Revision Surgery for Achilles Tendon Rupture: A Comprehensive Review of Treatment Options, Outcomes, and Complications and the Role of Artificial Intelligence
Abstract
1. Introduction
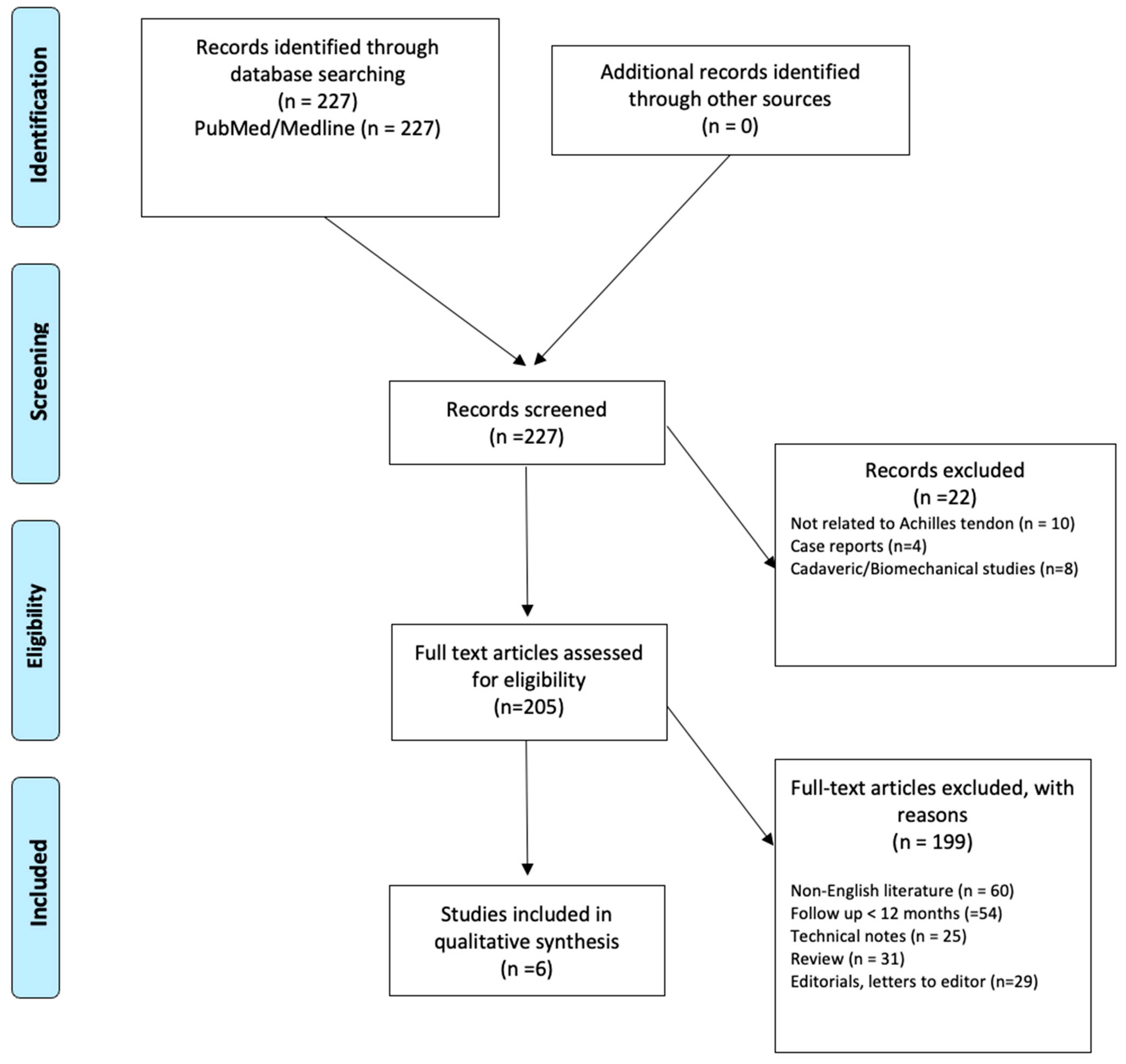
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria and Quality Assessment
3. Results
3.1. Treatment Algorithms
- Defects measuring 1–2 cm can typically be treated with a simple end-to-end anastomosis. Buda also suggests considering augmentation or tendon transfer, if necessary, with the plantaris tendon being his preferred option in such cases.
- Defects between 2–5 cm are best managed with V-Y myotendinous lengthening in combination with end-to-end repair. When tendon quality is compromised, flexor hallux longus (FHL) transfer may be warranted. In these instances, Buda favors a turndown flap if feasible.
- Defects greater than 5 cm require more extensive reconstruction. Myerson recommends combining turndown flaps and FHL transfer with other techniques as needed. Buda proposes a more aggressive approach, advocating for one or two tendon transfers in addition to V-Y lengthening, with optional use of allografts when necessary.
3.1.1. End-to-End Anastomosis
3.1.2. V-Y Myotendinous Lengthening
3.1.3. Fascial Turndown
3.1.4. Tendon Transfer
3.1.5. Autografts
3.1.6. Allografts
3.1.7. Synthetic Materials
3.1.8. Xenograft
4. Discussion
4.1. End-to-End Anastomosis
4.2. V-Y Myotendinous Lengthening
4.3. Fascial Turndown
4.4. Tendon Transfer
4.5. Autografts
4.6. Allografts
4.7. Synthetic Materials and Xenografts
4.8. The Role of Artificial Intelligence
4.9. Rehabilitation
4.10. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ganestam, A.; Kallemose, T.; Troelsen, A.; Barfod, K.W. Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013. A nationwide registry study of 33,160 patients. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3730–3737. [Google Scholar] [CrossRef]
- Huttunen, T.T.; Kannus, P.; Rolf, C.; Felländer-Tsai, L.; Mattila, V.M. Acute Achilles tendon ruptures. Incidence of injury and surgery in Sweden between 2001 and 2012. Am. J. Sports Med. 2014, 42, 2419–2423. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, T.J.; Choi, G.W.; Kim, H.J. Age is a risk factor for contralateral tendon rupture in patients with acute Achilles tendon rupture. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.; Spezia, M.; Elli, S.; Schiaffini, G.; Chisari, E. Obesity increases the risk of tendinopathy, tendon tear and rupture, and postoperative complications: A systematic review of clinical studies. Clin. Orthop. Relat. Res. 2020, 478, 1839–1847. [Google Scholar] [CrossRef]
- Lui, P.P.Y. Tendinopathy in diabetes mellitus patients—Epidemiology, pathogenesis, and management. Scand. J. Med. Sci. Sports 2017, 27, 776–787. [Google Scholar] [CrossRef]
- Trivedi, N.N.; Varshneya, K.; Calcei, J.B.; Lin, K.; Sochaki, K.R.; Voos, J.E. Achilles tendon repairs: Identification of risk factors for and economic impact of complications and reoperation. Sports Health 2023, 15, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kwon, T.H.; Choi, J.H.; Han, H.S.; Lee, K.M. Factors associated with Achilles tendon re-rupture following operative fixation. Bone Joint Res. 2024, 13, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.; Gasparini, G.; Cofano, E.; Colace, S.; Galasso, O. Revision surgery for shoulder infection after arthroscopic rotator cuff repair: Functional outcomes and eradication rate—A systematic review. Healthcare 2024, 12, 1291. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Palermi, S.; Sirico, F.; Corrado, B. Achilles Tendon Rupture: Mechanisms of Injury, Principles of Rehabilitation and Return to Play. J. Funct. Morphol. Kinesiol. 2020, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.; Castricini, R.; Castioni, D.; Cofano, E.; Familiari, F.; Gasparini, G.; Galasso, O. Better functional outcomes and a lower infection rate can be expected after superior capsular reconstruction in comparison with latissimus dorsi tendon transfer for massive, irreparable posterosuperior rotator cuff tears: A systematic review. J. Shoulder Elb. Surg. 2023, 32, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Khiami, F.; Di Schino, M.; Sariali, E.; Cao, D.; Rolland, E.; Catonné, Y. Treatment of chronic Achilles tendon rupture by shortening suture and free sural triceps aponeurosis graft. Orthop. Traumatol. Surg. Res. 2013, 99, 585–591. [Google Scholar] [CrossRef][Green Version]
- Maffulli, N.; Oliva, F.; Del Buono, A.; Florio, A.; Maffulli, G. Surgical management of Achilles tendon re-ruptures: A prospective cohort study. Int. Orthop. 2015, 39, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Del Buono, A.; Loppini, M.; Denaro, V. Ipsilateral free semitendinosus tendon graft with interference screw fixation for minimally invasive reconstruction of chronic tears of the Achilles tendon. Oper. Orthop. Traumatol. 2014, 26, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Danford, N.C.; Freibott, C.E.; Shoap, S.C.; Polzer, H.; Vosseller, J.T. Revision Surgery and Wound Complications with Minimally Invasive Compared to Open Achilles Tendon Repair: A Retrospective Comparative Study of 116 Patients. J. Surg. Orthop. Adv. 2023, 32, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Grassi, A.; Amendola, A.; Romagnoli, M.; Di Paolo, S.; Macchiarola, L.; Mosca, M. Flexor hallucis longus tendon transfer for chronic Achilles tendon rupture. Joints 2018, 6, 187–194. [Google Scholar]
- Park, J.Y.; Chang, M.J.; Kim, S.H.; Lee, H.I.; Ahn, J.H. Surgical reconstruction of chronic Achilles tendon rupture using various methods. Orthopedics 2014, 37, e888–e893. [Google Scholar] [CrossRef][Green Version]
- Myerson, M.S. Achilles tendon ruptures. Instr. Course Lect. 1999, 48, 219–230. [Google Scholar] [PubMed][Green Version]
- Buda, R.; Castagnini, F.; Pagliazzi, G.; Giannini, S. Treatment algorithm for chronic Achilles tendon lesions. J. Am. Podiatr. Med. Assoc. 2017, 107, 144–149. [Google Scholar] [CrossRef]
- Maruccia, M.; Tedeschi, P.; Caizzi, G.; Palmiotto, F.; Di Summa, P.G.; Vicenti, G.; Moretti, B.; Giudice, G.; Elia, R. Graft and flap. A novel orthoplastic approach to Achilles tendon secondary rupture. Plast. Reconstr. Surg. 2023, 152, 1359–1364. [Google Scholar] [CrossRef]
- Dogar, F.; Gurbuz, K.; Topak, D.; Ozdemir, M.A.; Kuşçu, B.; Ekinci, Y.; Batin, S.; Yaykasli, H.; Bilal, O. Comparison of suture types and techniques in Achilles tendon repair: An ex vivo biomechanical animal experiment. J. Am. Podiatr. Med. Assoc. 2024, 114, 2. [Google Scholar] [CrossRef] [PubMed]
- Feeley, A.A.; Feeley, I.H.; Roopnarinesingh, R.; Bayer, T. Rates of complications in Achilles tendon rupture repair using absorbable and nonabsorbable suture material: A systematic review. Foot 2022, 51, 101875. [Google Scholar] [CrossRef]
- Yasuda, T.; Shima, H.; Mori, K.; Kizawa, M.; Neo, M. Direct repair of chronic Achilles tendon ruptures using scar tissue located between the tendon stumps. J. Bone Jt. Surg. Am. 2016, 98, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Pankovich, A.M. Neglected rupture of the Achilles tendon: Treatment by V-Y tendinous flap. J. Bone Jt. Surg. Am. 1975, 57, 253–255. [Google Scholar] [CrossRef]
- Elias, I.; Besser, M.; Nazarian, L.N.; Raikin, S.M. Reconstruction for missed or neglected Achilles tendon rupture with V-Y lengthening and flexor hallucis longus tendon transfer through one incision. Foot Ankle Int. 2007, 28, 1238–1248. [Google Scholar] [CrossRef]
- Padanilam, T.G. Chronic Achilles tendon ruptures. Foot Ankle Clin. 2009, 14, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Us, A.K.; Bilgin, S.S.; Aydin, T.; Mergen, E. Repair of neglected Achilles tendon ruptures: Procedures and functional results. Arch. Orthop. Trauma Surg. 1997, 116, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Kissel, C.G.; Blacklidge, D.K.; Crowley, D.L. Repair of neglected Achilles tendon ruptures—Procedure and functional results. J. Foot Ankle Surg. 1994, 33, 46–52. [Google Scholar]
- Xu, T.; Liu, X.; Tian, J.; Liu, S.; Mi, J.; Xu, Y.; Chen, X.; Zhang, Y. Endoscopic-assisted locking block modified Krackow technique combined with a V-Y flap for chronic Achilles tendon rupture. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 86–93. [Google Scholar] [CrossRef]
- Bosworth, D.M. Repair of defects in the tendo Achillis. J. Bone Jt. Surg. Am. 1956, 38, 111–114. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lin, C.C.; Chen, C.N.; Chen, S.H.; Liao, W.Y.; Huang, C.R. Reconstruction for neglected Achilles tendon rupture: The modified Bosworth technique. Orthopedics 2005, 28, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Singhi, P.K.; Somashekar, V.; Ajari, A.; Chidambaram, M. Long-term outcomes of gastrocnemius V-Y plasty gastrosoleus fascial turndown flap for chronic tendo-Achilles injuries with complex gap (Kuwada type IV injuries). Indian J. Orthop. 2022, 56, 421–428. [Google Scholar] [CrossRef]
- Aynardi, M.C.; Atwater, L.C.; Melvani, R.; Parks, B.G.; Paez, A.G.; Miller, S.D. Is dual semitendinosus allograft stronger than turndown for Achilles tendon reconstruction? An in vitro analysis. Clin. Orthop. Relat. Res. 2017, 475, 2588–2596. [Google Scholar] [CrossRef][Green Version]
- Amlang, M.H.; Mittlmeier, T.; Rammelt, S. Weniger invasive Umkippplastik der Achillessehne bei chronischer Ruptur. Oper. Orthop. Traumatol. 2022, 34, 381–391. [Google Scholar] [CrossRef]
- Wapner, K.L.; Pavlock, G.S.; Hecht, P.J.; Naselli, F.; Walther, R. Repair of chronic Achilles tendon rupture with flexor hallucis longus tendon transfer. Foot Ankle 1993, 14, 443–449. [Google Scholar] [CrossRef]
- Lin, J.L. Tendon transfers for Achilles reconstruction. Foot Ankle Clin. 2009, 14, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Ziello, S.; Maisto, G.; Migliorini, F.; Oliva, F. Local Tendon Transfers For Chronic Ruptures Of The Achilles Tendon: A Systematic Review. J. Clin. Med. 2023, 12, 707. [Google Scholar] [CrossRef] [PubMed]
- Lui, T.H.; Chan, W.C.; Maffulli, N. Endoscopic Flexor Hallucis Longus Tendon Transfer For Chronic Achilles Tendon Rupture. Sports Med. Arthrosc. Rev. 2016, 24, 38–41. [Google Scholar] [CrossRef]
- Ahmad, C.S.; Gardner, T.R.; Groh, M.; Arnouk, J.; Levine, W.N. Mechanical Properties Of Soft Tissue Femoral Fixation Devices For Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2004, 32, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, C.A.; Brand, E.J.; Nuckley, D.J.; Johansen, S.; Laprade, R.F.; Engebretsen, L. Biomechanical Evaluation Of A Medial Knee Reconstruction With Comparison Of Bioabsorbable Interference Screw Constructs And Optimization With A Cortical Button. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.D.; Burton, K.J.; Romeo, A.A.; Santangelo, S.; Adams, D.A.; Arciero, R.A. Biomechanical Evaluation Of 4 Techniques Of Distal Biceps Brachii Tendon Repair. Am. J. Sports Med. 2007, 35, 252–258. [Google Scholar] [CrossRef]
- Sutton, K.M.; Dodds, S.D.; Ahmad, C.S.; Sethi, P.M. Surgical Treatment Of Distal Biceps Rupture. Am. Acad. Orthop. Surg. 2010, 18, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.; Ferreira, A.S.; Figueiredo, L.; Batista, J.P.; Abdelatif, N.; Pereira, H. Early Analysis Shows That Endoscopic Flexor Hallucis Longus Transfer Has A Promising Cost-Effectiveness Profile In The Treatment Of Acute Achilles Tendon Ruptures. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Williams, C.; Lowrey, C.; Gould, G.; Markert, R.; Laughlin, R. Flexor Hallucis Longus Tendon Transfer Fixation. Foot Ankle Spec. 2017, 10, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Pretterklieber, B. The High Variability Of The Chiasma Plantare And The Long Flexor Tendons: Anatomical Aspects Of Tendon Transfer In Foot Surgery. Ann. Anat. Anat. Anz. 2017, 211, 21–32. [Google Scholar] [CrossRef]
- Gonera, B.; Kurtys, K.; Paulsen, F.; Polguj, M.; Laprade, R.F.; Grzelecki, D. The Plantaris Muscle—Anatomical Curiosity Or A Structure With Important Clinical Value?—A Comprehensive Review Of The Current Literature. Ann. Anat.-Anat. Anz. 2021, 235, 151681. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Dutta, P.; Goswami, P.; Patel, A.; Purwar, S.; Jain, V. Management Of Neglected Achilles Tendon Division: Assessment Of Two Novel And Innovative Techniques. Adv. Orthop. Surg. 2014, 2014, 729397. [Google Scholar] [CrossRef]
- Sadek, A.F.; Fouly, E.H.; Laklok, M.A.; Amin, M.F. Functional And MRI Follow-Up After Reconstruction Of Chronic Ruptures Of The Achilles Tendon Myerson Type III Using The Triple-Loop Plantaris Tendon Wrapped With Central Turndown Flap: A Case Series. J. Orthop. Surg. Res. 2015, 10, 109. [Google Scholar] [CrossRef]
- Pérez Teuffer, A. Traumatic Rupture Of The Achilles Tendon. Reconstruction By Transplant And Graft Using The Lateral Peroneus Brevis. Orthop Clin. North Am. 1974, 5, 89–93. [Google Scholar] [PubMed]
- Turco, V.; Spinella, A.J. Peroneus Brevis Transfer For Achilles Tendon Rupture In Athletes. Orthop. Rev. 1988, 17, 827–828. [Google Scholar]
- Lo Torto, F.; Kaciulyte, J.; Marcasciano, M.; Casella, D.; Bernetti, A.; Mangone, M. Peroneus Brevis Flap In Achilles Tendon Reconstruction. Clinical, Radiological And Functional Analysis. Foot Ankle Surg. 2020, 26, 218–223. [Google Scholar] [CrossRef]
- Poeta, N.; Maffulli, N.; Bucolo, F.; Charpail, C.; Migliorini, F.; Guillo, S. Endoscopic Peroneus Brevis Tendon Transfer For Chronic Ruptures Of The Achilles Tendon: Surgical Technique. J. Orthop. Surg. Res. 2024, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Elliott, A.J.; Ellis, S.J. Reconstruction Of Achilles Rerupture With Peroneus Longus Tendon Transfer. Foot Ankle Int. 2013, 34, 898–903. [Google Scholar] [CrossRef]
- Mann, R.A.; Holmes, G.B.; Seale, K.S.; Collins, D.N. Chronic Rupture Of The Achilles Tendon: A New Technique Of Repair. J. Bone Joint Surg Am. 1991, 73, 214–219. [Google Scholar] [CrossRef]
- Wegrzyn, J.; Luciani, J.F.; Philippot, R.; Brunet-Guedj, E.; Moyen, B.; Besse, J.L. Chronic Achilles tendon rupture reconstruction using a modified flexor hallucis longus transfer. Int. Orthop. 2010, 34, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Kocaoglu, B.; Guven, O.; Gereli, A.; Karaoglan, O.; Bezer, M. The use of peroneus longus tendon in the treatment of Achilles tendon ruptures: An experimental study in rabbits. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 9–14. [Google Scholar]
- Cerciello, S.; Vasso, M.; De Cupis, V.; Maffulli, N. Dual graft reconstruction for chronic Achilles tendon tears. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2117–2123. [Google Scholar]
- Aktas, S.; Kocaoglu, B. Open versus minimally invasive repair with Achillon device. Foot Ankle Int. 2009, 30, 391–397. [Google Scholar] [CrossRef]
- Guclu, B.; Basarir, K.; Yilmaz, I.; Saglam, N.; Mutlu, S. Open versus minimal invasive repair with Achillon device. Foot Ankle Int. 2015, 36, 127–132. [Google Scholar]
- Pinto, M.; Fazal, M.A.; Ghosh, S.; Nicolaides, A.N. Vascular anatomy of the Achilles tendon. Foot Ankle Int. 2009, 30, 457–461. [Google Scholar]
- Lang, J. Clinical Anatomy of the Foot; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Maffulli, N.; Leadbetter, W.B. Free gracilis tendon graft in neglected tears of the Achilles tendon. Clin. J. Sport Med. 2005, 15, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lin, C.C.; Wang, C.J.; Chen, C.Y.; Huang, C.C. Free semitendinosus tendon graft for neglected Achilles tendon rupture. Foot Ankle Int. 2007, 28, 1208–1214. [Google Scholar]
- Ecker, M.L.; Lubowitz, J.H. Achilles tendon reconstruction with hamstring autograft: Technique tip. Arthroscopy 2011, 27, 446–450. [Google Scholar]
- Gigante, A.; Moschini, A.; Verdenelli, A.; Del Torto, M.; Ulisse, S.; de Palma, L. Open versus percutaneous repair in the treatment of acute Achilles tendon rupture: A randomized prospective study. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Ajis, A. Management of chronic ruptures of the Achilles tendon. J. Bone Jt. Surg. Am. 2008, 90, 1348–1360. [Google Scholar] [CrossRef] [PubMed]
- Doral, M.N.; Alam, M.; Bozkurt, M.; Turhan, E.; Donmez, G.; Maffulli, N. Functional anatomy of the Achilles tendon. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Bareither, D.; Neal, D.S.; Gerdes, M.H. Magnetic resonance and histological evaluation of the healing Achilles tendon in a rabbit model. J. Foot Ankle Surg. 2014, 53, 192–198. [Google Scholar]
- Regan, D.M.; Wong, M.W.; Young, D.A.; Fyfe, I.S. Surgical management of chronic Achilles tendon ruptures: A review of 21 cases. J. Foot Ankle Surg. 1997, 36, 161–167. [Google Scholar]
- Wilkins, R.; Bisson, L.J. Operative versus nonoperative management of acute Achilles tendon ruptures: A quantitative systematic review of randomized controlled trials. Am. J. Sports Med. 2012, 40, 2154–2160. [Google Scholar] [CrossRef]
- Gabel, S.; Manoli, A. Neglected rupture of the Achilles tendon. Foot Ankle Int. 1994, 15, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Krahe, M.A.; Berlet, G.C. Achilles tendon rupture: Pathophysiology, evaluation, and treatment. Clin. Podiatr. Med. Surg. 2010, 27, 151–162. [Google Scholar]
- Maffulli, N.; Ajis, A.; Longo, U.G.; Denaro, V. Chronic rupture of tendo Achillis. Foot Ankle Clin. 2007, 12, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Kuwada, G.T. Classification of tendo Achillis rupture with consideration of surgical repair techniques. J. Foot Surg. 1990, 29, 361–365. [Google Scholar] [PubMed]
- Elias, I.; Besser, M.P.; Nazarian, L.N.; Raikin, S.M. Imaging modalities for Achilles tendon rupture: A review. Clin. Orthop. Relat. Res. 2007, 454, 83–91. [Google Scholar]
- De Cesar Netto, C.; Chinanuvathana, A.; Fonseca LFDa Dein, E.J.; Tan, E.W.; Schon, L.C. Outcomes Of Flexor Digitorum Longus (FDL) Tendon Transfer In The Treatment Of Achilles Tendon Disorders. Foot Ankle Surg. 2019, 25, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Recio, D.; Ibañez, M.; Hormigo-Garcia, H.; Jimeno-Torres, E.; Vilá-Rico, J.; Alberti-Fito, G. Arthroscopic Flexor Halluces Longus Transfer and Percutaneous Achilles Tendon Repair for Distal Traumatic Ruptures. Arthrosc Tech. 2021, 10, 2435–2442. [Google Scholar] [CrossRef]
- Cetti, R.; Christensen, S.E.; Ejsted, R.; Jensen, N.M.; Jorgensen, U. Operative versus nonoperative treatment of Achilles tendon rupture: A prospective randomized study and review of the literature. Am. J. Sports Med. 1993, 21, 791–799. [Google Scholar] [CrossRef]
- Tallon, C.; Maffulli, N.; Ewen, S.W. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med. Sci. Sports Exerc. 2001, 33, 1983–1990. [Google Scholar] [CrossRef]
- Koulouris, G.; Connell, D. Evaluation of the Achilles tendon with ultrasound and magnetic resonance imaging. Semin. Musculoskelet. Radiol. 2005, 9, 243–254. [Google Scholar]
- Khoury, J.G.; Eisenhauer, A.C.; Shindle, M.K.; Polatsch, D.B. MRI diagnosis of Achilles tendon disorders. HSS J. 2008, 4, 117–123. [Google Scholar]
- Raikin, S.M.; Garras, D.N.; Krapchev, P.V. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013, 34, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Valkering, K.P.; Aufwerber, S.; Ranuccio, F.; Lunini, E.; Edman, G.; Ackermann, P.W. Functional weight-bearing mobilization after Achilles tendon rupture enhances early healing response: A single-blinded randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Marsilio, E.; Asparago, G.; Giai Via, A.; Biz, C.; Padulo, J.; Spoliti, M.; Foti, C.; Oliva, G.; Mannarini, S.; et al. Achilles Tendon Rupture and Dysmetabolic Diseases: A Multicentric, Epidemiologic Study. J. Clin. Med. 2022, 11, 3698. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A. Surgical treatment of chronic Achilles tendon rupture. J. Foot Ankle Surg. 2009, 48, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Kapiński, N.; Zieliński, J.; Borucki, B.A.; Trzciński, T.; Ciszkowska-łysoń, B.; Zdanowicz, U.; Śmigielski, R.; Nowiński, K.S. Monitoring of the achilles tendon healing process: Can artificial intelligence be helpful? Acta Bioeng. Biomech. 2019, 21, 103–111. [Google Scholar]
- Kwon, M.P.; Hullfish, T.J.; Humbyrd, C.J.; Boakye, L.A.T.; Baxter, J.R. Wearable sensor and machine learning estimate tendon load and walking speed during immobilizing boot ambulation. Sci. Rep. 2023, 13, 18086. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lin, C.I.; Henschke, J.; Quarmby, A.; Engel, T.; Cassel, M. Effects of exercise treatment on functional outcome parameters in mid-portion achilles tendinopathy: A systematic review. Front. Sports Act. Living. 2023, 17, 1144484. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Oliva, F.; Maffulli, G.D.; Buono, A.D.; Gougoulias, N. Chronic Achilles tendon rupture reconstructed with flexor hallucis longus transfer. J. Bone Jt. Surg. Am. 2010, 92, 187–194. [Google Scholar]
- Xu, Y.; Li, C.; Liu, T.; Xiang, F.; Deng, Y.; Li, Z.; Wei, D. Long-term outcome of flexor hallucis longus tendon transfer for chronic Achilles tendon rupture with large defect: A retrospective series. Medicine 2023, 102, 35302. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Hua, Y.H. Tendon allograft for treatment of chronic Achilles tendon rupture: A systematic review. Foot Ankle Surg. 2019, 25, 252–257. [Google Scholar] [CrossRef]

| Study Author (Year) | Criteria | Total | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| Trivedi et al. (2022) [6] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High |
| Choi et al. (2024) [7] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High |
| Khiami et al. (2013) [11] | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | High |
| Maffulli et al. (2015) [12] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High |
| Maffulli et al. (2014) [13] | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | High |
| Danford et al. (2023) [14] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High |
| Author | Year | Journal | N. | Sex | Age | Follow-Up | Surgery | Complications | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Mean | SD | ||||||||
| Choi YH et al. [7] | 2024 | Bone & Joint Research | 43,287 | 33,276 | 10,011 | 42.2 | 11.8 | 2y | NA | Infection and wound complication (0.5%) | NA |
| Danford NC et al. [14] | 2023 | Journal of surgical orthopaedic advances | 116 | 96 | 20 | 41.85 | 14.02 | 2y | End to end anastomosis open vs minimally invasive | Wound complications (11%), Infection (19%) | Revision surgery rate and complication rate |
| Maffulli N et al. [13] | 2014 | Operative Orthopaedics Traumatology | 28 | 21 | 7 | 46 | 9.3 | 2y | ipsilateral ST transfer | Infection (7.1%) | ATRS (median; ds) 86; 3.63 |
| Maffulli N et al. [12] | 2015 | International Orthopaedics | 21 | 17 | 4 | 38 | 8.5 | 3.25y | ipsilateral PB and ST transfer | Weakness calf muscle (19%) | ATRS (median; ds) 82; 3.25 |
| Khiami F et al. [11] | 2013 | Orthopaedics & Traumatology Surgery & Research | 23 | 20 | 3 | 52.1 | 13 | 2y | sural triceps aponeurosis transfer | Sural nerve hypoesthesia (0.23%) | AOFAS (median; ds) 96.1; 6.8 |
| Trivedi NN et al. [6] | 2023 | Sports Health | 50,279 | 34,161 | 16,118 | 43.6 | 11.7 | 2y | NA | Infection (0.4%), wound complication (0.52%), VTE (1.19%) | Identify risk factors for complications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delmastro, E.; Colace, S.; Longo, U.G.; D’Hooghe, P.; Marangon, A.; Galasso, O.; Gasparini, G.; Mercurio, M. Revision Surgery for Achilles Tendon Rupture: A Comprehensive Review of Treatment Options, Outcomes, and Complications and the Role of Artificial Intelligence. Medicina 2025, 61, 1684. https://doi.org/10.3390/medicina61091684
Delmastro E, Colace S, Longo UG, D’Hooghe P, Marangon A, Galasso O, Gasparini G, Mercurio M. Revision Surgery for Achilles Tendon Rupture: A Comprehensive Review of Treatment Options, Outcomes, and Complications and the Role of Artificial Intelligence. Medicina. 2025; 61(9):1684. https://doi.org/10.3390/medicina61091684
Chicago/Turabian StyleDelmastro, Elena, Stefano Colace, Umile Giuseppe Longo, Pieter D’Hooghe, Alberto Marangon, Olimpio Galasso, Giorgio Gasparini, and Michele Mercurio. 2025. "Revision Surgery for Achilles Tendon Rupture: A Comprehensive Review of Treatment Options, Outcomes, and Complications and the Role of Artificial Intelligence" Medicina 61, no. 9: 1684. https://doi.org/10.3390/medicina61091684
APA StyleDelmastro, E., Colace, S., Longo, U. G., D’Hooghe, P., Marangon, A., Galasso, O., Gasparini, G., & Mercurio, M. (2025). Revision Surgery for Achilles Tendon Rupture: A Comprehensive Review of Treatment Options, Outcomes, and Complications and the Role of Artificial Intelligence. Medicina, 61(9), 1684. https://doi.org/10.3390/medicina61091684








