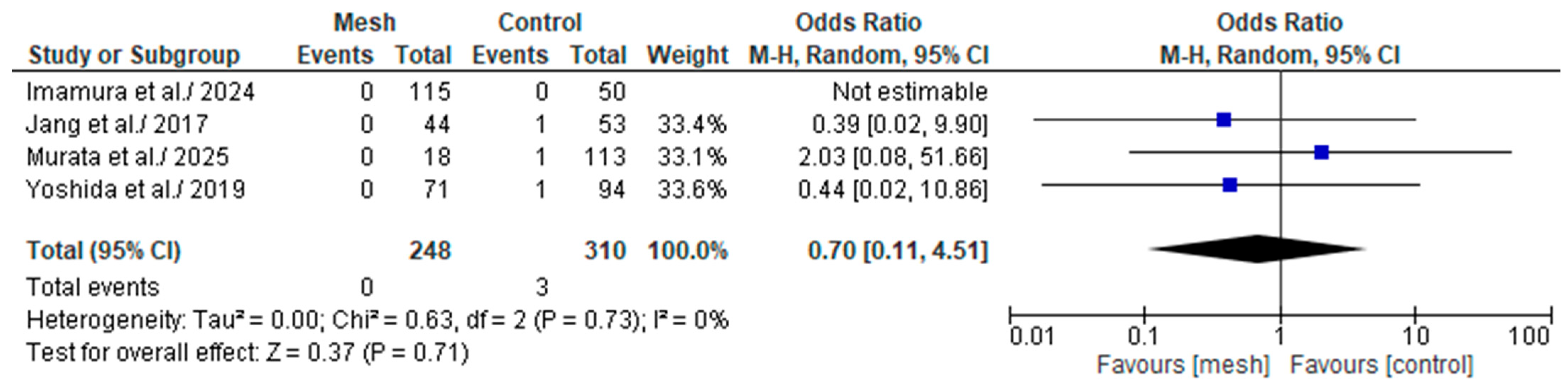
3.4.7. Grade C Post-Operative Pancreatic Fistula
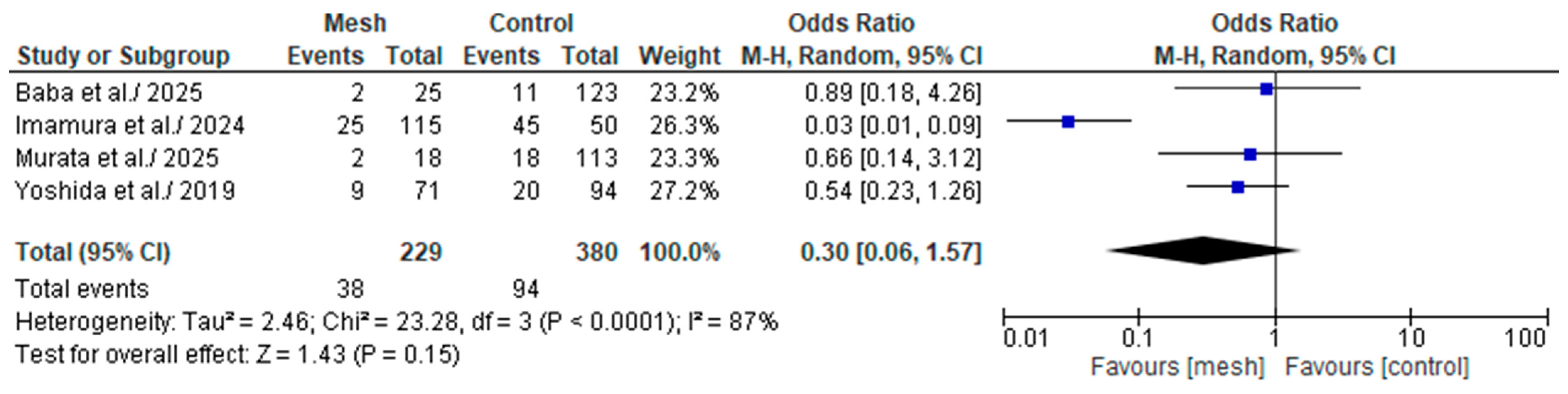
3.4.8. Major (Clavien-Dindo or CD ≥ III) Postoperative Complications
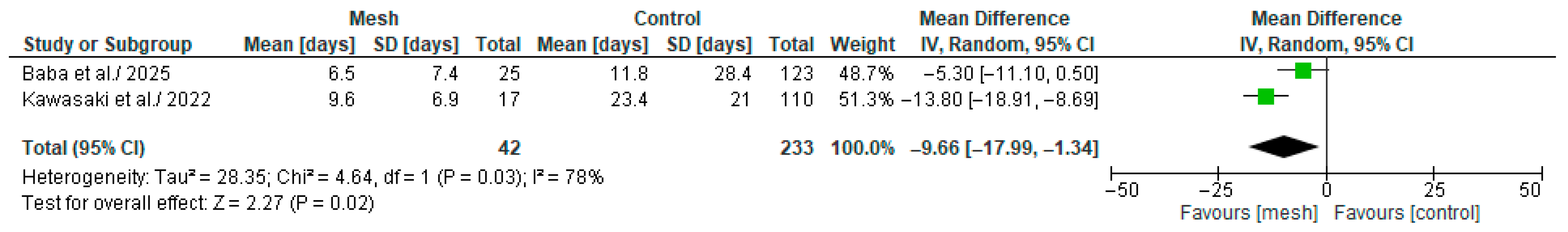
3.4.9. Time Required to Remove the Drain
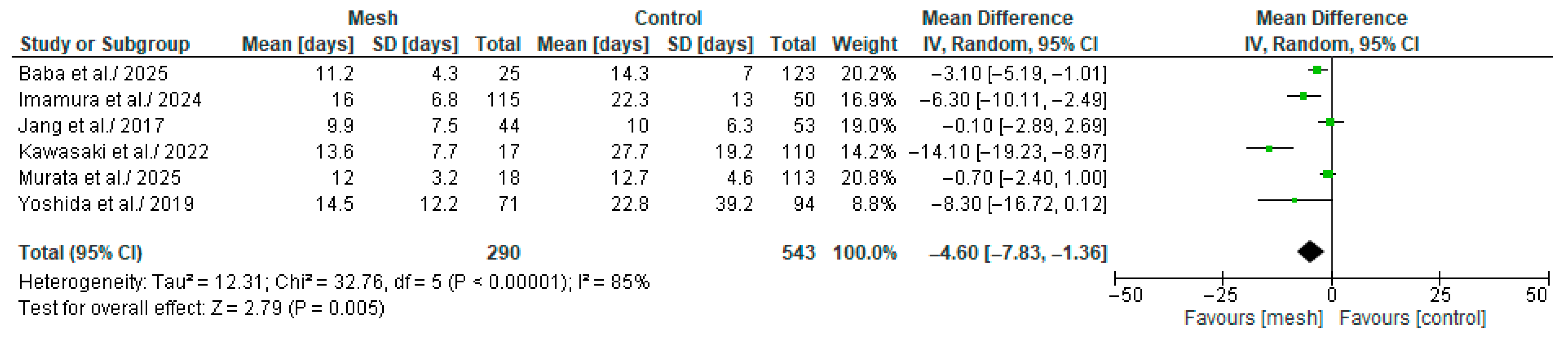
3.4.10. Length of Hospital Stay
3.4.11. Readmission
3.4.12. Publication Bias
4. Discussion
4.1. Discussion
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SoF | summary of findings |
| RCS | retrospective cohort studies |
| RCT | randomized controlled trial |
| PGA | polyglycolic acid |
| MPD | Main Pancreatic Duct |
| RSSC | Reinforced Stapler Suture Closure |
| NRU | Not Routinely Used |
| n | number |
| N/A | Not available |
| POPF | Post-Operative Pancreatic Fistula |
| BL | Biochemical Leak |
| CD | Clavien-Dindo |
| CR | Clinically Relevant |
| RevMan | Review Manager |
| OR | odds ratios |
| Cis | confidence intervals |
| MH | Mantel-Haenszel |
| WMD | weighted mean differences |
| SD | standard deviation |
| IQR | interquartile range |
| OP | operative time |
| WMG | Wrapping Mesh Group |
| CG | Control group |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Partyka, O.; Pajewska, M.; Kwaśniewska, D.; Czerw, A.; Deptała, A.; Budzik, M.; Cipora, E.; Gąska, I.; Gazdowicz, L.; Mielnik, A.; et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers 2023, 15, 3634. [Google Scholar] [CrossRef] [PubMed]
- Knaebel, H.P.; Diener, M.K.; Wente, M.N.; Büchler, M.W.; Seiler, C.M. Systematic Review and Meta-Analysis of Technique for Closure of the Pancreatic Remnant after Distal Pancreatectomy. Br. J. Surg. 2005, 92, 539–546. [Google Scholar] [CrossRef]
- Rodríguez, J.R.; Germes, S.S.; Pandharipande, P.V.; Gazelle, G.S.; Thayer, S.P.; Warshaw, A.L.; Fernández-del Castillo, C. Implications and Cost of Pancreatic Leak Following Distal Pancreatic Resection. Arch. Surg. 2006, 141, 361–365; discussion 366. [Google Scholar] [CrossRef] [PubMed]
- Paye, F.; Micelli Lupinacci, R.; Bachellier, P.; Boher, J.-M.; Delpero, J.-R. French Surgical Association (AFC) Distal Pancreatectomy for Pancreatic Carcinoma in the Era of Multimodal Treatment. Br. J. Surg. 2015, 102, 229–236. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 Update of the International Study Group (ISGPS) Definition and Grading of Postoperative Pancreatic Fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M. Postoperative Pancreatic Fistula: An International Study Group (ISGPF) Definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef]
- Miao, Y.; Lu, Z.; Yeo, C.J.; Vollmer, C.M.; Fernandez-del Castillo, C.; Ghaneh, P.; Halloran, C.M.; Kleeff, J.; De Rooij, T.; Werner, J.; et al. Management of the Pancreatic Transection Plane after Left (Distal) Pancreatectomy: Expert Consensus Guidelines by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2020, 168, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; (Updated August 2024); Cochrane: London, UK, 2024; Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current (accessed on 10 May 2025).
- Zhang, W.; Wei, Z.; Che, X. Effect of Polyglycolic Acid Mesh for Prevention of Pancreatic Fistula after Pancreatectomy: A Sytematic Review and Meta-Analysis. Medicine 2020, 99, e21456. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence (last updated August 2023). In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2024; Available online: https://www.training.cochrane.org/handbook (accessed on 10 May 2025).
- Baba, H.; Oba, A.; Maekawa, A.; Kiritani, S.; Kobayashi, H.; Takahashi, A.; Omiya, K.; Kobayashi, K.; Ono, Y.; Sato, T.; et al. Polyglycolic Acid Mesh and Fibrin Glue in Minimally Invasive Left Pancreatectomy: Video Technique and Single-Center Data. Ann. Surg. Oncol. 2025, 32, 5102–5103. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Kimura, Y.; Kukita, K.; Murakami, T.; Kato, T.; Kyuno, D.; Takemasa, I. Powered Stapler and Polyglycolic Acid Sheet for Pancreatic Fistula after Distal Pancreatectomy. J. Gastrointest. Surg. 2024, 28, 2008–2014. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Shin, Y.C.; Han, Y.; Park, J.S.; Han, H.-S.; Hwang, H.K.; Yoon, D.S.; Kim, J.K.; Yoon, Y.S.; Hwang, D.W.; et al. Effect of Polyglycolic Acid Mesh for Prevention of Pancreatic Fistula Following Distal Pancreatectomy: A Randomized Clinical Trial. JAMA Surg. 2017, 152, 150. [Google Scholar] [CrossRef]
- Kawasaki, K.; Hayashi, T.; Takahashi, M.; Morita, Y. Covering Reinforced Staples with Polyethylene Glycolic Acid Felt-Covered Fibrin Sealant to Prevent Pancreatic Fistula after Distal Pancreatomy: A Retrospective Comparative Study. BMC Surg. 2022, 22, 349. [Google Scholar] [CrossRef]
- Murata, Y.; Komatsubara, H.; Noguchi, D.; Ito, T.; Hayasaki, A.; Iizawa, Y.; Fujii, T.; Tanemura, A.; Kuriyama, N.; Kishiwada, M.; et al. Effect of Transpancreatic Mattress Suture with Polyglycolic Acid Sheet in Pancreatic Stump Closure for the Prevention of Postoperative Pancreatic Fistula in Robotic Distal Pancreatectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2025, 35, e1345. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Sonoyama, T.; Soga, K.; Inoue, K.; Ikoma, H.; Shiozaki, A.; Kuriu, Y.; Kubota, T.; Nakanishi, M.; Kikuchi, S.; et al. Application of Polyethylene Glycolic Acid Felt with Fibrin Sealant to Prevent Postoperative Pancreatic Fistula in Pancreatic Surgery. J. Gastrointest. Surg. 2010, 14, 884–890. [Google Scholar] [CrossRef]
- Yoshida, Y.; Matsumoto, I.; Matsumoto, M.; Kawaguchi, K.; Murase, T.; Kamei, K.; Satoi, S.; Takebe, A.; Nakai, T.; Takeyama, Y. Transpancreatic Mattress Suture with Vicryl Mesh around the Stump Decreases Postoperative Pancreatic Fistula after Distal Pancreatectomy. J. Hepato Biliary Pancreat. 2019, 26, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Ban, D.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Kudo, A.; Tanaka, S.; Tanabe, M. The Clinical Implications of Peripancreatic Fluid Collection After Distal Pancreatectomy. World J. Surg. 2019, 43, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Egger, M.; Moher, D.; on Behalf of the Cochrane Bias Methods Group. Cochrane Handbook for Systematic Reviews of Interventions [Version 5.1.0]; Chapter 10: Addressing Reporting Biases; Cochrane: London, UK, 2011. [Google Scholar]
- Park, S.-J.; Seo, H.-I.; Go, S.-H.; Yun, S.-P.; Lee, J.-Y. Complication Analysis of Distal Pancreatectomy Based on Early Personal Experience. Korean J. Hepatobiliary Pancreat. Surg. 2011, 15, 243–247. [Google Scholar] [CrossRef]
- Ecker, B.L.; McMillan, M.T.; Allegrini, V.; Bassi, C.; Beane, J.D.; Beckman, R.M.; Behrman, S.W.; Dickson, E.J.; Callery, M.P.; Christein, J.D.; et al. Risk Factors and Mitigation Strategies for Pancreatic Fistula After Distal Pancreatectomy: Analysis of 2026 R sections From the International, Multi-Institutional Distal Pancreatectomy Study Group. Ann. Surg. 2019, 269, 143. [Google Scholar] [CrossRef]
- Chen, J.W.; van Ramshorst, T.M.E.; Lof, S.; Al-Sarireh, B.; Bjornsson, B.; Boggi, U.; Burdio, F.; Butturini, G.; Casadei, R.; Coratti, A.; et al. Robot-Assisted Versus Laparoscopic Distal Pancreatectomy in Patients with Resectable Pancreatic Cancer: An International, Retrospective, Cohort Study. Ann. Surg. Oncol. 2023, 30, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Silva Nascimento, A.; Silva, O.D.C.; Duarte, B.N.; De Carvalho Baptista Barbosa, I.; Bajou, C.M.O.; Taba, J.V.; Pipek, L.Z.; Iuamoto, L.R.; Hsing, W.T.; Carneiro-D’Albuquerque, L.A.; et al. Suture versus Stapler in Distal Pancreatectomy and Its Impact on Postoperative Pancreatic Fistula. Sci. Rep. 2025, 15, 6052. [Google Scholar] [CrossRef]
- Golling, M.; Schaudt, A.; Mehrabi, A.; Mood, Z.A.; Bechstein, W.O. Clinical Application of Soft Polyglycolic Acid Felt for Hmostasis and Repair of a Lacerated Liver: Report of Two Cases. Surg. Today 2008, 38, 188–192. [Google Scholar] [CrossRef]
- Itano, H. The Optimal Technique for Combined Application of Fibrin Sealant and Bioabsorbable Felt against Alveolar Air Lea age. Eur. J. Cardiothorac. Surg. 2008, 33, 457–460. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, I.; Takeyama, Y.; Kamei, K.; Satoi, S.; Nakata, Y.; Ishikawa, H.; Murase, T.; Matsumoto, M.; Nakai, T. Transpancratic Mattress Suture with Vicryl Mesh Around the Stump During Distal Pancreatectomy: A Novel Technique for Preventing Postoerative Pancreatic Fistula. J. Am. Coll. Surg. 2016, 223, e1–e5. [Google Scholar] [CrossRef]
- Elkomos, B.E.; Elkomos, P.E.; Salem, A.A.; Adly, P.B. The Outcome of Bioabsorbable Staple Line Reinforcement versus Standard Stapler for Distal Pancreatectomy: A Systematic Review and Meta-Analysis. J. Minimal Access Surg. 2022, 18, 338–345. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic Acid Induced Inflammation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef]
- Verheggen, R.; Schulte-Baumann, W.J.; Hahm, G.; Lang, J.; Freudenthaler, S.; Schaake, T.; Markakis, E. A New Technique of Dural Closure--Experience with a Vicryl Mesh. Acta Neurochir. 1997, 139, 1074–1079. [Google Scholar] [CrossRef]
- Tobias, A.M.; Low, D.W. The Use of a Subfascial Vicryl Mesh Buttress to Aid in the Closure of Massive Ventral Hernias Folloing Damage-Control Laparotomy. Plast. Reconstr. Surg. 2003, 112, 766–776. [Google Scholar] [CrossRef]
- Madhuvrata, P.; Glazener, C.; Boachie, C.; Allahdin, S.; Bain, C. A Randomised Controlled Trial Evaluating the Use of Polyglatin (Vicryl) Mesh, Polydioxanone (PDS) or Polyglactin (Vicryl) Sutures for Pelvic Organ Prolapse Surgery: Outcomes at 2 Years. J. Obstet. Gynaecol. 2011, 31, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Abudalu, M.; Aqawi, M.; Sionov, R.V.; Friedman, M.; Gati, I.; Munz, Y.; Ohana, G.; Steinberg, D. Polyglactin 910 Meshes Coated with Sustained-Release Cannabigerol Varnish Inhibit Staphylococcus Aureus Biofilm Formation and Macrophage Cytokine Secrtion: An In Vitro Study. Pharmaceuticals 2023, 16, 745. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Y.; Yang, S.; Nie, G.; Wen, N.; Zhang, Y.; Cheng, N.; Xiong, X.; Lu, J.; Liu, G.; et al. Surgery-Related Factors for Pancreatic Fistula after Pancreatectomy: An Umbrella Review. Hepatobiliary Surg. Nutr. 2025, 14, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Misawa, T.; Shiba, H.; Usuba, T.; Nojiri, T.; Uwagawa, T.; Ishida, Y.; Ishii, Y.; Yanaga, K. Safe and Quick Distal Pancreatectomy Using a Staggered Six-Row Stapler. Am. J. Surg. 2008, 195, 115–118. [Google Scholar] [CrossRef]
- Chang, Y.R.; Kang, J.S.; Jang, J.-Y.; Jung, W.H.; Kang, M.J.; Lee, K.B.; Kim, S.-W. Prediction of Pancreatic Fistula After Distal Pancreatectomy Based on Cross-Sectional Images. World J. Surg. 2017, 41, 1610–1617. [Google Scholar] [CrossRef]
- Okano, K.; Oshima, M.; Kakinoki, K.; Yamamoto, N.; Akamoto, S.; Yachida, S.; Hagiike, M.; Kamada, H.; Masaki, T.; Suzuki, Y. Pancreatic Thickness as a Predictive Factor for Postoperative Pancreatic Fistula after Distal Pancreatectomy Using an Endopath Stpler. Surg. Today 2013, 43, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.-Y.; Son, D.; Lee, S.; Han, Y.; Shin, Y.C.; Kim, J.R.; Kwon, W.; Kim, S.-W. Optimal Stapler Cartridge Selection According to the Thickness of the Pancreas in Distal Pancreatectomy. Medicine 2016, 95, e4441. [Google Scholar] [CrossRef] [PubMed]
- van Bodegraven, E.A.; Balduzzi, A.; van Ramshorst, T.M.E.; Malleo, G.; Vissers, F.L.; van Hilst, J.; Festen, S.; Hilal, M.A.; Asbun, H.J.; Michiels, N.; et al. Prophylactic Abdominal Drainage after Distal Pancreatectomy (PANDORINA): An International, Multicetre, Open-Label, Randomised Controlled, Non-Inferiority Trial. Lancet Gastroenterol. Hepatol. 2024, 9, 438–447. [Google Scholar] [CrossRef]
- Li, T.; D’Cruz, R.T.; Lim, S.Y.; Shelat, V.G. Somatostatin Analogues and the Risk of Post-Operative Pancreatic Fistulas after Pancreatic Resection—A Systematic Review & Meta-Analysis. Pancreatology 2020, 20, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Schorn, S.; Vogel, T.; Demir, I.E.; Demir, E.; Safak, O.; Friess, H.; Ceyhan, G.O. Do Somatostatin-Analogues Have the Same Impact on Postoperative Morbidity and Pancreatic Fistula in Patients after Pancreaticoduodenectomy and Distal Pancreatectomy? A Systematic Review with Meta-Analysis of Randomized-Controlled Trials. Pancreatology 2020, 20, 1770–1778. [Google Scholar] [CrossRef] [PubMed]





| Authors/Year | Study Type | Study Period | Study Country | Type of Mesh | Cohort Sample | Study Groups | No of Patients [n] | Sex (m/f) [n] | Age [Years] Mean (SD) | BMI [kg/m2] Mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Baba et al., 2025 [18] | RCS | 2020 to 2023 | Japan | PGA | 148 | PGA mesh + Reinforced stapler suture closure + fibrin glue | 25 | 11/14 | 67.4 (8.9) | 21.3 (4.5) |
| Reinforced stapler suture closure | 123 | 55/68 | 67.6 (13.6) | 22.1 (2.9) | ||||||
| Imamura et al., 2024 [19] | RCS | Jan 2013 to Aug 2023 | Japan | PGA | 165 | PGA mesh + powered stapler closure + fibrin glue | 115 | 58/57 | 68.5 (11.6) | 23.1 (3.8) |
| Manual stapler closure or hand-sewn closure | 50 | 23/27 | 67.1 (14.4) | 21.7 (2.5) | ||||||
| Jang et al., 2017 [20] | RCT | Nov 2011 to Apr 2014 | Korea | PGA | 97 | PGA + fibrin glue | 44 | 19/25 | 59.9 (12) | N/A |
| Stapler closure | 53 | 20/33 | 54.5 (14.1) | N/A | ||||||
| Kawasaki et al., 2022 [21] | RCS | Jan 2018 to Jun 2021 | Japan | PGA | 127 | PGA + fibrin glue | 17 | N/A | N/A | N/A |
| Reinforced stapler suture closure or stapler closure or MPD ligation | 110 | N/A | N/A | N/A | ||||||
| Murata et al., 2025 [22] | RCS | Feb 2011 to Jul 2024 | Japan | PGA | 131 | PGA + transpancreatic mattress suture | 18 | 11/7 | 63.1 (23.3) | 22.5 (3.5) |
| Hand-sewn closure (fish–mouth manner) | 22 | 13/9 | 58.0 (20.6) | 22.4 (3.1) | ||||||
| Reinforced stapler suture closure | 91 | 43/48 | 66.2 (19.6) | 22.1 (3.2) | ||||||
| Ochiai et al., 2010 [23] | RCS | May 2003 to Apr 2008 | Japan | PGA | 63 | PGA mesh + fibrin glue + Reinforced stapler suture closure (NRU) | 26 | N/A | N/A | N/A |
| Hand-sewn or stapler closure | 37 | N/A | N/A | N/A | ||||||
| Yoshida et al., 2019 [24] | RCS | Jan 2010 to May 2018 | Japan | Polyglactin | 165 | Polyglactin mesh + transpancreatic mattress suture | 71 | 44/27 | 64.9 (11.8) | 21.4 (3.7) |
| Hand-sewn closure | 94 | 48/46 | 67.1 (13.5) | 22.3 (4.6) | ||||||
| Yoshino et al., 2019 [25] | RCS | Jan 2006 to Dec 2017 | Japan | PGA | 146 | PGA mesh + fibrin glue | 114 | N/A | N/A | N/A |
| Hand-sewn or stapler closure | 32 | N/A | N/A | N/A |
| Authors/Year | Study Groups | No of Patients [n] | Overall POPF [n] | BL/Grade A POPF [n] | Grade B POPF [n] | Grade C POPF [n] | CR-POPF [n] |
|---|---|---|---|---|---|---|---|
| Baba et al., 2025 [18] | PGA mesh + Reinforced stapler suture closure + fibrin glue | 25 | 11 | 6 | N/A | N/A | 5 |
| Reinforced stapler suture closure | 123 | 78 | 24 | N/A | N/A | 54 | |
| Imamura et al., 2024 [19] | PGA mesh + powered stapler closure + fibrin glue | 115 | 56 | 32 | 24 | 0 | 24 |
| Manual stapler closure or hand-sewn closure | 50 | 25 | 5 | 20 | 0 | 20 | |
| Jang et al., 2017 [20] | PGA + fibrin glue | 44 | 29 | 24 | 5 | 0 | 5 |
| Stapler closure | 53 | 29 | 14 | 14 | 1 | 15 | |
| Kawasaki et al., 2022 [21] | PGA + fibrin glue | 17 | 7 | 3 | N/A | N/A | 4 |
| Reinforced stapler suture closure or stapler closure or MPD ligation | 110 | 75 | 23 | N/A | N/A | 52 | |
| Murata et al., 2025 [22] | PGA + transpancreatic mattress suture | 18 | 7 | 6 | 1 | 0 | 1 |
| Hand-sewn closure (fish–mouth manner) | 22 | 11 | 5 | 5 | 1 | 6 | |
| Reinforced stapler suture closure | 91 | 41 | 28 | 13 | 0 | 13 | |
| Ochiai et al., 2010 [23] | PGA mesh + fibrin glue + Reinforced stapler suture closure (NRU) | 26 | 11 | 10 | N/A | N/A | 1 |
| Hand-sewn or stapler closure | 37 | 21 | 11 | N/A | N/A | 10 | |
| Yoshida et al., 2019 [24] | Polyglactin mesh + transpancreatic mattress suture | 71 | 49 | 45 | 4 | 0 | 4 |
| Hand-sewn closure | 94 | 82 | 66 | 15 | 1 | 16 | |
| Yoshino et al., 2019 [25] | PGA mesh + fibrin glue | 114 | 15 | N/A | N/A | N/A | N/A |
| Hand-sewn or stapler closure | 32 | 11 | N/A | N/A | N/A | N/A |
| Authors/Year | Study Groups | No of Patients [n] | Operative Time [min] Mean (SD) | Estimated Blood Loss [ml] Mean (SD) | Time Required to Drain Removal [Days] Mean (SD) | Post-op. Complication (CD ≥ III) [n] | Length of Hospital Stay [Days] Mean (SD) | No of Re-Admission [n] |
|---|---|---|---|---|---|---|---|---|
| Baba et al., 2025 [18] | PGA mesh + Reinforced stapler suture closure + fibrin glue | 25 | 348.5 (135.4) | 68.1 (100.5) | 6.5 (7.4) | 2 | 11.2 (4.3) | 1 |
| Reinforced stapler suture closure | 123 | 347.5 (74.7) | 98.6 (121.3) | 11.8 (28.4) | 11 | 14.3 (7.0) | 4 | |
| Imamura et al., 2024 [19] | PGA mesh + powered stapler closure + fibrin glue | 115 | 347.7 (25.3) | 58.4 (27.5) | N/A | 25 | 16 (6.8) | 3 |
| Manual stapler closure or hand-sewn closure | 50 | 338.5 (27.9) | 109.3 (39.7) | N/A | 45 | 22.3 (13) | 1 | |
| Jang et al., 2017 [20] | PGA + fibrin glue | 44 | 152.6 (58.3) | 175 (175.9) | N/A | N/A | 9.9 (7.5) | N/A |
| Stapler closure | 53 | 157.9 (57.6) | 206.8 (166.3) | N/A | N/A | 10 (6.3) | N/A | |
| Kawasaki et al., 2022 [21] | PGA + fibrin glue | 17 | N/A | N/A | 9.6 (6.9) | N/A | 13.6 (7.7) | N/A |
| Reinforced stapler suture closure or stapler closure or MPD ligation | 110 | N/A | N/A | 23.4 (21) | N/A | 27.7 (19.2) | N/A | |
| Murata et al., 2025 [22] | PGA + transpancreatic mattress suture | 18 | 501.9 (118.6) | 130.1 (183.4) | N/A | 2 | 12 (3.2) | 1 |
| Hand-sewn closure (fish–mouth manner) | 22 | 372.2 (242.5) | 223 (450.1) | N/A | 3 | 14 (4.8) | 1 | |
| Reinforced stapler suture closure | 91 | 352.8 (95.7) | 106.5 (163.5) | N/A | 15 | 12.4 (4.5) | 9 | |
| Ochiai et al., 2010 [23] | PGA mesh + fibrin glue + Reinforced stapler suture closure (NRU) | 26 | N/A | N/A | N/A | N/A | N/A | N/A |
| Hand-sewn or stapler closure | 37 | N/A | N/A | N/A | N/A | N/A | N/A | |
| Yoshida et al., 2019 [24] | Polyglactin mesh + transpancreatic mattress suture | 71 | 232.5 (96.8) | 1107.7 (1787.9) | N/A | 9 | 14.5 (12.2) | 4 |
| Hand-sewn closure | 94 | 205.9 (86.2) | 781.6 (879.4) | N/A | 20 | 22.8 (39.2) | 7 | |
| Yoshino et al., 2019 [25] | PGA mesh + fibrin glue | 114 | N/A | N/A | N/A | N/A | N/A | N/A |
| Hand-sewn or stapler closure | 32 | N/A | N/A | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morini, A.; Zizzo, M.; Zanelli, M.; Dell’Atti, L.; Mereu, F.; Palicelli, A.; Giuffrida, M.; Orlandi, E.; Fabozzi, M. Impact of Pancreatic Stump Wrapping with Mesh on Post-Operative Pancreatic Fistula in Patients Undergoing Distal/Left Pancreatectomy for Malignant or Benign Diseases: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1688. https://doi.org/10.3390/medicina61091688
Morini A, Zizzo M, Zanelli M, Dell’Atti L, Mereu F, Palicelli A, Giuffrida M, Orlandi E, Fabozzi M. Impact of Pancreatic Stump Wrapping with Mesh on Post-Operative Pancreatic Fistula in Patients Undergoing Distal/Left Pancreatectomy for Malignant or Benign Diseases: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(9):1688. https://doi.org/10.3390/medicina61091688
Chicago/Turabian StyleMorini, Andrea, Maurizio Zizzo, Magda Zanelli, Lorenzo Dell’Atti, Federica Mereu, Andrea Palicelli, Mario Giuffrida, Elena Orlandi, and Massimiliano Fabozzi. 2025. "Impact of Pancreatic Stump Wrapping with Mesh on Post-Operative Pancreatic Fistula in Patients Undergoing Distal/Left Pancreatectomy for Malignant or Benign Diseases: A Systematic Review and Meta-Analysis" Medicina 61, no. 9: 1688. https://doi.org/10.3390/medicina61091688
APA StyleMorini, A., Zizzo, M., Zanelli, M., Dell’Atti, L., Mereu, F., Palicelli, A., Giuffrida, M., Orlandi, E., & Fabozzi, M. (2025). Impact of Pancreatic Stump Wrapping with Mesh on Post-Operative Pancreatic Fistula in Patients Undergoing Distal/Left Pancreatectomy for Malignant or Benign Diseases: A Systematic Review and Meta-Analysis. Medicina, 61(9), 1688. https://doi.org/10.3390/medicina61091688








