Clinical and Biological Relevance of Kidney Injury Molecule-1 and Beta-2 Microglobulin in Monitoring Patients with Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical and Biological Characteristics of the Studied Groups
2.3. Laboratory Tests
2.4. Statistical Analysis
3. Results
4. Discussion
- (a)
- The elevated levels of KIM-1 and β2MG in patients with SLE compared to controls suggest that these biomarkers play a significant role in the pathophysiology of SLE. The measurement of KIM-1 and β2MG could serve as a molecular prognostic model for individuals diagnosed with SLE.
- (b)
- Both serum and urinary levels of KIM-1 and β2MG are associated with the clinical and biological manifestations of SLE.
- (c)
- KIM-1 is a marker that defines the renal phenotype in SLE patients.
- (d)
- β2MG reflects the progression of cumulative lesions in individuals with SLE.
- (e)
- Serum β2MG levels can distinguish between patients with renal lesions and those without renal lesions.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.-J.; Xu, R.-Y.; Kang, L.-L. Biomarkers for Systemic Lupus Erythematosus: A Scoping Review. Immun. Inflamm. Dis. 2024, 12, e70022. [Google Scholar] [CrossRef]
- Siegel, C.H.; Sammaritano, L.R. Systemic Lupus Erythematosus: A Review. JAMA 2024, 331, 1480–1491. [Google Scholar] [CrossRef]
- Roveta, A.; Parodi, E.L.; Brezzi, B.; Tunesi, F.; Zanetti, V.; Merlotti, G.; Francese, A.; Maconi, A.G.; Quaglia, M. Lupus Nephritis from Pathogenesis to New Therapies: An Update. Int. J. Mol. Sci. 2024, 25, 8981. [Google Scholar] [CrossRef]
- Musa, R.; Rout, P.; Qurie, A. Lupus Nephritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- De Vriese, A.S.; Sethi, S.; Fervenza, F.C. Lupus Nephritis: Redefining the Treatment Goals. Kidney Int. 2025, 107, 198–211. [Google Scholar] [CrossRef]
- Rossi, G.M.; Vaglio, A. New Treatment Regimens, New Drugs, and New Treatment Goals for Lupus Nephritis. J. Clin. Med. 2025, 14, 584. [Google Scholar] [CrossRef]
- Ene, C.-D.; Penescu, M.N.; Nicolae, I. Sialoglyco-Conjugate Abnormalities, IL-6 Trans-Signaling and Anti-Ganglioside Immune Response-Potential Interferences in Lupus Nephritis Pathogenesis. Diagnostics 2021, 11, 1129. [Google Scholar] [CrossRef]
- Ene, C.D.; Georgescu, S.R.; Tampa, M.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Penescu, M.N.; Nicolae, I. Cellular Response against Oxidative Stress, a Novel Insight into Lupus Nephritis Pathogenesis. J. Pers. Med. 2021, 11, 693. [Google Scholar] [CrossRef]
- Kim, Y.; Koopman, J.J.E.; Choi, M.; Feldman, C.H.; Costenbader, K.H. Environmental Risk Factors for Systemic Lupus Erythematosus Through the Lens of Social Determinants of Health. Arthritis Care Res. 2025, 77, 689–699. [Google Scholar] [CrossRef]
- You, T.; Lin, X.; Zhang, C.; Wang, W.; Lei, M. Correlation between Serum Β2-Microglobulin Level and Systemic Lupus Erythematosus Disease Activity: A PRISMA-Compliant Meta-Analysis. Medicine 2022, 101, e30594. [Google Scholar] [CrossRef]
- Skopelja-Gardner, S.; Tai, J.; Sun, X.; Tanaka, L.; Kuchenbecker, J.A.; Snyder, J.M.; Kubes, P.; Mustelin, T.; Elkon, K.B. Acute Skin Exposure to Ultraviolet Light Triggers Neutrophil-Mediated Kidney Inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019097118. [Google Scholar] [CrossRef]
- Aldea, P.L.; Turbuleasa-Jurje, R.A.; Bulata, B.; Delean, D.; Elec, F.I.; Ciumarnean, L.; Bot (Rachisan), A.L. The Evaluation of Serum KIM-1 in a Pediatric Cohort of Renal Transplantation—A Pilot Study. Children 2025, 12, 63. [Google Scholar] [CrossRef]
- Tutunea-Fatan, E.; Arumugarajah, S.; Suri, R.S.; Edgar, C.R.; Hon, I.; Dikeakos, J.D.; Gunaratnam, L. Sensing Dying Cells in Health and Disease: The Importance of Kidney Injury Molecule-1. J. Am. Soc. Nephrol. JASN 2024, 35, 795–808. [Google Scholar] [CrossRef]
- Fathy, H.M.; Mohamed, R.A.; Korany, M.G.; Abd-ElAzeem, M.I. Relation of Serum Beta 2 Microglobulin Levels to Systemic Lupus Disease Manifestations and Disease Activity. Egypt. J. Hosp. Med. 2023, 90, 3209–3214. [Google Scholar] [CrossRef]
- Żychowska, I.; Suszek, D.; Dryglewska, M.; Majdan, M. Β2-Microglobulin as a Marker of Systemic Lupus Erythematosus Activity. Adv. Clin. Exp. Med. Off. Organ. Wroclaw Med. Univ. 2018, 27, 379–382. [Google Scholar] [CrossRef]
- Gamal, D.M.; Badr, F.M.; Taha, S.I.A.E.F.; Moustafa, N.M.; Teama, M.A.E.M. Serum Beta-2 Microglobulin as a Predictor of Nephritis, Disease Activity, and Damage Score in Systemic Lupus Erythematosus: A Cross-Sectional Study. Rheumatol. Int. 2023, 43, 323–333. [Google Scholar] [CrossRef]
- Puia, D.; Ivănuță, M.; Pricop, C. Kidney Injury Molecule-1 as a Biomarker for Renal Cancer: Current Insights and Future Perspectives—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3431. [Google Scholar] [CrossRef]
- Tampa, M.; Georgescu, S.R.; Mitran, M.I.; Mitran, C.I.; Matei, C.; Caruntu, A.; Scheau, C.; Nicolae, I.; Matei, A.; Caruntu, C.; et al. Current Perspectives on the Role of Matrix Metalloproteinases in the Pathogenesis of Basal Cell Carcinoma. Biomolecules 2021, 11, 903. [Google Scholar] [CrossRef]
- Raju, M.C.; Hossain, R.M.; Ahmed, A.H.H.; Islam, M.N.; Faroque, O.; Uddin, B.; Rafi, S.R.; Daisy, A.A.; Jahan, N.; Amin, N. Urine KIM-1 as Predictor of Renal Histopathology and Treatment Response in Lupus Nephritis Patients. Arch. Nephrol. Urol. 2025, 8, 1–8. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.-H.; Roumelioti, M.-E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Puthiyottil, D.; Priyamvada, P.S.; Kumar, M.N.; Chellappan, A.; Zachariah, B.; Parameswaran, S. Role of Urinary Beta 2 Microglobulin and Kidney Injury Molecule-1 in Predicting Kidney Function at One Year Following Acute Kidney Injury. Int. J. Nephrol. Renov. Dis. 2021, 14, 225–234. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Tang, F.; Yang, J.-Z.; Chen, X.; Wang, Z.-F.; Li, Z.-Q. The Role of Beta2-Microglobulin in Central Nervous System Disease. Cell Mol. Neurobiol. 2024, 44, 46. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Yao, T.; Zhou, J.; Wang, Z. The Immune-Related Role of Beta-2-Microglobulin in Melanoma. Front. Oncol. 2022, 12, 944722. [Google Scholar] [CrossRef]
- Chen, C.-H.; Su, C.-Y.; Chien, C.-Y.; Huang, C.-C.; Chuang, H.-C.; Fang, F.-M.; Huang, H.-Y.; Chen, C.-M.; Chiou, S.-J. Overexpression of Β2-Microglobulin Is Associated with Poor Survival in Patients with Oral Cavity Squamous Cell Carcinoma and Contributes to Oral Cancer Cell Migration and Invasion. Br. J. Cancer 2008, 99, 1453–1461. [Google Scholar] [CrossRef]
- Kim, H.-A.; Jeon, J.-Y.; Yoon, J.-M.; Suh, C.-H. Beta2-Microglobulin Can Be a Disease Activity Marker in Systemic Lupus Erythematosus. Am. J. Med. Sci. 2010, 339, 337–340. [Google Scholar] [CrossRef]
- Chan, O.T.; Paliwal, V.; McNiff, J.M.; Park, S.H.; Bendelac, A.; Shlomchik, M.J. Deficiency in Beta(2)-Microglobulin, but Not CD1, Accelerates Spontaneous Lupus Skin Disease While Inhibiting Nephritis in MRL-Fas(Lpr) Nice: An Example of Disease Regulation at the Organ Level. J. Immunol. 2001, 167, 2985–2990. [Google Scholar] [CrossRef]
- Yang, L.; Brooks, C.R.; Xiao, S.; Sabbisetti, V.; Yeung, M.Y.; Hsiao, L.-L.; Ichimura, T.; Kuchroo, V.; Bonventre, J.V. KIM-1-Mediated Phagocytosis Reduces Acute Injury to the Kidney. J. Clin. Investig. 2015, 125, 1620–1636. [Google Scholar] [CrossRef]
- Al-Bataineh, M.M.; Kinlough, C.L.; Mi, Z.; Jackson, E.K.; Mutchler, S.M.; Emlet, D.R.; Kellum, J.A.; Hughey, R.P. KIM-1-Mediated Anti-Inflammatory Activity Is Preserved by MUC1 Induction in the Proximal Tubule during Ischemia-Reperfusion Injury. Am. J. Physiol. Renal Physiol. 2021, 321, F135–F148. [Google Scholar] [CrossRef]
- Abd-Elbaky, N.M.; Albeltagy, E.S.; Hammour, A.E.; Ibrahim, A.S. Evaluation of Serum Β2-Microglobulin in Egyptian Patients with Systemic Lupus Erythematosus. Egypt. J. Immunol. 2019, 26, 143–153. [Google Scholar]
- Karmakova, T.A.; Sergeeva, N.S.; Kanukoev, K.Y.; Alekseev, B.Y.; Kaprin, A.D. Kidney Injury Molecule 1 (KIM-1): A Multifunctional Glycoprotein and Biological Marker (Review). Sovrem. Tekhnologii V Meditsine 2021, 13, 64–78. [Google Scholar] [CrossRef]
- Muneshige, K.; Onuma, K.; Sukegawa, K.; Otake, Y.; Inoue, G.; Takaso, M.; Uchida, K. Β2-Microglobulin Elevates COL5A1 mRNA in the Subsynovial Connective Tissue of Patients Receiving Hemodialysis With Carpal Tunnel Syndrome. Cureus 2022, 4, e32423. [Google Scholar] [CrossRef]
- Tinajero-Sánchez, D.N.; Zúñiga-González, E.Y.; Zavala-Miranda, M.F.; Hernández-Andrade, A.; Navarro-Sánchez, V.; Nordmann-Gomes, A.; Rivero-Otamendi, E.; Uribe-Uribe, N.O.; Mejia-Vilet, J.M. Histologic Predictors of Kidney Outcomes in Lupus Nephritis: Reevaluating the Role of Segmental Glomerulosclerosis in the Chronicity Index. Rheumatology 2025, 64, keaf194. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V. Kidney Injury Molecule-1: A Translational Journey. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 293–299, discussion 299. [Google Scholar]
- Steiner, C.; Saad, E.; Saliby, R.M.; Eid, M.; Semaan, K.; Machaalani, M.; Yekeduz, E.; Horst, J.T.; Lee, J.; Phillips, N.; et al. Circulating Kidney Injury Molecule-1 (KIM-1) in Association with Kidney Injury Biomarkers and Outcomes in Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2025, 43 (Suppl. S5), 582. [Google Scholar] [CrossRef]
- Hofbauer, D.; Mougiakakos, D.; Broggini, L.; Zaiss, M.; Büttner-Herold, M.; Bach, C.; Spriewald, B.; Neumann, F.; Bisht, S.; Nolting, J.; et al. Β2-Microglobulin Triggers NLRP3 Inflammasome Activation in Tumor-Associated Macrophages to Promote Multiple Myeloma Progression. Immunity 2021, 54, 1772–1787.e9. [Google Scholar] [CrossRef] [PubMed]
- Riitano, G.; Capozzi, A.; Recalchi, S.; Caissutti, D.; Longo, A.; Mattei, V.; Conti, F.; Misasi, R.; Garofalo, T.; Sorice, M.; et al. Anti-Β2-GPI Antibodies Induce Endothelial Cell Expression of Tissue Factor by LRP6 Signal Transduction Pathway Involving Lipid Rafts. Cells 2022, 11, 1288. [Google Scholar] [CrossRef] [PubMed]
| Variable | SLE Patients (n = 80) | Controls (n = 30) | p-Value |
|---|---|---|---|
| uKIM-1 (ng/mL) | 3.1 ± 0.7 | 1.4 ± 0.3 | <0.01 |
| sKIM-1 (ng/dL) | 14.7 ± 1.3 | 8.8 ± 0.5 | <0.001 |
| uβ2MG (mg/L) | 2.1 ± 0.4 | 0.1 ± 0.1 | <0.001 |
| sβ2MG (ng/dL) | 5.3 ± 2.2 | 1.3 ± 0.4 | <0.001 |
| Variable | eGFR ≥90 (A) (n = 31) | eGFR 75–90 (B) (n = 29) | eGFR 60–75 (C) (n = 20) |
|---|---|---|---|
| uKIM-1 (ng/mL) | 2.2 ± 0.3 | 2.7 ± 0.5 | 4.7 ± 1.2 |
| sKIM-1 (ng/dL) | 9.2 ± 0.5 | 15.5 ± 1.0 * | 22.7 ± 1.5 |
| uβ2MG (mg/L) | 0.3 ± 0.1 | 2.2 ± 0.4 * | 4.8 ± 1.1 |
| sβ2MG (ng/dL) | 3.4 ± 0.9 | 4.9 ± 1.1 * | 8.7 ± 1.8 |
| Parameter | Albuminuria < 30 mg/24 h (A) | Albuminuria 30–299 mg/24 h (B) | Albuminuria 300–500 mg/24 h (C) |
|---|---|---|---|
| No of patients | 33 | 31 | 16 |
| uKIM-1 (ng/mL) | 2.1 ± 0.3 | 3.5 ± 0.5 | 4.5 ± 1.1 |
| sKIM-1 (ng/dL) | 9.8 ± 0.4 | 17.7 ± 1.1 * | 20.2 ± 1.3 |
| uβ2MG (mg/L) | 0.2 ± 0.1 | 2.9 ± 0.6 * | 4.4 ± 1.3 */** |
| sβ2MG (ng/dL) | 2.4 ± 0.3 | 5.9 ± 1.0 * | 9.9 ± 2.0 */** |
| Parameter | SLEDAI < 5 (A) | SLEDAI 5–11 (B) | SLEDAI ≥ 11 (C) |
|---|---|---|---|
| No of patients | 14 | 49 | 17 |
| uKIM-1 (ng/mL) | 2.8 ± 0.4 | 2.9 ± 0.9 | 3.9 ± 1.1 |
| sKIM-1 (ng/dL) | 13.4 ± 1.1 | 13.1 ± 1.9 * | 20.9 ± 2.2 |
| uβ2MG (mg/L) | 0.3 ± 0.2 | 2.5 ± −0.6 * | 2.4 ± 0.4 |
| sβ2MG (ng/dL) | 3.9 ± 0.7 | 4.8 ± 1.3 * | 7.7 ± 2.1 |
| Variable | No. of Cases | uβ2MG (mg/L) | sβ2MG (ng/dL) |
|---|---|---|---|
| Alopecia | Present (18) | 3.0 ± 0.6 | 5.4 ± 1.0 |
| Absent (11) | 2.9 ± 0.8 | 4.9 ± 1.3 | |
| p | p > 0.05 | p < 0.01 | |
| Oral ulcers | Present (14) | 2.7 ± 0.6 | 4.0 ± 0.8 |
| Absent (22) | 2.6 ± 0.8 | 3.4 ± 0.5 | |
| p | p > 0.05 | p < 0.01 | |
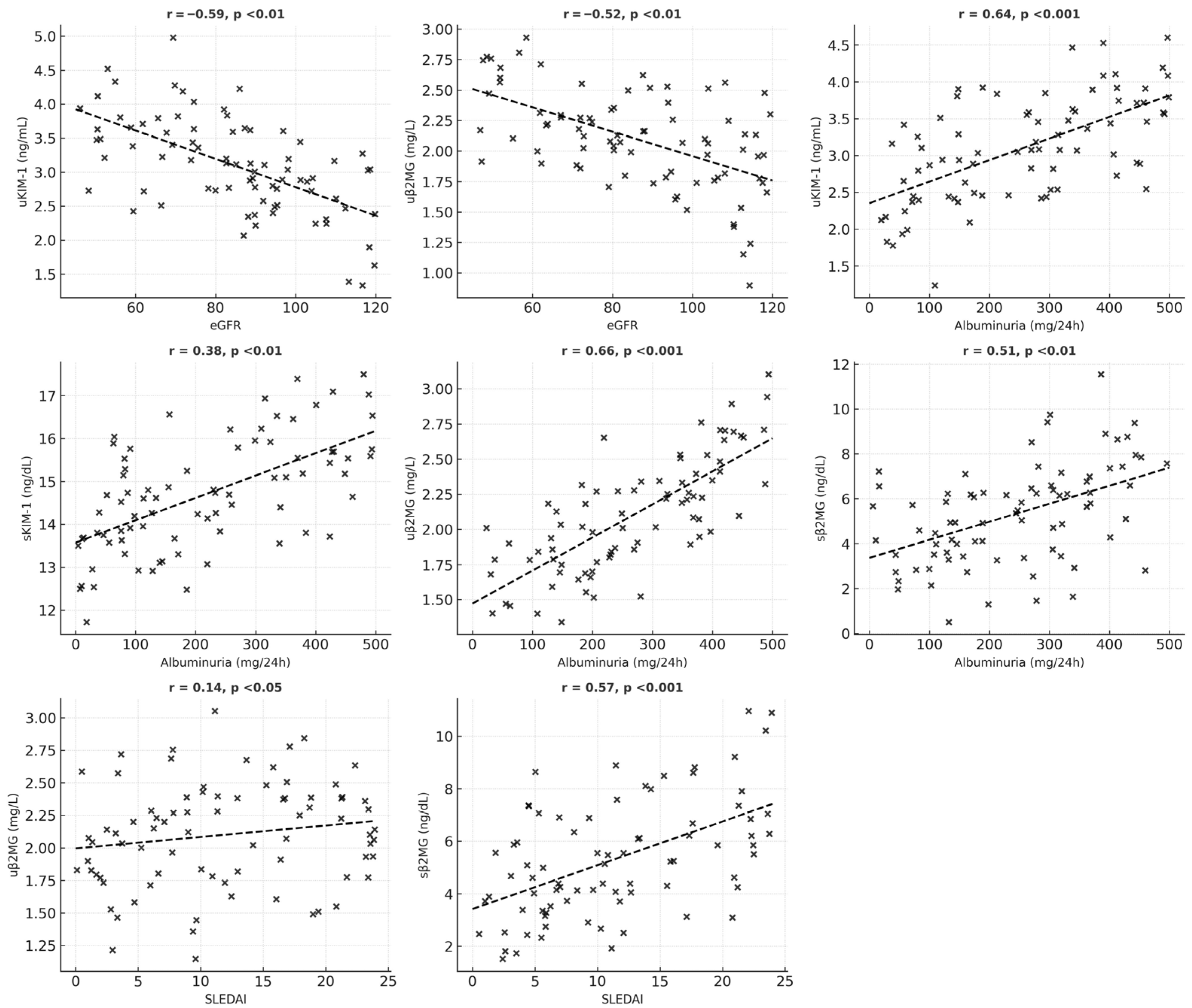
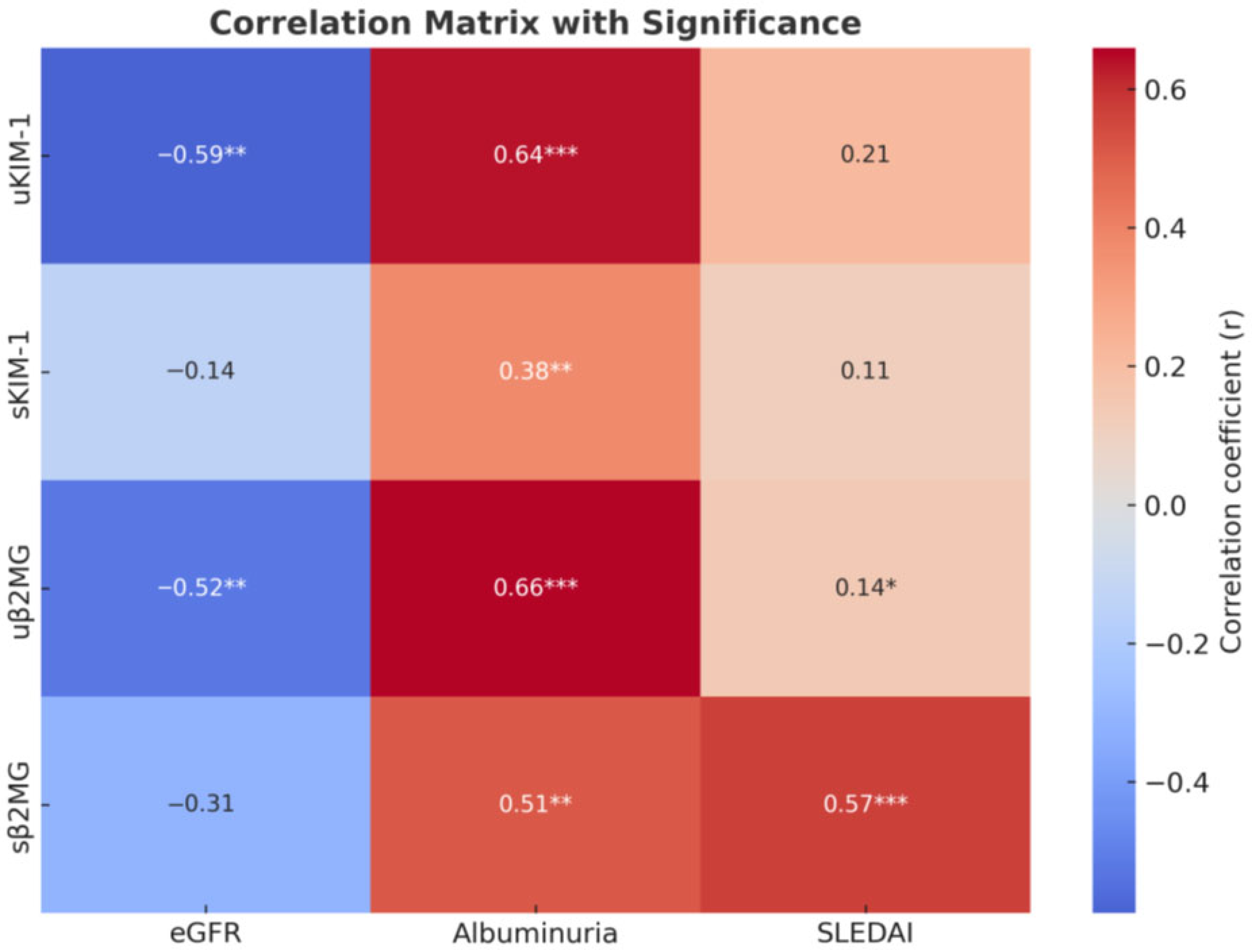
| Variable | uKIM-1 | sKIM-1 | uβ2MG | sβ2MG |
|---|---|---|---|---|
| eGFR | r = −0.59 p < 0.01 | r = −0.144 p = 0.05 | r = −0.52 p < 0.01 | r = −0.31 p > 0.05 |
| Albuminuria | r = 0.64 p < 0.001 | r = 0.38 p < 0.01 | r = 0.66 p < 0.001 | r = 0.51 p < 0.01 |
| SLEDAI | r = 0.21 p = 0.05 | r = 0.11 p > 0.05 | r = 0.14 p < 0.05 | r = 0.57 p < 0.001 |
| Hematuria | r = 0.25 p < 0.05 | r = 0.19 p < 0.05 | r = 0.10 p > 0.05 | r = 0.16 p > 0.05 |
| Leukocyturia | r = 0.14 p < 0.05 | r = 0.27 p < 0.05 | r = 0.33 p < 0.05 | r = 0.07 p > 0.05 |
| Cylindruria | r = 0.165 p > 0.05 | r = 0.102 p > 0.05 | r = 0.13 p > 0.05 | r = 0.17 p > 0.05 |
| Hemoglobin | r = 0.04 p = 1.0 | r = 0.08 p > 0.05 | r = 0.18 p > 0.05 | r = −0.12 p > 0.05 |
| Leukocytes | r = −0.07 p = 0.97 | r = 0.02 p > 0.05 | r = −0.11 p > 0.05 | r = −0.39 p < 0.05 |
| Thrombocytes | r = 0.02 p = 1.0 | r = −0.11 p > 0.05 | r = 0.07 p > 0.05 | r = 0.04 p > 0.05 |
| Mucocutaneous manifestations | r = 0.056 p > 0.05 | r = 0.133 p > 0.05 | r = 0.038 p > 0.05 | r = 0.491 p < 0.001 |
| ESR | r = 0.401 p < 0.001 | r = 0.278 p < 0.010 | r = 0.211 p < 0.010 | r = 0.364 p < 0.010 |
| AGP | r = 0.398 p < 0.010 | r = 0.103 p < 0.05 | r = 0.277 p < 0.010 | r = 0.201 p < 0.010 |
| sKIM | r = 0.58 p < 0.05 | - | r = 0.129 p < 0.05 | r = 0.146 p > 0.05 |
| sβ2MG | r = 0.13 p = 0.05 | - | r = 0.425 p < 0.05 | - |
| uβ2MG | r = 0.216 p < 0.05 | - | - | - |
| Role of KIM-1 and β2MG in SLE | KIM-1 Profile | β2MG Profile | Potential Mechanisms |
|---|---|---|---|
| Pathogenesis | Serum levels of KIM-1 show significant increase in SLE (Table 1). KIM-1 could represent (a) a molecular factor for detecting lupus nephritis before clinical diagnosis; (b) a prognostic model for patients with SLE. | β2MG is elevated in the serum of patients with SLE. β2MG substantially improved discrimination between cases and controls. | The KIM-1 upregulation in SLE would be explained by the accelerated proteolytic cleavage of the KIM-1 ectodomain in the early stage of the renal disease [27,28]. Increased lymphocyte turnover could explain β2MG upregulation in SLE [10,15]. |
| Skin and/or mucosal lesions | Invariable serum KIM-1 values in patients with SLE with preserved renal function and cutaneous determinations. KIM-1 could be an indicator of renal status in SLE. | Serum β2MG levels are high in patients with SLE and skin complications. β2MG has a potential pathogenic element in non-renal diseases. | β2MG and MHC-I/HLA-I orchestrate mucosal immunity, production of immunosuppressive molecules, and melanocyte stimulating hormone activity [16,20,29]. |
| Development of renal manifestations | KIM-1 levels increase with worsening proteinuria, and decrease eGFR and abnormal urinary findings (leukocyturia, hematuria, and cylindruria). | β2MG levels increase progressively with decreasing eGFR and rising proteinuria. | KIM-1 mediates adaptive reactions of the renal epithelium to ischemic lesions [30]. The overproduction of β2MG favors the formation of immune complexes and impairs glomerular function [10]. |
| Inflammatory profile | KIM-1 levels increase with serum pro-inflammatory factors (ESR, AGP) in SLE. | β2MG levels increase in inflammatory processes. | KIM-1 regulates NF κB, STAT3/ERK1/2. β-MG promotes M1/M2 polarization [28,31]. |
| Disease activity | KIM-1 correlates with disease severity. | β2MG levels vary significantly with SLEDAI. | KIM-1 and β2MG levels vary with the clinical course of SLE [13,16]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ene, C.D.; Capusa, C.; Nicolae, I.; Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Nicolae, G.; Tampa, M.; Matei, C. Clinical and Biological Relevance of Kidney Injury Molecule-1 and Beta-2 Microglobulin in Monitoring Patients with Systemic Lupus Erythematosus. Medicina 2025, 61, 1663. https://doi.org/10.3390/medicina61091663
Ene CD, Capusa C, Nicolae I, Georgescu SR, Mitran CI, Mitran MI, Nicolae G, Tampa M, Matei C. Clinical and Biological Relevance of Kidney Injury Molecule-1 and Beta-2 Microglobulin in Monitoring Patients with Systemic Lupus Erythematosus. Medicina. 2025; 61(9):1663. https://doi.org/10.3390/medicina61091663
Chicago/Turabian StyleEne, Corina Daniela, Cristina Capusa, Ilinca Nicolae, Simona Roxana Georgescu, Cristina Iulia Mitran, Madalina Irina Mitran, Gheorghe Nicolae, Mircea Tampa, and Clara Matei. 2025. "Clinical and Biological Relevance of Kidney Injury Molecule-1 and Beta-2 Microglobulin in Monitoring Patients with Systemic Lupus Erythematosus" Medicina 61, no. 9: 1663. https://doi.org/10.3390/medicina61091663
APA StyleEne, C. D., Capusa, C., Nicolae, I., Georgescu, S. R., Mitran, C. I., Mitran, M. I., Nicolae, G., Tampa, M., & Matei, C. (2025). Clinical and Biological Relevance of Kidney Injury Molecule-1 and Beta-2 Microglobulin in Monitoring Patients with Systemic Lupus Erythematosus. Medicina, 61(9), 1663. https://doi.org/10.3390/medicina61091663







