Elevated Levels of sLAG-3 as a Possible Biomarker in Graves’ Disease With and Without Thyroid Eye Disease: A Prospective Observational Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
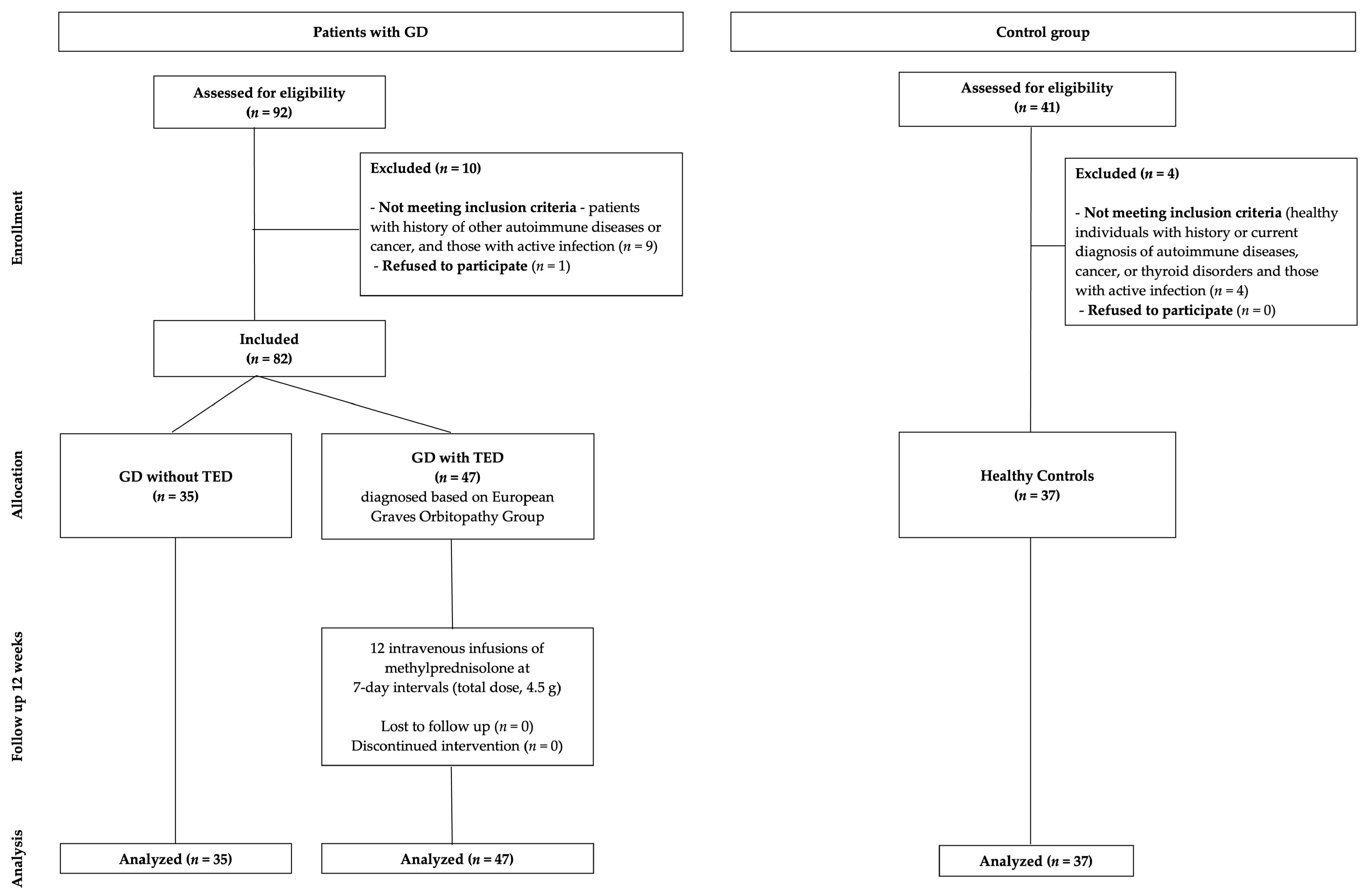
2.1. Study Population
2.2. Measurement and Analysis of Serum sLAG-3 Concentration
2.3. Additional Tests Including Routine Thyroid Assessment
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
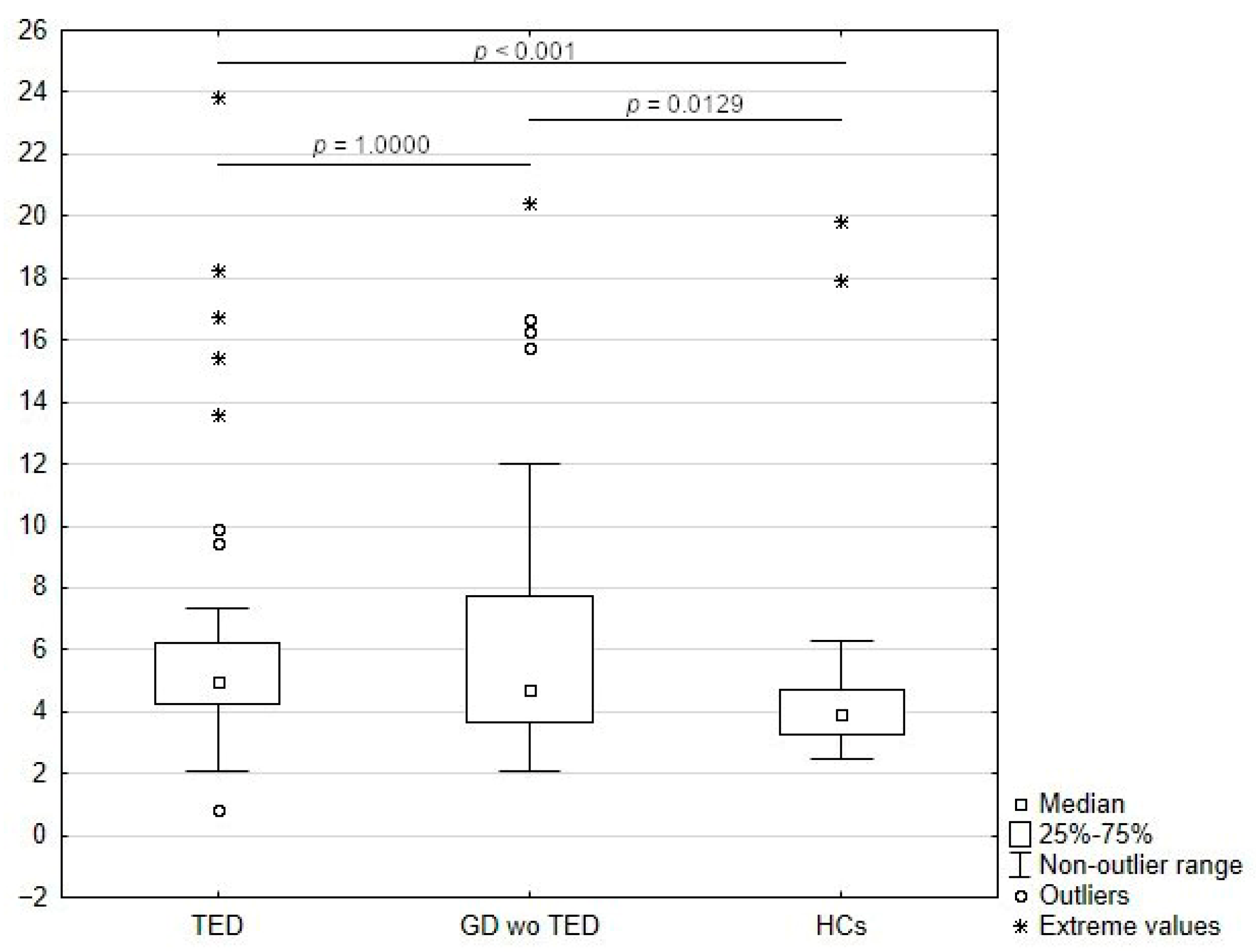
3.2. Comparison of sLAG -3 Concentrations Among Study Groups
3.3. Effects of IVGC Treatment
3.4. Analysis of Correlation of sLAG-3 Levels with Clinical and Laboratory Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cells |
| ATD | Antithyroid drugs |
| CAS | Clinical Activity Score |
| EUGOGO | European Graves’ Orbitopathy Group |
| GD | Graves’ disease |
| HCs | Healthy control subjects |
| hTSHR | Recombinant human TSH receptors |
| ICs | Immune checkpoint molecules |
| IVGC | Intravenous glucocorticosteroid |
| MHC class II | Major histocompatibility complex class II |
| NA | Not applicable |
| OF | Orbital fibroblast |
| Q1 | First quartile |
| Q3 | Third quartile |
| RA | Rheumatoid arthritis |
| rhLAG-3 | Recombinant human LAG-3 |
| SD | Standard deviation |
| sICP | Soluble immune checkpoint |
| sICs | Soluble immune checkpoint molecules |
| sLAG-3 | Soluble LAG-3 |
| TCR | T cell antigen receptor |
| TED | Thyroid eye disease |
| TSH | Thyroid-stimulating hormone |
| TSH-R | Thyrotropin hormone receptor |
| TSI | Thyroid-stimulating immunoglobulin |
References
- Kahaly, G.J.; Bartalena, L.; Hegedüs, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid. J. 2018, 7, 167–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kustrimovic, N.; Gallo, D.; Piantanida, E.; Bartalena, L.; Lai, A.; Zerbinati, N.; Tanda, M.L.; Mortara, L. Regulatory T Cells in the Pathogenesis of Graves’ Disease. Int. J. Mol. Sci. 2023, 24, 16432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallo, D.; Piantanida, E.; Gallazzi, M.; Bartalena, L.; Tanda, M.L.; Bruno, A.; Mortara, L. Immunological Drivers in Graves’ Disease: NK Cells as a Master Switcher. Front. Endocrinol. 2020, 11, 406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallo, D.; Tanda, M.L.; Piantanida, E. Predicting the Risk of Graves Disease Relapse: Commentary on “Thyroid Peroxidase Antibody Positivity is Associated with Relapse-Free Survival Following Antithyroid Drug Treatment for Graves Disease”. Endocr. Pract. 2020, 26, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Cieplińska, K.; Niedziela, E.; Kowalska, A. Immunological Processes in the Orbit and Indications for Current and Potential Drug Targets. J. Clin. Med. 2023, 13, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marino, M.; Vaidya, B.; Wiersinga, W.M. The 2021 European Group on Graves’ Orbitopathy (Eugogo) Clinical Practice Guidelines for the Medical Management of Graves’ Orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Tanda, M.L. Current concepts regarding Graves’ orbitopathy. J. Intern. Med. 2022, 292, 692–716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rotondo Dottore, G.; Torregrossa, L.; Caturegli, P.; Ionni, I.; Sframeli, A.; Sabini, E.; Menconi, F.; Piaggi, P.; Sellari-Franceschini, S.; Nardi, M.; et al. Association of T and B Cells Infiltrating Orbital Tissues with Clinical Features of Graves Orbitopathy. JAMA Ophthalmol. 2018, 136, 613–619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartalena, L.; Piantanida, E.; Gallo, D.; Lai, A.; Tanda, M.L. Epidemiology, Natural History, Risk Factors, and Prevention of Graves’ Orbitopathy. Front. Endocrinol. 2020, 11, 615993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marinò, M.; Rotondo Dottore, G.; Ionni, I.; Lanzolla, G.; Sabini, E.; Ricci, D.; Sframeli, A.; Mazzi, B.; Menconi, F.; Latrofa, F.; et al. Serum antibodies against the insulin-like growth factor-1 receptor (IGF-1R) in Graves’ disease and Graves’ orbitopathy. J. Endocrinol. Investig. 2019, 42, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Tanda, M.L.; Gallo, D.; Ippolito, S.; Bartalena, L.; Piantanida, E. Treatment of moderate-to-severe and active Graves’ orbitopathy: A step forward from the OPTIC study. J. Endocrinol. Investig. 2020, 43, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Rotondo Dottore, G.; Bucci, I.; Lanzolla, G.; Dallan, I.; Sframeli, A.; Torregrossa, L.; Casini, G.; Basolo, F.; Figus, M.; Nardi, M.; et al. Genetic Profiling of Orbital Fibroblasts from Patients with Graves’ Orbitopathy. J. Clin. Endocrinol. Metab. 2021, 106, e2176–e2190. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.M.; Feldon, S.E.; Smith, T.J.; Phipps, R.P. Immune mechanisms in thyroid eye disease. Thyroid 2008, 18, 959–965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, S.; Lu, Y.; Huang, Y.; Zhou, H.; Fan, X. Mechanisms That Underly T Cell Immunity in Graves’ Orbitopathy. Front. Endocrinol. 2021, 12, 648732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the third checkpoint inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burnell, S.E.A.; Capitani, L.; MacLachlan, B.J.; Mason, G.H.; Gallimore, A.M.; Godkin, A. Seven mysteries of LAG-3: A multi-faceted immune receptor of increasing complexity. Immunother. Adv. 2021, 2, ltab025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Klein, C.; Cochran, J.R.; Sockolosky, J.; Lippow, S.M. Exploring new frontiers in LAG-3 biology and therapeutics. Trends Pharmacol. Sci. 2025, 46, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor. Semin. Immunol. 2019, 42, 101305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Triebel, F. LAG-3: A regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003, 24, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Forbes, K.; Vignali, K.M.; Heale, B.S.; Saftig, P.; Hartmann, D.; Black, R.A.; Rossi, J.J.; Blobel, C.P.; et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007, 26, 494–504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malinga, N.Z.; Siwele, S.C.; Steel, H.C.; Kwofie, L.L.; Meyer, P.W.; Smit, T.; Anderson, R.; Rapoport, B.L.; Kgokolo, M.C. Systemic levels of the soluble co-inhibitory immune checkpoints, CTLA-4, LAG-3, PD-1/PD-L1 and TIM-3 are markedly increased in basal cell carcinoma. Transl. Oncol. 2022, 19, 101384. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Wu, X.; Liu, Y.; Wu, Q.; Wu, J.; Zhang, H.; Zhou, M.; Qu, J. Soluble Immune-related proteins as new candidate serum biomarkers for the diagnosis and progression of Lymphangioleiomyomatosis. Front. Immunol. 2022, 13, 844914. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Tian, J.; Zhou, Y.; Shen, Y.; Wang, M.; Tang, L.; Liu, C.; Zhang, X.; Shen, F.; et al. Clinical significance of Soluble LAG-3 (sLAG-3) in patients with Cervical Cancer determined via enzyme-linked immunosorbent assay with monoclonal antibodies. Technol. Cancer Res. Treat. 2023, 22, 15330338231202650. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Tu, H.; Liang, D.; Chang, D.W.; Ye, Y.; Wu, X. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J. Immunother. Cancer. 2019, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Gorgulho, J.; Roderburg, C.; Beier, F.; Bokemeyer, C.; Brümmendorf, T.H.; Loosen, S.H.; Luedde, T. Soluble lymphocyte activation gene-3 (sLAG3) and CD4/CD8 ratio dynamics as predictive biomarkers in patients undergoing immune checkpoint blockade for solid malignancies. Br. J. Cancer. 2024, 130, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Botticelli, A.; Zizzari, I.G.; Scagnoli, S.; Pomati, G.; Strigari, L.; Cirillo, A.; Cerbelli, B.; Di Filippo, A.; Napoletano, C.; Scirocchi, F.; et al. The Role of Soluble LAG3 and Soluble Immune Checkpoints Profile in Advanced Head and Neck Cancer: A Pilot Study. J. Pers. Med. 2021, 11, 651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landeira-Viñuela, A.; Arias-Hidalgo, C.; Juanes-Velasco, P.; Alcoceba, M.; Navarro-Bailón, A.; Pedreira, C.E.; Lecrevisse, Q.; Díaz-Muñoz, L.; Sánchez-Santos, J.M.; Hernández, Á.P.; et al. Unravelling soluble immune checkpoints in chronic lymphocytic leukemia: Physiological immunomodulators or immune dysfunction. Front. Immunol. 2022, 13, 965905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, L.; Wang, Y.; Shen, X.; Ma, F.; Wang, J.; Yan, F. Soluble form of immune checkpoints in autoimmune diseases. J. Autoimmun. 2024, 147, 103278. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Shao, Y.; Chen, Y.; Zeng, C.; Huang, X.; Wei, R. Immune checkpoints: New insights into the pathogenesis of thyroid eye disease. Front. Immunol. 2024, 15, 1392956. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khamisi, S.; Anders Karlsson, F.; Ljunggren, Ö.; Thulin, M.; Larsson, A. Increased plasma levels of soluble programmed death ligand 1 (sPD-L1) and fibroblast growth factor 23 (FGF-23) in patients with Graves’ ophthalmopathy in comparison to hyperthyroid patients without Graves’ ophthalmopathy. Cytokine 2023, 169, 156269. [Google Scholar] [CrossRef] [PubMed]
- Cieplińska, K.; Niedziela, E.; Rdzanek, A.K.; Słuszniak, A.; Chrapek, M.; Pałyga, I.; Kowalska, A. Association between clinical activity score and serum sPD-1 and sPD-L1 levels during systemic glucocorticoid treatment for active moderate-to-severe thyroid eye disease. Cytokine 2025, 187, 156862. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhu, T.; Song, D.; Fang, S.; Zhou, H.; Guan, H. Soluble immune checkpoints associated with disease activity and treatment response in GD and TED. J. Clin. Endocrinol. Metab. 2024, 110, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Luo, Q.; Liu, N.; Wei, G.; Wu, X.; Lu, J.; Tang, K.; Wu, Y.; Zi, J.; Li, X.; et al. Increased ADAM10 expression in patients with immune thrombocytopenia. Int. Immunopharmacol. 2018, 55, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Saadati, N.; Khodashahi, M.; Rezaieyazdi, Z.; Sahebari, M.; Saremi, Z.; Mohammadian Haftcheshmeh, S.; Rafatpanah, H.; Salehi, M. Serum Level of Soluble Lymphocyte-Activation Gene 3 Is Increased in Patients with Rheumatoid Arthritis. Iran. J. Immunol. 2020, 17, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.Y.; Yoon, T.; Ahn, S.S.; Song, J.J.; Park, Y.B.; Lee, S.W. Soluble immune checkpoint molecules in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Sci. Rep. 2022, 12, 21319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, X.; Wang, X.; Guan, Y.; Wang, L.; Gao, Y.; Niu, J. Soluble immune checkpoints are elevated in patients with primary biliary cholangitis. Eur. J. Med. Res. 2023, 28, 477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dana Yehudai-Ofir Nasren Eiza Israel, J. Henig, Tsila Zuckerman, Zahava Vadasz, Soluble LAG3: A Potential Marker for the Development and Activity of Acute Graft-Versus-Host Disease. Blood 2023, 142 (Suppl. S1), 4795. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, T.; Yang, L.; Qian, W.; Fang, L.; Zhang, W.; Zhang, H.; Wang, Y.; Yu, B.; Sun, J.; et al. Cigarette Smoking Drives Thyroid Eye Disease Progression via RAGE Signaling Activation. Thyroid 2025, 35, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Mastrangeli, R.; Prigent, P.; Bruniquel, D.; Donini, S.; El-Tayar, N.; Maigret, B.; Dréano, M.; Triebel, F. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc. Natl. Acad. Sci. USA 1997, 94, 5744–5749. [Google Scholar] [CrossRef]
- Li, N.; Jilisihan, B.; Wang, W.; Tang, Y.; Keyoumu, S. Soluble LAG3 acts as a potential prognostic marker of gastric cancer and its positive correlation with CD8+T cell frequency and secretion of IL-12 and INF-γ in peripheral blood. Cancer Biomark. 2018, 23, 341–351. [Google Scholar] [CrossRef] [PubMed]
- El Mir, S.; Triebel, F. A soluble lymphocyte activation gene-3 molecule used as a vaccine adjuvant elicits greater humoral and cellular immune responses to both particulate and soluble antigens. J. Immunol. 2000, 164, 5583–5589. [Google Scholar] [CrossRef] [PubMed]
- Andreae, S.; Piras, F.; Burdin, N.; Triebel, F. Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223). J. Immunol. 2002, 168, 3874–3880. [Google Scholar] [CrossRef] [PubMed]
- Prigent, P.; El Mir, S.; Dréano, M.; Triebel, F. Lymphocyte activation gene-3 induces tumor regression and antitumor immune responses. Eur. J. Immunol. 1999, 29, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.; Caldera, O.; Smith, T.J. Therapeutic IGF-I receptor inhibition alters fibrocyte immune phenotype in thyroid-associated ophthalmopathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2114244118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riva, A. Editorial: Soluble immune checkpoints: Novel physiological immunomodulators. Front. Immunol. 2023, 14, 1178541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Characteristic | Patients with GD Without TED (N = 35) | Patients with GD and TED (N = 47) |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 53.09 (17.78) | 55.13 (12.02) |
| Median (Q1, Q3) | 57 (39.00, 67.00) | 54 (46.00, 64.00) |
| Range | 20–80 | 20–78 |
| Sex | ||
| Female | 26 (74.29%) | 30 (68.09%) |
| Male | 9 (25.71%) | 15 (31.92%) |
| Smoking history | ||
| Non-smoker | 25 (75.76%) | 20 (42.55%) |
| Former smoker | 3 (9.10%) | 11 (23.40%) |
| Active smoker | 5 (15.15%) | 16 (34.04%) |
| Duration of GD (months) | ||
| Mean (SD) | 55.60 (63.43) | 23.72 (37.40) |
| Median (Q1, Q3) | 36 (4.00, 72.00) | 12 (7.00, 24.00) |
| Range | 1–240 | 2–240 |
| Duration of GD (months) | ||
| ≤12 | 12 (35.29%) | 26 (55.32%) |
| >12 | 22 (64.71%) | 21 (44.68%) |
| Duration of TED (months) | ||
| Mean (SD) | NA | 6.25 (3.98) |
| Median (Q1, Q3) | NA | 6 (3.00, 8.00) |
| Range | NA | 1–18 |
| Duration of TED (months) | ||
| ≤12 | NA | 45 (95.75%) |
| >12 | NA | 2 (4.26%) |
| TSH (µIU/mL) | ||
| Mean (SD) | 1.36 (2.62) | 4.49 (12.15) |
| Median (Q1, Q3) | 0.39 (0.01, 1.41) | 0.92 (0.11–1.98) |
| Range | 0–12.49 | 0–71.08 |
| TSH (µIU/mL) | ||
| <0.35 | 15 (46.88%) | 16 (34.04%) |
| 0.35–4.94 | 14 (43.75%) | 25 (53.19%) |
| >4.94 | 3 (9.38%) | 6 (12.77%) |
| Maximum CAS at baseline | ||
| 1 | NA | 2 (4.25%) |
| 2 | NA | 4 (8.51%) |
| 3 | NA | 13 (27.66%) |
| 4 | NA | 14 (29.79%) |
| 5 | NA | 9 (19.15%) |
| 6 | NA | 4 (8.51%) |
| 7 | NA | 1 (2.13%) |
| Maximum CAS at baseline | ||
| Mean (SD) | NA | 3.85 (1.32) |
| Median (Q1, Q3) | NA | 4 (3, 5) |
| Range | NA | 1–7 |
| TSI at baseline (IU/L) | ||
| Mean (SD) | 4.15 (4.12) | 6.40 (7.93) |
| Median (Q1, Q3) | 2.17 (1.33, 5.98) | 2.90 (0.72, 10.3) |
| Range | 0.31–15.20 | 0.1–37.4 |
| TSI at baseline > 2 (IU/L) | ||
| No | 17 (48.57%) | 19 (40.43%) |
| Yes | 18 (51.43%) | 28 (59.57%) |
| sLAG-3 at baseline (ng/mL) | ||
| Mean (SD) | 6.73 (4.52) | 6.86 (5.06) |
| Median (Q1, Q3) | 4.73 (3.63, 7.75) | 4.94 (4.23, 6.23) |
| Range | 2.10–20.43 | 0.848–23.84 |
| IL-6 at baseline (pg/mL) | ||
| Mean (SD) | 3.37 (2.40) | 3.30 (2.37) |
| Median (Q1, Q3) | 2.40 (2.00, 3.36) | 2.2 (2, 3.51) |
| Range | 2–11.6 | <2–12.5 |
| IL-6 at baseline (pg/mL) | ||
| <3.4 | 27 (77.14%) | 29 (61.70%) |
| >3.4 | 8 (22.86%) | 10 (38.30%) |
| Antithyroid drugs | ||
| Treatment-naïve | 2 (5.71%) | 6 (12.77%) |
| Currently under treatment | 23 (65.71%) | 38 (80.85%) |
| Previously treated, not currently | 10 (28.57%) | 3 (6.38%) |
| HCs (N = 37) | Patients with GD Without TED (N = 35) | Patients with GD and TED (N = 47) | p-Value (HC vs. GD Without TED) | p-Value (HC vs. TED) | p-Value (GD Without TED vs. TED) | |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Mean (SD) | 55.11 (13.89) | 53.09 (17.78) | 55.13 (12.02) | 0.5913 * | 0.9945 * | 0.5597 ** |
| Median (Q1, Q3) | 55 (45, 67) | 57 (39, 67) | 54 (46, 64) | |||
| Range | 23–78 | 20–80 | 20–78 | |||
| Sex | 0.2702 *** | 0.5708 *** | 0.5416 *** | |||
| Female | 23 (62.16%) | 26 (74.29%) | 32 (68.09%) | |||
| Male | 14 (37.84%) | 9 (25.71%) | 15 (31.92%) | |||
| sLAG-3 at baseline (ng/mL) | 0.0129 **** | <0.001 **** | 1.000 **** | |||
| Mean (SD) | 4.76 (3.56) | 6.73 (4.52) | 6.68 (5.06) | |||
| Median (Q1, Q3) | 3.94 (3.28, 4.71) | 4.73 (3.63, 7.75) | 5.06 (4.23, 6.16) | |||
| Range | 2.46–19.85 | 2.10–20.43 | 0.85–23.84 |
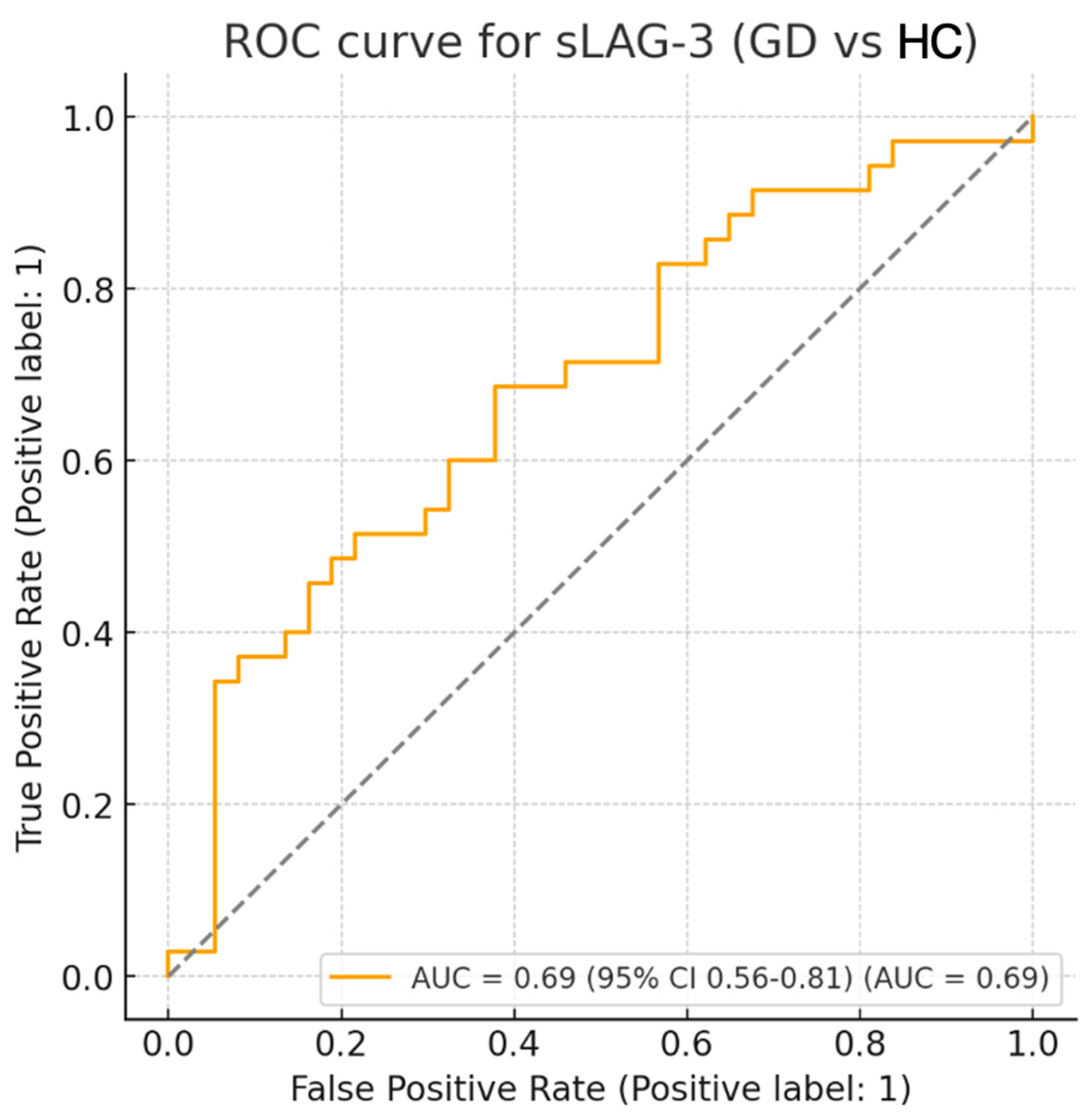
| Parameter | Value | 95% CI | p-Value |
|---|---|---|---|
| AUC | 0.69 | 0.56–0.81 | 0.004 |
| Optimal cut-off (ng/mL) | 4.18 | – | – |
| Sensitivity (%) | 68.6 | – | – |
| Specificity (%) | 62.2 | – | – |
| False positives (n) | 14 | – | – |
| False negatives (n) | 11 | – | – |
| Youden index | 0.307 | – | – |
| At Baseline (N = 47) | sLAG-3 at Baseline (ng/mL) Mean (SD) Median (Q1, Q3) Range | sLAG-3 at 12 Weeks (ng/mL) Mean (SD) Median (Q1, Q3) Range | p-Value (for Difference in sLAG-3 Concentration) |
|---|---|---|---|
| All patients | 6.68 (5.06) | 6.91 (4.86) | 0.0536 |
| 4.94 (4.23, 6.23) | 5.13 (4.62, 6.54) | ||
| 0.85–23.84 | 0.79–23.84 | ||
| TSH 0.35–4. 94 (µIU/mL) (N = 25) | 7.12 (5.92) | 7.34 (5.61) | 0.0777 |
| 5.07 (4.19–6.44) | 5.19 (4.79–6.89) | ||
| 0.85–23.84 | 0.79–23.84 | ||
| TSH < 0.35 (µIU/mL) (N = 16) | 6.78 (4.47) | 7.05 (4.43) | 0.6051 |
| 5.19 (4.38–6.50) | 5.42 (4.68–7.30) | ||
| 2.92–18.25 | 3.10–17.95 | ||
| TSI > 2 (IU/L) (N = 28) | 7.36 (6.07) | 7.46 (5.81) | 0.1213 |
| 4.91 (4.29–5.98) | 5.04 (4.61–6.64) | ||
| 2.92–23.84 | 3.10–23.84 | ||
| TSI ≤ 2 (IU/L) (N = 19) | 5.67 (2.89) | 6.10 (2.96) | 0.2432 |
| 5.07 (4.17–6.90) | 5.35 (4.68–6.54) | ||
| 0.85–13.59 | 0.79–13.32 | ||
| IL-6 < 3.4 (pg/mL) (N = 29) | 6.62 (4.80) | 6.60 (4.52) | 0.1658 |
| 4.94 (4.23–6.09) | 5.03 (4.64–5.64) | ||
| 2.09–23.84 | 2.99–23.84 | ||
| IL-6 > 3.4 (pg/mL) (N = 10) | 7.53 (5.98) | 7.94 (5.81) | 0.4413 |
| 5.19 (4.69–7.33) | 6.07 (4.68–8.69) | ||
| 4.10–23.84 | 4.60–23.84 | ||
| Non-smokers (N = 20) | 8.12 (6.79) | 8.71 (6.64) | 0.1570 |
| 5.07 (4.29–8.61) | 5.42 (4.68–9.96) | ||
| 2.09–23.84 | 2.99–23.84 | ||
| Active Smokers (N = 16) | 6.01 (3.43) | 5.78 (2.36) | 0.3794 |
| 4.95 (4.29–5.72) | 5.01 (4.61–5.99) | ||
| 3.15–15.41 | 3.60–13.32 | ||
| Duration of TED ≤ 6 months (N = 28) | 7.61 (6.03) | 8.00 (5.95) | 0.0734 |
| 5.20 (4.21–8.38) | 5.35 (4.59–9.87) | ||
| 2.09–23.84 | 2.99–23.84 | ||
| Duration of TED > 6 months (N = 19) | 5.30 (2.74) | 5.31 (1.66) | 0.3144 |
| 4.84 (4.36–5.44) | 5.13 (4.64–5.92) | ||
| 0.85–15.41 | 0.79–8.83 | ||
| Duration of GD ≤ 12 months (N = 26) | 6.80 (4.69) | 7.29 (4.72) | 0.1094 |
| 5.14 (4.36–6.23) | 5.27 (4.68–8.69) | ||
| 2.09–23.84 | 2.99–23.84 | ||
| Duration of GD > 12 months (N = 21) | 6.53 (5.60) | 6.43 (5.11) | 0.2471 |
| 4.76 (4.10–5.44) | 5.13 (4.60–5.76) | ||
| 0.848–23.84 | 0.787–23.84 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| lag3 | 0.99 | 0.85–1.15 | 0.89 |
| TSI | 1.04 | 0.93–1.17 | 0.46 |
| IL6 | 1.00 | 0.72–1.39 | 1.00 |
| Age | 1.02 | 0.96–1.10 | 0.51 |
| Smoking (former vs. current) | 1.98 | 0.23–17.1 | 0.54 |
| Smoking (never vs. current) | 0.44 | 0.07–2.76 | 0.38 |
| ATD status (former vs. current) | – | – | 1.00 * |
| ATD status (never vs. current) | 0.46 | 0.01–16.2 | 0.67 |
| GD duration (months) | 1.03 | 0.98–1.08 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieplińska, K.; Niedziela, E.; Jagielska, E.; Pałyga, I.; Słuszniak, A.; Kowalska, A. Elevated Levels of sLAG-3 as a Possible Biomarker in Graves’ Disease With and Without Thyroid Eye Disease: A Prospective Observational Case–Control Study. Medicina 2025, 61, 1664. https://doi.org/10.3390/medicina61091664
Cieplińska K, Niedziela E, Jagielska E, Pałyga I, Słuszniak A, Kowalska A. Elevated Levels of sLAG-3 as a Possible Biomarker in Graves’ Disease With and Without Thyroid Eye Disease: A Prospective Observational Case–Control Study. Medicina. 2025; 61(9):1664. https://doi.org/10.3390/medicina61091664
Chicago/Turabian StyleCieplińska, Katarzyna, Emilia Niedziela, Edyta Jagielska, Iwona Pałyga, Anna Słuszniak, and Aldona Kowalska. 2025. "Elevated Levels of sLAG-3 as a Possible Biomarker in Graves’ Disease With and Without Thyroid Eye Disease: A Prospective Observational Case–Control Study" Medicina 61, no. 9: 1664. https://doi.org/10.3390/medicina61091664
APA StyleCieplińska, K., Niedziela, E., Jagielska, E., Pałyga, I., Słuszniak, A., & Kowalska, A. (2025). Elevated Levels of sLAG-3 as a Possible Biomarker in Graves’ Disease With and Without Thyroid Eye Disease: A Prospective Observational Case–Control Study. Medicina, 61(9), 1664. https://doi.org/10.3390/medicina61091664






