Synchronous/Metachronous Prostate Cancer with Other Cancer Sites—The Experience of a Single Center in Romania
Abstract
1. Introduction
2. Materials and Methods
Inclusion and Exclusion Criteria
- -
- Age > 18 years;
- -
- Patients with a histopathologically confirmed diagnosis of prostate cancer;
- -
- Patients with a histopathologically confirmed diagnosis of a second primary cancer;
- -
- Comprehensive pretherapeutic assessment;
- -
- Patients who signed the informed consent form.
- -
- Patients without a histopathologically confirmed diagnosis of prostate cancer and a second or third primary cancer;
- -
- <6 months of available followup data;
- -
- Patients who did not sign the informed consent form.
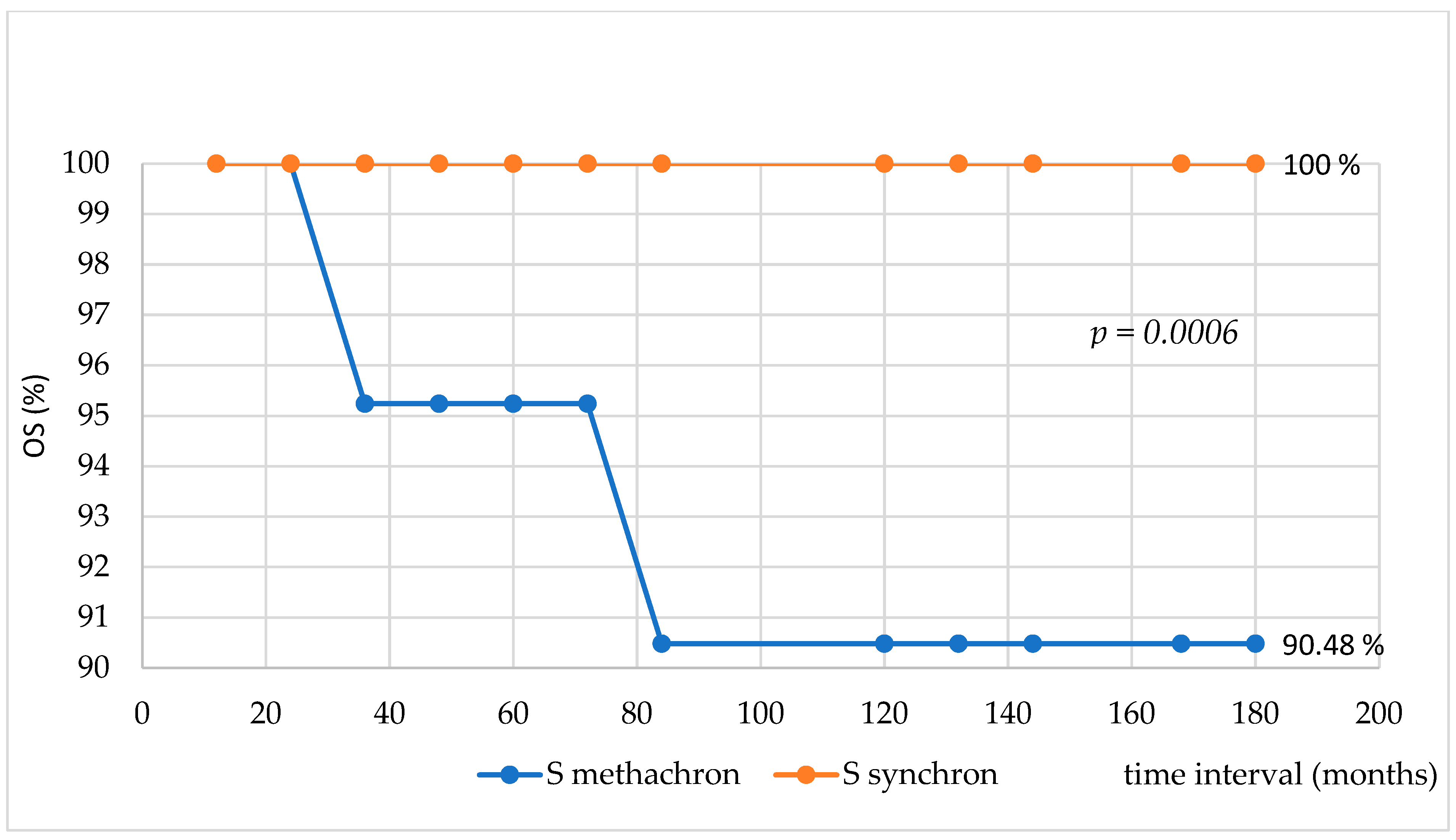
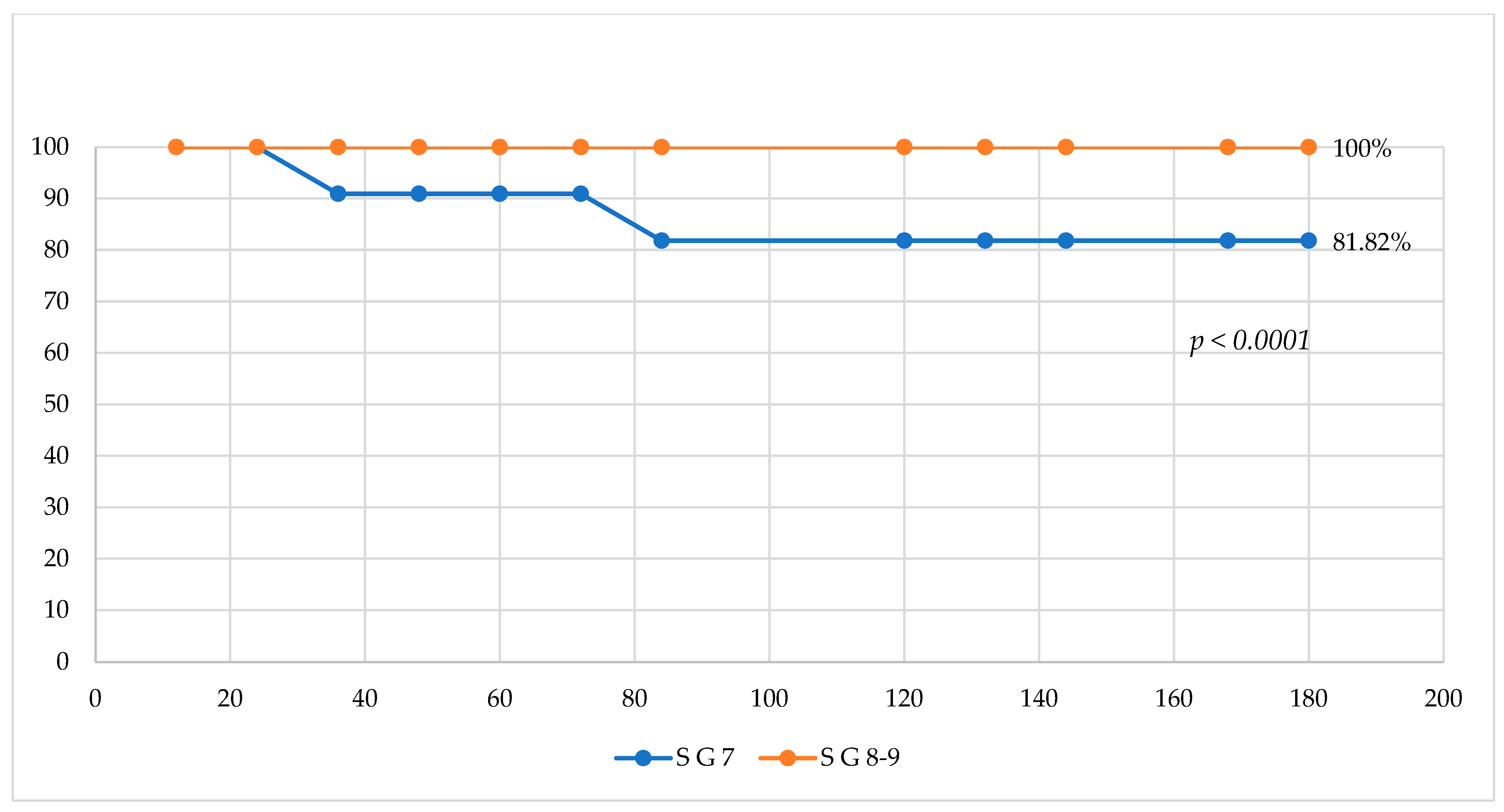
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BRCA2 | breast cancer-related gene 2 |
| MPMs | multiple primary malignancies |
| SEER | Surveillance, Epidemiology, and End Results Program |
| IACR/IARC | International Association of Cancer Registries and International Agency for Research on Cancer |
| EBRT | external beam radiotherapy |
| ADT | androgen deprivation therapy |
| CHT | chemotherapy |
| TKI | tyrosine kinase inhibitor |
| GIST | Gastrointestinal Stromal Tumor |
| ARPI | Androgen receptor pathway inhibitors |
References
- Warren, S.; Gates, O. Multiple Primary Malignant Tumors: A Survey of the Literature and Statistical Study. Am. J. Cancer 1932, 16, 1358–1414. [Google Scholar]
- Pan, S.Y.; Huang, C.P.; Chen, W.C. Synchronous/Metachronous Multiple Primary Malignancies: Review of Associated Risk Factors. Diagnostics 2022, 12, 1940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amer, M.H. Multiple neoplasms, single primaries, and patient survival. Cancer Manag. Res. 2014, 6, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Coyte, A.; Morrison, D.S.; McLoone, P. Second primary cancer risk—The impact of applying different definitions of multiple primaries: Results from a retrospective population-based cancer registry study. BMC Cancer 2014, 14, 272. [Google Scholar] [CrossRef]
- Tanjak, P.; Suktitipat, B.; Vorasan, N.; Juengwiwattanakitti, P.; Thiengtrong, B.; Songjang, C.; Therasakvichya, S.; Laiteerapong, S.; Chinswangwatanakul, V. Risks and cancer associations of metachronous and synchronous multiple primary cancers: A 25-year retrospective study. BMC Cancer 2021, 21, 1045. [Google Scholar] [CrossRef]
- Huang, S.F.; Li, H.F.; Liao, C.T.; Wang, H.M.; Chen, I.H.; Chang, J.T.; Chen, Y.J.; Cheng, A.J. Association of HPV infections with second primary tumors in early-staged oral cavity cancer. Oral Dis. 2012, 18, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Mazzoni, E.; Bononi, I.; Tognon, M.; Martini, F. Association Between Simian Virus 40 and Human Tumors. Front. Oncol. 2019, 9, 670. [Google Scholar] [CrossRef]
- Schlenker, B.; Schneede, P. The Role of Human Papilloma Virus in Penile Cancer Prevention and New Therapeutic Agents. Eur. Urol. Focus 2019, 5, 42–45. [Google Scholar] [CrossRef]
- Biller, L.H.; Syngal, S.; Yurgelun, M.B. Recent advances in Lynch syndrome. Fam. Cancer 2019, 18, 211–219. [Google Scholar] [CrossRef]
- Pilarski, R. The Role of BRCA Testing in Hereditary Pancreatic and Prostate Cancer Families. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Concolino, P.; Costella, A.; Capoluongo, E.D. Multiple endocrine neoplasia type 1 (MEN1): An update of 208 new germline variants reported in the last nine years. Cancer Genet. 2016, 209, 36–41. [Google Scholar] [CrossRef]
- Parsa, N. Environmental Factors Inducing Human Cancers. Iran. J. Public Health 2012, 41, 1–9. [Google Scholar]
- Chen, P.-H.; Mahmood, Q.; Mariottini, G.L.; Chiang, T.-A.; Lee, K.-W. Adverse Health Effects of Betel Quid and the Risk of Oral and Pharyngeal Cancers. BioMed Res. Int. 2017, 2017, 3904098. [Google Scholar] [CrossRef] [PubMed]
- Elicin, O.; Sermaxhaj, B.; Bojaxhiu, B.; Shelan, M.; Giger, R.; Rauch, D.; Aebersold, D.M. Incidence of second primary cancers after radiotherapy combined with platinum and/or cetuximab in head and neck cancer patients. Strahlenther. Und Onkol. 2019, 195, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Law, N.L.W.; Hong, L.W.; Tan, S.S.N.; Foo, C.J.; Lee, D.; Voon, P.J. Barriers and Challenges of Multidisciplinary Teams in Oncology Management: A Scoping Review Protocol. BMJ Open 2024, 14, e079559. [Google Scholar] [CrossRef]
- Ghinescu, M.; Olaroiu, M.; Aurelian, S.; Halfens, R.J.; Dumitrescu, L.; Schols, J.M.; Rahnea-Nita, G.; Curaj, A.; Alexa, I.; van den Heuvel, W.J. Assessment of care problems in Romania: Feasibility and exploration. J. Am. Med. Dir. Assoc. 2015, 16, 86.e9–86.e12. [Google Scholar] [CrossRef]
- He, C. Multidisciplinary Team Meetings: Barriers to Implementation in Cancer Care. Oncology 2024, 38, 339–344. [Google Scholar] [CrossRef] [PubMed]
- de Castro Jr, G.; Souza, F.H.; Lima, J.; Bernardi, L.P.; Teixeira, C.H.A.; Prado, G.F.; Grupo Brasileiro de Oncologia Torácica (GBOT). Does Multidisciplinary Team Management Improve Clinical Outcomes in NSCLC? A Systematic Review with Meta-Analysis. JTO Clin. Res. Rep. 2023, 4, 100580. [Google Scholar] [CrossRef]
- Alfieri, S.; Brunelli, C.; Borreani, C.; Capri, G.; Angi, M.; Bianchi, G.V.; Lo Dico, S.; Spada, P.; Fusetti, V.; Zecca, E.; et al. Characterizing Different Multidisciplinary Team Models Implemented Within One Comprehensive Cancer Center. J. Multidiscip. Healthc. 2023, 16, 1845–1855. [Google Scholar] [CrossRef]
- Rahnea-Nita, R.A.; Stoian, A.R.; Anghel, R.M.; Rebegea, L.F.; Ciuhu, A.N.; Bacinschi, X.E.; Zgura, A.F.; Trifanescu, O.G.; Toma, R.V.; Constantin, G.B.; et al. The Efficacy of Immunotherapy in Long-Term Survival in Non-Small Cell Lung Cancer (NSCLC) Associated with the Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH). Life 2023, 13, 1279. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Yan, W.; Yang, D.; Shen, B. Translational Bioinformatics for Diagnostic and Prognostic Prediction of Prostate Cancer in the Next-Generation Sequencing Era. BioMed Res. Int. 2013, 2013, 901578. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Rahnea-Nita, R.-A.; Rebegea, L.-F.; Nechifor, A.; Mareș, C.; Toma, R.-V.; Stoian, A.-R.; Ciuhu, A.-N.; Andronache, L.-F.; Constantin, G.B.; Rahnea-Nita, G. The Complexity of Treatments and the Multidisciplinary Team—A Rare Case of Long-Term Progression—Free Survival in Prostate Cancer until Development of Liver and Brain Metastases. J. Clin. Med. 2023, 12, 5579. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rebegea, L.; Stefan, A.M.; Firescu, D.; Miron, D.; Romila, A. Paraneoplastic pemphigus associated with a hypopharynx squamous cell carcinoma. Case report authors. Acta Medica Mediterr. 2018, 34, 1265. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Xu, L.; Hu, X.; Zeng, H.; Liu, Z. Case report: Synchronous prostate cancer and renal cell carcinoma with prostate cancer-origin metastases to adrenal and renal hilar lymph nodes. Front. Oncol. 2024, 14, 1412067. [Google Scholar] [CrossRef]
- Bunnag, N.; Wongwijitsook, J.; Vachatimanont, S. Synchronous Pulmonary Malignancy Detected During PSMA Ligand PET/CT for Initial Staging of Prostate Cancer: A Case Report. Nucl. Med. Mol. Imaging 2023, 57, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chaudhuri, T.; Roy, S.; Bose, S. A rare synchronous presentation of double primary malignancies—Lung and prostate. J. Cancer Res. Ther. 2021, 17, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Wali, L.; Husain, F.; Shah, A.; Tahir, H.; Alam, F.; Khan, M.; Ghosh, S. Prostate cancer and sarcoma: Challenges of synchronous malignancies. Radiol. Case Rep. 2020, 15, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Edfelt, E.; Shahrivar, M.; Holmsten, K.; Radkiewicz, C. Rising incidence trends of synchronous prostate and rectal cancers: A population-based study. Acta Oncol. 2025, 64, 374–379. [Google Scholar] [CrossRef]
- Sidiqi, B.; Nosrati, J.; Wu, V.; Kobritz, M.; La Gamma, N.; Whelan, R.; Parashar, B.; King, D.; Tchelebi, L.; Herman, J. The Prevalence and Management of Synchronous Prostate and Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e339. [Google Scholar] [CrossRef]
- Jean, S.; Trésor, M.G.; Lionelle, F.; Félicien, H.Y.; Inès, Y.D.M.; Jean-Martin, H.F.; Georges, A.D.J. A Prostate Cancer Metachronous to A Breast Cancer in A 74-Year-Old Male. J. Med. Res. 2023, 9, 109–111. [Google Scholar] [CrossRef]
- Abhyankar, N.; Hoskins, K.F.; Abern, M.R.; Calip, G.S. Descriptive characteristics of prostate cancer in patients with a history of primary male breast cancer—A SEER analysis. BMC Cancer 2017, 17, 659. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Lori, E.; Forte, F.; Lauro, A.; Tripodi, D.; Amabile, M.I.; Cantisani, V.; Varanese, M.; Ferent, I.C.; Baldini, E.; et al. Thyroid and renal cancers: A bidirectional association. Front. Oncol. 2022, 12, 951976. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Meissner, V.H.; Jahnen, M.; Herkommer, K. Familial prostate cancer and genetic predisposition. Der Urol. 2021, 60, 567–575. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Q.; Ding, M.; Wang, T.; Chen, Y.; Zhang, K. BRCA2 mutations in familial breast cancer with prostate cancer: A case report and literature review. Front. Oncol. 2024, 14, 1428849. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, L.; Kim, P.; Uche, A.; Cobos, E. A Synchronous Diagnosis of Metastatic Male Breast Cancer and Prostate Cancer. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619847230. [Google Scholar] [CrossRef] [PubMed]
- Millican, J.; Wong, M. Treatment of rectal cancer after previous prostate cancer: A single institution experience. Oncol. Lett. 2022, 25, 20. [Google Scholar] [CrossRef]
- Hoshi, S.; Bilim, V.; Hoshi, K.; Ogawa, Y.; Kato, T.; Urano, K.; Yamada, T.; Sakagami, R.; Kudo, T.; Numahata, K.; et al. Double Primary Cancer of the Prostate and Urothelial Cancer: A Single Institution Experience. J. Pers. Med. 2024, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tian, Y.; Ding, X.; Liu, L.; Tan, B.; Yang, B.; Wu, J.; Lei, T.; Wang, R.; Ding, Y. Genetic Analysis and Targeted Therapy Using Buparlisib and MK2206 in a Patient with Triple Metachronous Cancers of the Kidney, Prostate, and Squamous Cell Carcinoma of the Lung: A Case Report. Onco Targets Ther. 2021, 14, 2839–2845. [Google Scholar] [CrossRef]
- Fan, C.Y.; Huang, W.Y.; Lin, C.S.; Su, Y.F.; Lo, C.H.; Tsao, C.C.; Liu, M.Y.; Lin, C.L.; Kao, C.H. Risk of second primary malignancies among patients with prostate cancer: A population-based cohort study. PLoS ONE 2017, 12, e0175217. [Google Scholar] [CrossRef]
- Omer, D.M.; Thompson, H.M.; Verheij, F.S.; Yuval, J.B.; Rosen, R.; Beets, N.R.A.; Luthra, A.; Romesser, P.B.; Paty, P.B.; Garcia-Aguilar, J.; et al. Rectal Cancer after Prostate Radiation: A Complex and Controversial Disease. Cancers 2023, 15, 2214. [Google Scholar] [CrossRef] [PubMed]
- Neugut, A.I.; Ahsan, H.; Robinson, E.; Ennis, R.D. Bladder carcinoma and other second malignancies after radiotherapy for prostate carcinoma. Cancer 1997, 79, 1600–1604. [Google Scholar] [CrossRef]
- Wallis, C.J.; Mahar, A.L.; Choo, R.; Herschorn, S.; Kodama, R.T.; Shah, P.S.; Danjoux, C.; Narod, S.A.; Nam, R.K. Second malignancies after radiotherapy for prostate cancer: Systematic review and meta-analysis. BMJ 2016, 352, i851. [Google Scholar] [CrossRef] [PubMed]


| Clinical and Therapeutic Parametrics | No. of Patients (%) |
|---|---|
| Median age (range) (years) | 72.5 (56–83) |
| First tumor site | |
| - Prostate | 14 (53.86) |
| - Bladder | 2 (7.69) |
| - Kidney | 3 (11.54) |
| - Colon-rectum | 2 (7.69) |
| - Breast | 1 (3.85) |
| - Larynx | 2 (7.69) |
| - Liver | 1 (3.85) |
| - Nasopharynx | 1 (3.85) |
| Second tumor site | |
| - Prostate | 10 (38.46) |
| - Lung | 4 (15.38) |
| - Bladder | 2 (7.69) |
| - Colon-rectum | 5 (19.23) |
| - Stromal gastric tumor | 1 (3.85) |
| - Thyroid | 2 (7.69) |
| - Kidney | 1 (3.85) |
| - Soft tissues | 1 (3.85) |
| Third tumor site | |
| - Prostate | 2 (7.69) |
| - Colon-rectum | 2 (7.69) |
| - Pancreas | 1 (3.85) |
| Patient No. | Diagnosis | HP (Histopathological Exam); Molecular Markers | Year | Stage of the Disease | Treatment | ||
|---|---|---|---|---|---|---|---|
| 1 | Prostate | Adenocarcinoma Gleason 8 (4 + 4) | 2022 | IV | EBRT | ADT + ARPI (Abiraterone) | |
| 2 | Prostate | Adenocarcinoma Gleason 7 (3 + 4) | 2022 | III | Surgery | ||
| 3 | Prostate | Adenocarcinoma G2 Gleason 6 (3 + 3) | 2019 | III | EBRT | ADT + ARPI (Apalutamide) | |
| 4 | Prostate | Adenocarcinoma Gleason 7 (3 + 4) | 2023 | II | EBRT | ADT | |
| 5 | Prostate | Adenocarcinoma Gleason 7 (3 + 4) | 2023 | III | EBRT | ||
| 6 | Prostate | Adenocarcinoma Gleason 7 (4 + 3) | 2021 | IV | EBRT | ADT | |
| 7 | Prostate | Adenocarcinoma Gleason 7 (4 + 3) | 2024 | II | Surgery | ||
| 8 | Larynx | Squamous carcinoma G1 | 2011 | I | Surgery | ||
| 9 | Colon | Adenocarcinoma | 2014 | II | Surgery | ||
| 10 | Prostate | Adenocarcinoma Gleason 6 (3 + 3) | 2016 | III | ADT | ||
| 11 | Prostate | Adenocarcinoma Gleason 6 (3 + 3) | 2018 | II | EBRT | ADT | |
| 12 | Kidney | Clear cell carcinoma | 2015 | IV | Surgery | ||
| 13 | Larynx | Squamous cell carcinoma | 2013 | III | Surgery | EBRT adjuvant | |
| 14 | Bladder | Papillary carcinoma | 2010 | II | TUR-V | Instillation CHT | |
| 15 | Colon | adenocarcinoma | 2022 | II | Surgery | CHT | |
| 16 | Prostate | Adenocarcinoma Gleason 9 (4 + 5) | 2021 | IV | EBRT | ADT | |
| 17 | Prostate | Adenocarcinoma Gleason 8 (3 + 5) | 2014 | IV | EBRT | ADT (36 months) | |
| 18 | Kidney | Oncocytoma | 2010 | I | Surgery | ||
| 19 | Prostate | Adenocarcinoma Gleason 5 (2 + 3) | 2010 | II | EBRT | ADT | |
| 20 | Breast | Invasive breast carcinoma Luminal B, RE-80%, PR-30%, HER2-negative KI 67 25% | 2015 | II | Surgery | EBRT | CHT, HT (adjuvant, 5 years) |
| 21 | Bladder | Urothelial carcinoma | 2015 | II | TUR-V | EBRT at relapse | |
| 22 | Prostate | Adenocarcinoma Gleason 8 (4 + 4) | 2023 | III | EBRT at relapse | ADT | |
| 23 | Liver | Unresectable carcinoma | 2018 | Chemoembolization, Sorafenib | |||
| 24 | Prostate | Adenocarcinoma Gleason 7 (4 + 3) | 2018 | III | EBRT | ||
| 25 | Nasopharynx | Carcinoma | 2010 | II | EBRT | ||
| 26 | Kidney | Clear cell carcinoma | 2020 | III | Surgery | ||
| Patient No. | Diagnosis | HP (Histopathological Exam); Molecular Markers | Year | Stage | Treatment | ||
|---|---|---|---|---|---|---|---|
| 1 | Lung | Adenocarcinoma EGFR-negative, ALK-negative, PDL1-negative | 2023 | III | Surgery | CHT | |
| 2 | Lung | Large cell, EGFR-negative, ALK-negative, PDL1-negative | 2024 | IV | CHT | ||
| 3 | Bladder | Papillary carcinoma | 2024 | I | Surgery | ||
| 4 | Lung | Adenocarcinoma EGFR-negative, ALK-negative, PDLI-negative | 2024 | III | Surgery | CHT | |
| 5 | Lung | Adenocarcinoma G2, EGFR-positive, ALK-negative | 2024 | III | Anti-EGFR therapy-Osimertinib | ||
| 6 | Colon | Adenocarcinoma | 2024 | III | Surgery | ||
| 7. | Bladder | Urothelial carcinoma | 2024 | II | TUR-V | CHT | |
| 8. | Prostate | Adenocarcinoma Gleason 6 (3 + 3) | 2021 | II-INTERMEDIATE GROUP RISK | EBRT | ADT | |
| 9 | Prostate | Adenocarcinoma Gleason 9 (4 + 5) | 2022 | II | EBRT | ADT + ARPI (Enzalutamide) | |
| 10 | Colon | Adenocarcinoma | 2024 | II | Surgery | ||
| 11 | GIST Stomach | GIST | 2018 | Surgery | Imatinib | ||
| 12 | Prostate | Adenocarcinoma Gleason 8 (4 + 4) | 2022 | II | EBRT | ADT | |
| 13 | Prostate | Adenocarcinoma Gleason 6 (3 + 3) | 2016 | III | EBRT | ADT (36 months) | |
| 14 | Prostate | Adenocarcinoma Gleason 7 (3 + 4) | 2017 | II | Surgery | ||
| 15 | Prostate | Adenocarcinoma Gleason 9 (4 + 5) | 2024 | IV | EBRT | ADT + ARPI (Apalutamide) | |
| 16 | Colon | Adenocarcinoma | 2024 | I | Surgery | ||
| 17 | Rectum | Adenocarcinoma | 2024 | III | Surgery | CHT | |
| 18 | Thyroid | Papillary carcinoma | 2019 | Surgery-thyroidectomy | Radioactive Iodine Therapy | Sorafenib | |
| 19 | Sarcoma | Leiomyosarcoma | 2014 | Surgery | adjuvant EBRT | ||
| 20 | Prostate | Adenocarcinoma Gleason 6 (3 + 3) | 2023 | IV | EBRT | ADT | |
| 21 | Prostate | Adenocarcinoma Gleason 6 (3 + 3) | 2024 | IV | EBRT | ADT | |
| 22 | Rectum | Adenocarcinoma | 2024 | III | Surgery | EBRT | CHT |
| 23 | Prostate | Adenocarcinoma Gleason 7 (4 + 3) | 2023 | II | Surgery | ADT (36 months) | |
| 24 | Thyroid | Papillary carcinoma | 2019 | Surgery | |||
| 25 | Kidney | Clear cell carcinoma | 2016 | II | Surgery | ||
| 26 | Prostate | Adenocarcinoma Gleason 9 (4 + 5) | 2023 | IV | EBRT | ADT | |
| Patient No. | Diagnosis | HP (Histopathological Exam) | Year | Stage | Treatment | ||
|---|---|---|---|---|---|---|---|
| 5 | Rectum | Adenocarcinoma | 2025 | IV | EBRT | ||
| 18 | Prostate | Adenocarcinoma Gleason 9 (4 + 5) | 2025 | IV | EBRT | ADT | |
| 19 | Colon | Adenocarcinoma | 2021 | II | Surgery | ||
| 24 | Pancreas | Adenocarcinoma | 2021 | II | Surgery | ||
| 25 | Prostate | Adenocarcinoma Gleason 7 (4 + 3) | 2020 | IV | EBRT | ADT | |
| No. | Combination | No. of Cases |
|---|---|---|
| 1 | Prostate Cancer plus Bladder/Renal Cancer | 7 |
| 2 | Prostate Cancer plus Colon/Rectum | 7 |
| 3 | Prostate Cancer plus Two Cancers | 5 |
| 4 | Prostate Cancer plus Lung Cancer | 4 |
| Patient No. | Time Period (Months) Between the First and the Second Cancer Site Occurrence | Synchronous/Metachronous Cancer |
|---|---|---|
| 1 | 12 | metachronous |
| 2 | 24 | metachronous |
| 3 | 60 | metachronous |
| 4 | 12 | metachronous |
| 5 | 3 | synchronous |
| 6 | 36 | metachronous |
| 7 | 4 | synchronous |
| 8 | 120 | metachronous |
| 9 | 96 | metachronous |
| 10 | 96 | metachronous |
| 11 | 8 | metachronous |
| 12 | 84 | metachronous |
| 13 | 36 | metachronous |
| 14 | 84 | metachronous |
| 15 | 24 | metachronous |
| 16 | 36 | metachronous |
| 17 | 120 | metachronous |
| 18 | 108 | metachronous |
| 19 | 48 | metachronous |
| 20 | 96 | metachronous |
| 21 | 108 | metachronous |
| 22 | 12 | metachronous |
| 23 | 60 | metachronous |
| 24 | 12 | metachronous |
| 25 | 72 | metachronous |
| 26 | 36 | metachronous |
| Patient No. | Time Period (Months) Between the First and the Third Cancer Site Occurrence | Synchronous/Metachronous |
|---|---|---|
| 5 | 24 | metachronous |
| 18 | 180 | metachronous |
| 19 | 132 | metachronous |
| 24 | 36 | metachronous |
| 25 | 120 | metachronous |
| Patient No. | Time Period (Months) Between the Second and the Third Cancer Site Occurrence | Synchronous/Metachronous |
|---|---|---|
| 5 | 12 | metachronous |
| 18 | 72 | metachronous |
| 19 | 84 | metachronous |
| 24 | 24 | metachronous |
| 25 | 48 | metachronous |
| Regression Summary for Survival | |||||
|---|---|---|---|---|---|
| Predictor | Standard Error | t Stat | p-Value | 95% Lower | 95% Upper |
| Intercept | 0.518 | 1.717 | 0.1001 | −0.185 | 1.964 |
| Gleason | 0.0713 | 0.076 | 0.9397 | −0.142 | 0.153 |
| Prostate tumor_P1 | 0.0954 | 0.687 | 0.8832 | −0.098 | 0.154 |
| Prostate tumor_P2 | 0.151 | 0.477 | 0.6381 | −0.242 | 0.386 |
| Prostate tumor_P3 | 0.277 | 0.251 | 0.8042 | −0.505 | 0.644 |
| No. | Author, Year | Subject: Prostate Cancer/Other Cancer; Synchronous/Metachronous | Reference No. |
|---|---|---|---|
| 1 | Zhang Y et al., 2024 | Prostate/renal; case report; synchronous | [27] |
| 2 | Bunnag N et al., 2023 | Prostate/lung; case report; synchronous | [28] |
| 3 | Sarkar S et al., 2021 | Prostate/Lung; case report; synchronous | [29] |
| 4 | Luqman W et al., 2020 | Prostate/sarcoma; case report; synchronous | [30] |
| 5 | Edfelt E et al., 2025 | Prostate/rectum; large cohort study; synchronous | [31] |
| 6 | B.U. Sidiqi et al., 2023 | Prostate/rectum; case series (10 cases); synchronous | [32] |
| 7 | Sossa J et al., 2023 | Prostate/breast; case report; metachronous | [33] |
| 8 | Millican J et al., 2022 | Prostate/rectum; single-institution experience; synchronous/metachronous | [40] |
| 9 | Hoshi S et al., 2024 | Prostate/urothelial; three cases; metachronous | [40] |
| 10 | Zhao T et al., 2021 | Kidney/prostate/lung; case report; metachronous | [41] |
| 11 | Omer DM et al., 2023 | Rectal cancer after prostate radiation; synchronous/metachronous | [44] |
| 12 | Neugut AI et al., 1997 | Second malignancies after radiotherapy for prostate carcinoma; synchronous/metachronous | [45] |
| 13 | Wallis CJ et al., 2019 | Second malignancies after radiotherapy for prostate cancer; synchronous/metachronous | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahnea-Nita, G.; Dumitru, M.; Nechifor, A.; Rahnea-Nita, R.-A.; Maier, A.-C.; Andronache, L.F.; Cernea, A.; Rebegea, A.; Rebegea, L.-F. Synchronous/Metachronous Prostate Cancer with Other Cancer Sites—The Experience of a Single Center in Romania. Medicina 2025, 61, 1666. https://doi.org/10.3390/medicina61091666
Rahnea-Nita G, Dumitru M, Nechifor A, Rahnea-Nita R-A, Maier A-C, Andronache LF, Cernea A, Rebegea A, Rebegea L-F. Synchronous/Metachronous Prostate Cancer with Other Cancer Sites—The Experience of a Single Center in Romania. Medicina. 2025; 61(9):1666. https://doi.org/10.3390/medicina61091666
Chicago/Turabian StyleRahnea-Nita, Gabriela, Mihaela Dumitru, Alexandru Nechifor, Roxana-Andreea Rahnea-Nita, Adrian-Cornel Maier, Liliana Florina Andronache, Andreea Cernea, Alexandru Rebegea, and Laura-Florentina Rebegea. 2025. "Synchronous/Metachronous Prostate Cancer with Other Cancer Sites—The Experience of a Single Center in Romania" Medicina 61, no. 9: 1666. https://doi.org/10.3390/medicina61091666
APA StyleRahnea-Nita, G., Dumitru, M., Nechifor, A., Rahnea-Nita, R.-A., Maier, A.-C., Andronache, L. F., Cernea, A., Rebegea, A., & Rebegea, L.-F. (2025). Synchronous/Metachronous Prostate Cancer with Other Cancer Sites—The Experience of a Single Center in Romania. Medicina, 61(9), 1666. https://doi.org/10.3390/medicina61091666





