Sentinel Lymph Node Dissection—Novelty, Trend, or a Paradigm Shift in Surgical Decision-Making for Early Cervical Cancer?
Abstract
1. Introduction
- In which clinical scenarios of early-stage cervical cancer should SLNB be performed?
- What is the preferred surgical approach for SLNB—minimally invasive or open surgery?
- Which tracer is most appropriate for intraoperative identification of the SLN, and what is the optimal technique for its administration?
- Can SLNB be replaced by preoperative imaging modalities for LN assessment?
- What is the detection rate of SLNB for identifying sentinel lymph nodes in early-stage cervical cancer?
- What are the most common anatomical locations of SLNs in patients with early-stage cervical cancer?
- What is the recommended intraoperative management strategy when no SLN is identified?
- How should excised SLNs be examined—via intraoperative frozen section or paraffin-embedded tissue sections? Are there discrepancies between these two diagnostic methods?
- What is the clinical relevance of ultrastaging in SLN assessment, particularly regarding micrometastases (MMs) and isolated tumor cells (ITCs)?
- Does performing SLNB intraoperatively influence oncological outcomes and overall survival?
- What is the cost-effectiveness ratio of SLNB, and how should procedural costs be calculated, especially in relation to histopathological processing and evaluation?
- Is there an association between SLNB and the incidence of early or late postoperative complications?
- Are there specific risk factors—such as high BMI, prior conization, or tumor size—that may serve as contraindications to intraoperative SLNB?
2. Methodology
Strength of Evidence
3. Discussion
3.1. In Which Cases of Early-Stage Cervical Cancer Should SLNB Be Performed?
3.2. What Is the Optimal Surgical Approach for Performing SLNB?
- Laparotomy: sensitivity of 0.86 (95% CI, 0.80–0.90); detection rate of 0.87 (95% CI, 0.83–0.91);
- Laparoscopy: sensitivity of 0.90 (95% CI, 0.86–0.94); detection rate of 0.93 (95% CI, 0.90–0.96);
- Robot-assisted surgery: sensitivity of 0.84 (95% CI, 0.72–0.92); detection of rate 0.92 (95% CI, 0.88–0.95) [45].
3.3. What Tracer Should Be Used for Intraoperative Sentinel Lymph Node Detection and How Should It Be Administered?
- Combined techniques (dye + 99 mTc): sensitivity of 0.88 (95% CI, 0.84–0.91); detection rate of 0.97 (95% CI, 0.96–0.98);
- Technetium-99 m alone: sensitivity of 0.87 (95% CI, 0.78–0.93); detection of rate 0.90 (95% CI, 0.87–0.93);
- Blue dye alone: sensitivity of 0.87 (95% CI, 0.79–0.93); detection rate of 0.87 (95% CI, 0.84–0.90).
3.4. Can Preoperative Imaging Techniques Replace Intraoperative SLNB?
3.5. What Is the Detection Rate of SLNB for Identifying SLNs?
- The SENTICOL study (n = 139, tumors ≤ 4 cm) using a combined tracer approach (blue dye + 99 mTc) reported a detection rate of 97.8% overall (95% CI, 93.8% to 99.6%) and 76.5% bilaterally, with a sensitivity of 92.0%, an NPV of 98.2% (95% CI, 74.0% to 99.0%), and an FNR of 1.4% [13].
- Kim et al. used ICG in 103 women with stages IA1 (LVSI+) to IIA, finding SLNs in 100% of the cases and bilaterally in 85.44%. The reported sensitivity was 76.92% (95% CI, 57.95–88.97%), the sensitivity was 100% (95% CI, 95.00–100%), the FNR was 23.08%, and the NPV was 92.41% (95% CI, 84.40–96.47%) [58]. For tumors < 2 cm without radiographic lymphadenopathy, the sensitivity and specificity reached 100% (95% CI 20.65–100% and 95% CI, 94.42–100%) [57].
- Dostálek et al. (n = 350) used blue dye + radiocolloid and found an overall detection rate of 93%, with bilateral detection in 80%. The sensitivity was 93–96%, with an FNR of 1.6–0% depending on the tumor size subgroup (<2 cm, 2–4 cm, >4 cm) [58].
- The SENTIREC trial used ICG in 245 women and reported a detection rate of 96.3% (95% CI, 81.0–99.9%) overall and 82% bilaterally. For tumors > 20 mm, the bilateral detection was 80.9%; for tumors ≤ 20 mm, the bilateral detection was 83.1% [28]. The sensitivity was 96.3%, and the NPV was 98.7% (95% CI, 93.0–100%).
- Papadia et al. (n = 60, stages IA1–IIA) found a sensitivity of 93%, a specificity of 100%, a PPV of 100%, and an NPV of 97%. The bilateral detection was 83.4%, and the overall detection was 91.7% [55]. In 22 patients (36.7%), the combination of Tc99 + blue dye was used, and in 38 patients (63.3%), −ICG was used.
- The SENTIX trial (n = 395, tumors ≤ 4 cm) reported bilateral detection in 91%, most commonly using blue dye + radiocolloid [59].
- Chiyoda et al.’s meta-analysis found unilateral SLN detection rates of 95.7–100% and bilateral rates of 80.4–90% for 99 mTc ± blue dye and ICG alone [60].
- Another meta-analysis of seven studies (589 patients with early-stage cervical cancer) found that ICG had a higher bilateral detection rate than the combined 99 mTc/blue dye method (90.3% vs. 73.5%). However, the evidence quality was low [61].
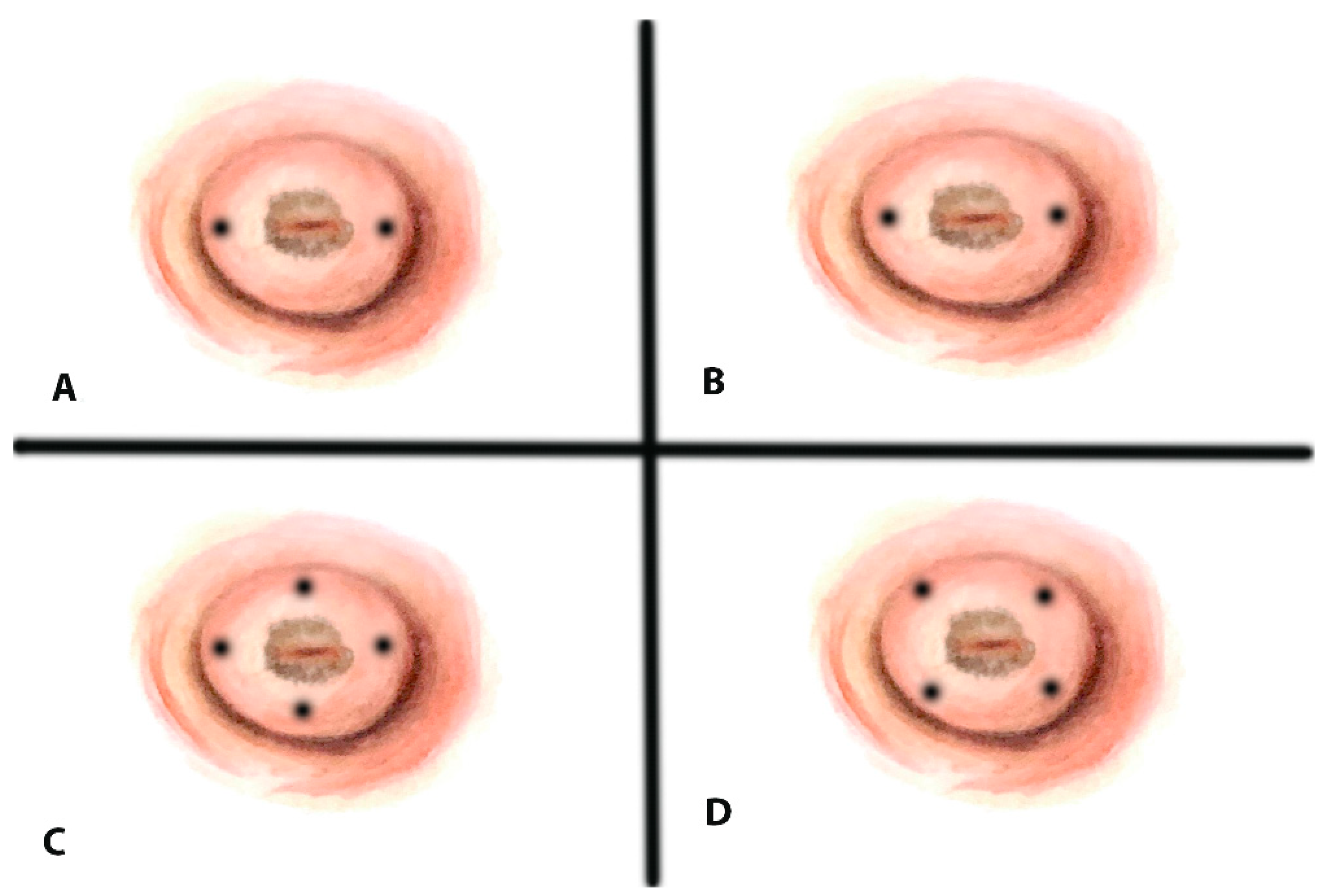
3.6. What Are the Most Common Anatomical Locations of SLNs in Early-Stage Cervical Cancer?
- Balaya et al. (n = 326) reported SLN detection in the interiliac or external iliac area in 83.2%, 9.2% in the common iliac area, 3.9% in the parametrium, 1.6% in the promontory area, 1.5% in the paraaortic area, and 0.5% in other areas [62]. In 10.7% of the patients, they found atypical SLN without SLN in the typical area on one or both sides and concluded that a tumor size of more than 20 mm and nulliparity increase the risk of having exclusive atypical SLN in early-stage cervical cancer [62].
- Lührs et al. (n = 145) reported bilateral obturator node detection in 69.4% (left) and 68.7% (right); external iliac nodes in 84.4% and 82.3%; common iliac nodes in 13.6% and 21.1%; and presacral nodes in 76.9% and 81.6% [64], possibly due to ICG use.
- Cibula et al. (n = 395) reported external iliac SLNs in 48% and 46% (left/right), internal iliac in 51% and 54%, and presacral nodes only on the right (6%) [59].
- Isolated SLNs in only presacral or common iliac areas were observed in 4% of cases [59].
- Ouldamer et al. presented a meta-analysis including 27 articles with 1301 patients and 3012 detected SLNs. They reported that 83.7% of the SLNs were found in classic areas of the pelvis (obturator, external iliac, and internal iliac), 6.6% in the common iliac area, 4.3% in the parametrial area, 2.0% in the paraaortic area, 1.3% in the presacral area, 0.2% in the hypogastric area, 0.07% in the inguinal area, and 0.07% in the cardinal ligament area [65].
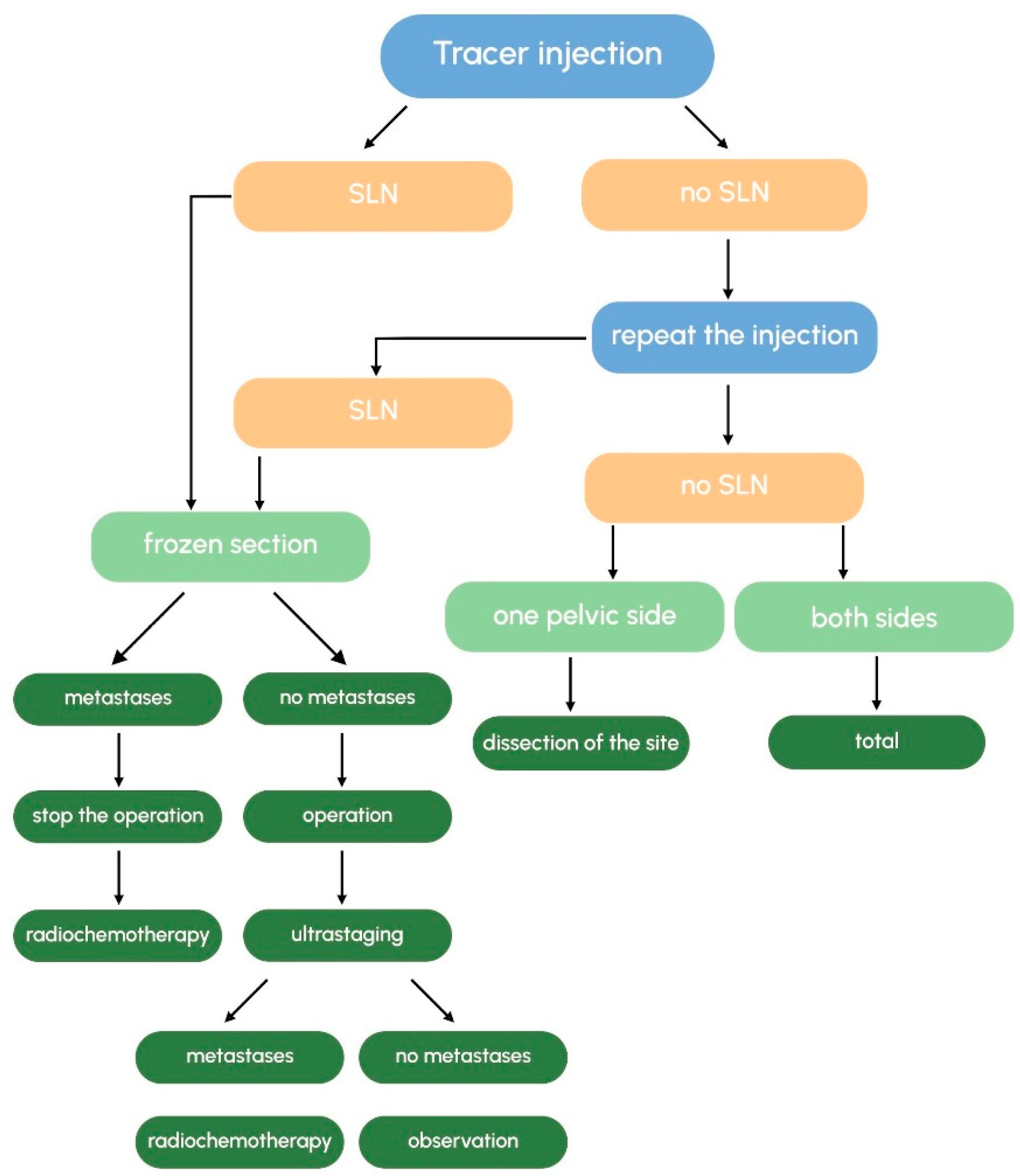
3.7. What Is the Recommended Surgical Approach When No SLNs Are Detected?
3.8. Should SLNs Be Examined Intraoperatively (Frozen Section) or as Paraffin-Embedded Tissue Sections? Are the Results Comparable?
- The Sophie Bats et al. (n = 139) trial (prospective) showed sensitivity, specificity, positive predictive, and negative predictive values for the diagnosis of macrometastases of 55.6% [95%CI: 21.2–86.3%], 100% [95%CI: 98.5–100.0%], 100% [95%CI: 47.8–100.0%], and 98.3% [95%CI: 95.8–99.5%], respectively [69].
- Slama et al. (n = 225) reported a sensitivity of 63% for macro/micrometastases and 81% for macrometastases, with an NPV of 91% [70].
- Martinez et al. (n = 225) found sensitivities of 88.9% (macro/micro) and 100% (macro) and an NPV of 98.8% [71].
- Rychlik et al. (n = 176) reported 76.9% sensitivity (macro) (95% CI, 49.7 to 91.8), 81.2% sensitivity (macro/micro) (95% CI, 57.0 to 93.4), and an NPV of 97.9% (95% CI, 93.9 to 99.3) [72].
- Sonoda et al. (n = 201) reported 100% sensitivity for macrometastases [73].
- SENTI-ENDO (n = 125) showed a sensitivity of 85.7% (95% CI, 42–99.6), an NPV of 96.8%95% CI, 83.8–99.9), and a specificity of 97.3% (95% CI, 85.8–99.9) [74].
- A meta-analysis of 14 studies (1270 patients) showed that frozen section detects 65% of nodal metastases (95% CI, 51–77%), increasing to 72% (95% CI, 60–82%) when isolated tumor cells (ITCs) are excluded [75].
3.9. What Is the Role of SLN Ultrastaging—Particularly Regarding MM and ITCs?
- The review article by Delomenie et al. analyzed the literature up to January 2019 and reported that the available data cannot determine how to treat patients with MMs and ITCs. They expressed an opinion that small nodal disease has to be treated as a high-risk group [86].
- Cibula et al. reported a detection rate of macrometastases, MMs, and ITCs in SLN by ultrastaging in 14.7%, 10.1%, and 4.5% of patients, respectively, and established significantly reduced overall survival (OS) in patients with MMs vs. negative lymph node status (p < 0.001). This pattern is not observed when comparing OS in MM vs. macrometastasis [83].
- Marchiolé et al. identified MMs as an independent risk factor for recurrence in early cervical cancer and reported that MMs occur only in LVSI-positive tumors [87].
- Zaal et al. noted that survival improves with dissection of >16 nodes in patients with MMs but found no prognostic value for ITCs [88].
- Horn et al. demonstrated significantly reduced 5-year disease-free survival in patients with MMs vs. N0 (68.9% vs. 91.4%, p < 0.001), and the 5-year OS rate was decreased in patients with MMs vs. N0 (63.8% vs. 86.6%. But it is important to note that the included patients were IB to IIB FIGO 1988 [89].
- Guani et al. reported no impact of MMs or ITCs on recurrence-free survival [90].
3.10. Does Intraoperative Sentinel Lymph Node Biopsy (SLNB) Influence Oncologic Outcomes and Survival?
3.11. What Is the Cost-Effectiveness Ratio, and How Should the Procedure Cost Be Determined, with a Focus on Pathological Assessment Activities?
3.12. Is Performing SLNB Associated with the Incidence of Early and Late Postoperative Complications?
3.13. Are There Risk Factors, Such as High BMI, Prior Conization, or Tumor Size, That Contraindicate Intraoperative SLNB?
3.14. Prespectives
Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 20 January 2025).
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 22–36. [Google Scholar] [CrossRef]
- Guida, F.; Kidman, R.; Ferlay, J.; Schüz, J.; Soerjomataram, I.; Kithaka, B.; Ginsburg, O.; Mailhot Vega, R.B.; Galukande, M.; Parham, G.; et al. Global and regional estimates of orphans attributed to maternal cancer mortality in 2020. Nat. Med. 2022, 28, 2563–2572. [Google Scholar] [CrossRef]
- ESGO. Cervical Cancer. Pocket Guidelines. Available online: https://www.esgo.org/media/2019/03/Pocket-Guidelines_Cervical-cancer_June2023.pdf (accessed on 20 January 2025).
- National Comprehensive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 20 January 2025).
- Ferrandina, G.; Pedone Anchora, L.; Gallotta, V.; Fagotti, A.; Vizza, E.; Chiantera, V.; De Iaco, P.; Ercoli, A.; Corrado, G.; Bottoni, C.; et al. Can we define the risk of lymph node metastasis in early-stage cervical cancer patients? A large-scale, Retrospective Study. Ann. Surg. Oncol. 2017, 24, 2311–2318. [Google Scholar] [CrossRef]
- Cabanas, R.M. An approach for the treatment of penile carcinoma. Cancer 1977, 39, 456–466. [Google Scholar] [CrossRef]
- Lyman, G.H.; Giuliano, A.E.; Somerfield, M.R.; Benson, A.B., 3rd; Bodurka, D.C.; Burstein, H.J.; Cochran, A.J.; Cody, H.S., 3rd; Edge, S.B.; Galper, S.; et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J. Clin. Oncol. 2005, 23, 7703–7720. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Slama, J.; Zikán, M.; Zaal, A.; Sevcik, L.; Kenter, G.; Querleu, D.; Jach, R.; et al. Bilateral ultrastaging of sentinel lymph node in cervical cancer: Lowering the falsenegative rate and improving the detection of micrometastasis. Gynecol. Oncol. 2012, 127, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Altgassen, C.; Hertel, H.; Brandstädt, A.; Köhler, C.; Dürst, M.; Schneider, A. Multicenter validation study of the sentinel lymph node concept in cervical cancer: AGO Study Group. J. Clin. Oncol. 2008, 26, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.P.; Gemignani, M.L.; Pandit-Taskar, N.; Park, K.J.; Murray, M.P.; Chi, D.S.; Sonoda, Y.; Barakat, R.R.; Abu-Rustum, N.R. Sentinel lymph node biopsy in the management of early-stage cervical carcinoma. Gynecol. Oncol. 2011, 120, 347–352. [Google Scholar] [CrossRef]
- Lécuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Daraï, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: Results of the SENTICOL study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef]
- Du, X.-L.; Sheng, X.-G.; Jiang, T.; Li, Q.-S.; Yu, H.; Pan, C.-X.; Lu, C.-H.; Wang, C.; Song, Q.-Q. Sentinel lymph node biopsy as guidance for radical trachelectomy in young patients with early stage cervical cancer. BMC Cancer 2011, 11, 157. [Google Scholar] [CrossRef]
- Tax, C.; Rovers, M.M.; de Graaf, C.; Zusterzeel, P.L.; Bekkers, R.L. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnosticreview. Gynecol. Oncol. 2015, 139, 559–567. [Google Scholar]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer, Version 2.2025; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025. [Google Scholar]
- Dargent, D.; Martin, X.; Mathevet, P. Laparoscopic assessment of the sentinel lymph node in early stage cervical cancer. Gynecol. Oncol. 2000, 79, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Medl, M.; Peters-Engl, C.; Schütz, P.; Vesely, M.; Sevelda, P. First report of lymphatic mapping with isosulfan blue dye and sentinel node biopsy in cervical cancer. Anticancer Res. 2000, 20 Pt 2B, 1133–1134. [Google Scholar]
- O’Boyle, J.D.; Coleman, R.L.; Bernstein, S.G.; Lifshitz, S.; Muller, C.Y.; Miller, D.S. Intraoperative lymphatic mapping in cervix cancer patients undergoing radical hysterectomy: A pilot study. Gynecol. Oncol. 2000, 79, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Kamprath, S.; Possover, M.; Schneider, A. Laparoscopic sentinel lymph node detection in patients with cervical cancer. Am. J. Obstet. Gynecol. 2000, 182, 1648. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Levenback, C. Sentinel nodes in gynecologic malignancies. Curr. Opin. Oncol. 2001, 13, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, R.H.; Pijpers, R.; van Diest, P.J.; Burger, C.W.; Buist, M.R.; Kenemans, P. Sentinel node detection in cervical cancer. Obstet. Gynecol. 2000, 96, 135–138. [Google Scholar] [CrossRef]
- Malur, S.; Krause, N.; Köhler, C.; Schneider, A. Sentinel lymph node detection in patients with cervical cancer. Gynecol. Oncol. 2001, 80, 254–257. [Google Scholar] [CrossRef]
- van Dam, P.A.; Hauspy, J.; Vanderheyden, T.; Sonnemans, H.; Spaepen, A.; Eggenstein, G.; Dirix, L.; Verkinderen, L. Intraoperative sentinel node identification with Technetium-99m-labeled nanocolloid in patients with cancer of the uterine cervix: A feasibility study. Int. J. Gynecol. Cancer 2003, 13, 182–186. [Google Scholar] [CrossRef]
- Levenback, C.; Coleman, R.L.; Burke, T.W.; Lin, W.M.; Erdman, W.; Deavers, M.; Delpassand, E.S. Lymphatic mapping and sentinel node identification in patients with cervix cancer undergoing radical hysterectomy and pelvic lymphadenectomy. J. Clin. Oncol. 2002, 20, 688–693. [Google Scholar] [CrossRef]
- Salvo, G.; Ramirez, P.T.; Levenback, C.F.; Munsell, M.F.; Euscher, E.D.; Soliman, P.T.; Frumovitz, M. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol. Oncol. 2017, 145, 96–101. [Google Scholar] [CrossRef]
- Sponholtz, S.E.; Mogensen, O.; Hildebrandt, M.G.; Schledermann, D.; Parner, E.; Markauskas, A.; Frøding, L.P.; Fuglsang, K.; Vilstrup, M.H.; Bjørnholt, S.M.; et al. Sentinel lymph node mapping in early-stage cervical cancer—A national prospective multicenter study (SENTIREC trial). Gynecol. Oncol. 2021, 162, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Bean, S.; Bradley, K.; Campos, S.M.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 660–666. [Google Scholar] [CrossRef]
- Mathevet, P.; Lécuru, F.; Uzan, C.; Boutitie, F.; Magaud, L.; Guyon, F.; Querleu, D.; Fourchotte, V.; Baron, M.; Bats, A.S.; et al. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentre randomised trial (SENTICOL-2). Eur. J. Cancer. 2021, 148, 307–315. [Google Scholar] [CrossRef]
- Lecuru, F.R.; McCormack, M.; Hillemanns, P.; Anota, A.; Leitao, M.; Mathevet, P.; Zweemer, R.; Fujiwara, K.; Zanagnolo, V.; Zahl Eriksson, A.G.; et al. SENTICOL III: An international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int. J. Gynecol. Cancer 2019, 29, 829–834. [Google Scholar] [CrossRef]
- Cibula, D.; Kuzel, D.; Sláma, J.; Fischerova, D.; Dundr, P.; Freitag, P.; Zikán, M.; Pavlista, D.; Tomancova, V. Sentinel node (SLN) biopsy in the management of locally advanced cervical cancer. Gynecol. Oncol. 2009, 115, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Slama, J.; Dundr, P.; Dusek, L.; Fischerova, D.; Pinkavova, I.; Zikan, M.; Vrzackova, P.; Kojanova, M.; Cibula, D. Sentinel lymph node status in patients with locally advanced cervical cancers and impact of neoadjuvant chemotherapy. Gynecol. Oncol. 2012, 125, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Sláma, J.; Zikán, M.; Fischerová, D.; Kocián, R.; Germanová, A.; Frühauf, F.; Cibula, D. Contribution of sentinel lymph-node biopsy to treatment of locally advanced stages of cervical cancers. Ceska Gynekol. 2016, 81, 165–170. (In Czech) [Google Scholar]
- Barranger, E.; Coutant, C.; Cortez, A.; Uzan, S.; Darai, E. Sentinel node biopsy is reliable in early-stage cervical cancer but not in locally advanced disease. Ann. Oncol. 2005, 16, 1237–1242. [Google Scholar] [CrossRef]
- Daraï, E.; Lavoué, V.; Rouzier, R.; Coutant, C.; Barranger, E.; Bats, A.S. Contribution of the sentinel node procedure to tailoring the radicality of hysterectomy for cervical cancer. Gynecol. Oncol. 2007, 106, 251–256. [Google Scholar] [CrossRef]
- Lavoué, V.; Bats, A.S.; Rouzier, R.; Coutant, C.; Barranger, E.; Daraï, E. Sentinel lymph node procedure followed by laparoscopic pelvic and paraaortic lymphadenectomy in women with IB2-II cervical cancer. Ann. Surg. Oncol. 2007, 14, 2654–2661. [Google Scholar] [CrossRef]
- Bray, F.; Loos, A.H.; McCarron, P.; Weiderpass, E.; Arbyn, M.; Møller, H.; Hakama, M.; Parkin, D.M. Trends in cervical squamous cell carcinoma incidence in 13 European countries: Changing risk and the effects of screening. Cancer Epidemiol. Biomark. Prev. 2005, 14, 677–686. [Google Scholar] [CrossRef]
- Wang, S.S.; Sherman, M.E.; Hildesheim, A.; Lacey, J.V.; Devesa, S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer 2004, 100, 10351044. [Google Scholar] [CrossRef]
- Castellsague, X.; Diaz, M.; de Sanjose, S.; Muñoz, N.; Herrero, R.; Franceschi, S.; Peeling, R.W.; Ashley, R.; Smith, J.S.; Snijders, P.J.F.; et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J. Natl. Cancer Inst. 2006, 98, 303315. [Google Scholar] [CrossRef]
- Sasieni, P.; Castanon, A.; Cuzick, J. Screening and adenocarcinoma of the cervix. Int. J. Cancer 2009, 125, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Stolnicu, S.; Barsan, I.; Hoang, L.; Patel, P.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Pike, M.C.; et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am. J. Surg. Pathol. 2018, 42, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Guani, B.; Pache, B.; Durand, Y.G.; Bonsang-Kitzis, H.; Ngô, C.; Mathevet, P.; Lécuru, F. Sentinel lymph node in cervical cancer: Time to move forward. Chin. Clin. Oncol. 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, M.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 189. [Google Scholar]
- Wang, X.J.; Fang, F.; Li, Y.F. Sentinel-lymph-node procedures in early stage cervical cancer: A systematic review and meta-analysis. Med. Oncol. 2015, 32, 385. [Google Scholar] [CrossRef]
- Margioula-Siarkou, C.; Almperis, A.; Gullo, G.; Almperi, E.A.; Margioula-Siarkou, G.; Nixarlidou, E.; Mponiou, K.; Papakotoulas, P.; Sardeli, C.; Guyon, F.; et al. Sentinel Lymph Node Staging in Early-Stage Cervical Cancer: A Comprehensive Review. J. Clin. Med. 2023, 13, 27. [Google Scholar] [CrossRef]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef]
- Ruscito, I.; Gasparri, M.L.; Braicu, E.I.; Bellati, F.; Raio, L.; Sehouli, J.; Mueller, M.D.; Panici, P.B.; Papadia, A. Sentinel Node Mapping in Cervical and Endometrial Cancer: Indocyanine Green Versus Other Conventional Dyes-A Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, B.; Wang, S.; Yi, M.; Jiang, L.; Fang, X. Sentinel lymph node biopsy in early stage cervical cancer: A meta-analysis. Cancer Med. 2021, 10, 2590–2600. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Xu, T.; Yuan, L.; Yang, X. Sentinel lymph node mapping in early-stage cervical cancer: Meta-analysis. Medicine 2021, 100, e27035. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Ten Eikelder, M.; Dhanis, J.; Moore, W.; Blake, D.; Zusterzeel, P.; Kucukmetin, A.; Ratnavelu, N.; Rundle, S. Finding the sentinel lymph node in early cervical cancer: When is unusual not uncommon? Gynecol. Oncol. 2023, 170, 84–92. [Google Scholar] [CrossRef]
- Hricak, H.; Gatsonis, C.; Chi, D.S.; Amendola, M.A.; Brandt, K.; Schwartz, L.H.; Koelliker, S.; Siegelman, E.S.; Brown, J.J.; McGhee, R.B.; et al. Role of Imaging in Pretreatment Evaluation of Early Invasive Cervical Cancer: Results of the Intergroup Study American College of Radiology Imaging Network 6651-Gynecologic Oncology Group 183. J. Clin. Oncol. 2005, 23, 9329–9337. [Google Scholar] [CrossRef]
- Kodama, J.; Mizutani, Y.; Hongo, A.; Yoshinouchi, M.; Kudo, T.; Okuda, H. Optimal Surgery and Diagnostic Approach of Stage IA2 Squamous Cell Carcinoma of the Cervix. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 101, 192–195. [Google Scholar] [CrossRef]
- Selman, T.J.; Mann, C.; Zamora, J.; Appleyard, T.-L.; Khan, K. Diagnostic Accuracy of Tests for Lymph Node Status in Primary Cervical Cancer: A Systematic Review and Meta-Analysis. Can. Med. Assoc. J. 2008, 178, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Papadia, A.; Gasparri, M.L.; Genoud, S.; Bernd, K.; Mueller, M.D. The Combination of Preoperative PET/CT and Sentinel Lymph Node Biopsy in the Surgical Management of Early-Stage Cervical Cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 2275–2281. [Google Scholar] [CrossRef]
- Tanaka, T.; Sasaki, S.; Tsuchihashi, H.; Terai, Y.; Yamamoto, K.; Yamada, T.; Ohmichi, M. Which Is Better for Predicting Pelvic Lymph Node Metastases in Patients with Cervical Cancer: Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography or a Sentinel Node Biopsy? A Retrospective Observational Study. Medicine 2018, 97, e0410. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. The efficacy of sentinel lymph node mapping with indocyanine green in cervical cancer. World J. Surg. Oncol. 2018, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, L.; Zikan, M.; Fischerova, D.; Kocian, R.; Germanova, A.; Frühauf, F.; Dusek, L.; Slama, J.; Dundr, P.; Nemejcova, K.; et al. SLN biopsy in cervical cancer patients with tumors larger than 2 cm and 4 cm. Gynecol. Oncol. 2018, 148, 456–460. [Google Scholar] [CrossRef]
- Cibula, D.; Kocian, R.; Plaikner, A.; Jarkovsky, J.; Klat, J.; Zapardiel, I.; Pilka, R.; Torne, A.; Sehnal, B.; Ostojich, M.; et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: The SENTIX trial. Eur. J. Cancer. 2020, 137, 69–80. [Google Scholar] [CrossRef]
- Chiyoda, T.; Yoshihara, K.; Kagabu, M.; Nagase, S.; Katabuchi, H.; Mikami, M.; Tabata, T.; Hirashima, Y.; Kobayashi, Y.; Kaneuchi, M.; et al. Sentinel node navigation surgery in cervical cancer: A systematic review and metaanalysis. Int. J. Clin. Oncol. 2022, 27, 1247–1255. [Google Scholar] [CrossRef]
- Baeten, I.G.T.; Hoogendam, J.P.; Jeremiasse, B.; Braat, A.J.A.T.; Veldhuis, W.B.; Jonges, G.N.; Jürgenliemk-Schulz, I.M.; van Gils, C.H.; Zweemer, R.P.; Gerestein, C.G. Indocyanine green versus technetium-99m with blue dye for sentinel lymph node detection in early-stage cervical cancer: A systematic review and meta-analysis. Cancer Rep. 2022, 5, e1401. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Mathevet, P.; Magaud, L.; Bonsang-Kitzis, H.; Delomenie, M.; Montero Macias, R.; Ngô, C.; Bats, A.S.; Lécuru, F. Predictive factors of unexpected lymphatic drainage pathways in early-stage cervical cancer. Gynecol. Oncol. 2019, 154, 102–109. [Google Scholar] [CrossRef]
- Marnitz, S.; Köhler, C.; Bongardt, S.; Braig, U.; Hertel, H.; Schneider, A. German Association of Gynecologic Oncologists (AGO). Topographic distribution of sentinel lymph nodes in patients with cervical cancer. Gynecol. Oncol. 2006, 103, 35–44. [Google Scholar] [CrossRef]
- Lührs, O.; Bollino, M.; Ekdahl, L.; Lönnerfors, C.; Geppert, B.; Persson, J. Similar distribution of pelvic sentinel lymph nodes and nodal metastases in cervical and endometrial cancer. A prospective study based on lymphatic anatomy. Gynecol. Oncol. 2022, 165, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Ouldamer, L.; Marret, H.; Acker, O.; Barillot, I.; Body, G. Unusual localizations of sentinel lymph nodes in early stage cervical cancer: A review. Surg. Oncol. 2012, 21, e153–e157. [Google Scholar] [CrossRef]
- Pavone, M.; Bizzarri, N.; Rychlik, A.; Persson, J.; Fagotti, A.; Fanfani, F.; Scambia, G.; Querleu, D. In-transit metastatic lymph nodes in cervical cancer: A new staging and therapeutic concept. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 312, 114523. [Google Scholar] [CrossRef]
- Maramai, M.; Achilarre, M.T.; Aloisi, A.; Betella, I.; Bogliolo, S.; Garbi, A.; Maruccio, M.; Quatrale, C.; Aletti, G.D.; Mariani, A.; et al. Cervical re-injection of indocyanine green to improve sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2021, 162, 38–42. [Google Scholar] [CrossRef]
- Cormier, B.; Diaz, J.P.; Shih, K.; Sampson, R.M.; Sonoda, Y.; Park, K.J.; Alektiar, K.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. Establishing a sentinel lymph node mapping algorithm for the treatment of early cervical cancer. Gynecol. Oncol. 2011, 122, 275–280. [Google Scholar] [CrossRef]
- Bats, A.-S.; Buénerd, A.; Querleu, D.; Leblanc, E.; Daraï, E.; Morice, P.; Marret, H.; Gillaizeau, F.; Mathevet, P.; Lécuru, F. Diagnostic value of intraoperative examination of sentinel lymph node in early cervical cancer: A prospective, multicenter study. Gynecol. Oncol. 2011, 123, 230–235. [Google Scholar] [CrossRef]
- Slama, J.; Dundr, P.; Dusek, L.; Cibula, D. High false negative rate of frozen section examination of sentinel lymph nodes in patients with cervical cancer. Gynecol. Oncol 2013, 129, 384–388. [Google Scholar] [CrossRef]
- Martínez, A.; Mery, E.; Filleron, T.; Boileau, L.; Ferron, G.; Querleu, D. Accuracy of intraoperative pathological examination of SLN in cervical cancer. Gynecol. Oncol. 2013, 130, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, A.; Angeles, M.A.; Migliorelli, F.; Croce, S.; Mery, E.; Martinez, A.; Ferron, G.; Guyon, F.; Querleu, D. Frozen section examination of sentinel lymph nodes can be used as a decisional tool in the surgical management of early cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, K.; Yahata, H.; Okugawa, K.; Kaneki, E.; Ohgami, T.; Yasunaga, M.; Baba, S.; Oda, Y.; Honda, H.; Kato, K. Value of intraoperative cytological and pathological sentinel lymph node diagnosis in fertility-sparing trachelectomy for early-stage cervical cancer. Oncology 2018, 94, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ballester, M.; Dubernard, G.; Bats, A.-S.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Comparison of diagnostic accuracy of frozen section with imprint cytology for intraoperative examination of sentinel lymph node in early-stage endometrial cancer: Results of Senti-Endo study. Ann. Surg. Oncol. 2012, 19, 3515–3521. [Google Scholar] [CrossRef]
- Agustí, N.; Viveros-Carreño, D.; Mora-Soto, N.; Ramírez, P.T.; Rauh-Hain, A.; Wu, C.F.; Rodríguez, J.; Grillo-Ardila, C.F.; Salazar, C.; Jorgensen, K.; et al. Diagnostic accuracy of sentinel lymph node frozen section analysis in patients with early-stage cervical cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2023, 177, 157–164. [Google Scholar] [CrossRef]
- Cibula, D.; McCluggage, W.G. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol. Oncol. 2019, 152, 202–207. [Google Scholar] [CrossRef]
- Roy, M.; Bouchard-Fortier, G.; Popa, I.; Grégoire, J.; Renaud, M.C.; Têtu, B.; Plante, M. Value of sentinel node mapping in cancer of the cervix. Gynecol. Oncol. 2011, 122, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Guani, B.; Benoit, L.; Magaud, L.; Bonsang-Kitzis, H.; Ngô, C.; Le Frère-Belda, M.A.; Mathevet, P.; Lécuru, F. Diagnostic value of frozen section examination of sentinel lymph nodes in early-stage cervical cancer at the time of ultrastaging. Gynecol. Oncol. 2020, 158, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Gortzak-Uzan, L.; Jimenez, W.; Nofech-Mozes, S.; Ismiil, N.; Khalifa, M.A.; Dubé, V.; Rosen, B.; Murphy, J.; Laframboise, S.; Covens, A. Sentinel lymph node biopsy vs. pelvic lymphadenectomy in early stage cervical cancer: Is it time to change the gold standard? Gynecol Oncol. 2010, 116, 28–32. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Kim, C.H.; Soslow, R.A.; Park, K.J.; Barber, E.L.; Khoury-Collado, F.; Barlin, J.N.; Sonoda, Y.; Hensley, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Pathologic Ultrastaging Improves Micrometastasis Detection in Sentinel Lymph Nodes during Endometrial Cancer Staging. Int. J. Gynecol. Cancer 2013, 23, 964–970. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Zikán, M.; Zaal, A.; Sevcik, L.; Kenter, G.G.; Querleu, D.; Jach, R.; Bats, A.S.; et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervicalcancer. Gynecol. Oncol. 2012, 124, 496–501. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Lentz, S.E.; Muderspach, L.I.; Felix, J.C.; Ye, W.; Groshen, S.; Amezcua, C.A. Identification of micrometastases in histologically negative lymph nodes of earlystage cervical cancer patients. Obstet. Gynecol. 2004, 103, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Delomenie, M.; Bonsang-Kitzis, H.; Bats, A.S.; Ngo, C.; Balaya, V.; Xuan, H.T.N.; Koual, M.; Mathevet, P.; Lecuru, F. The clinical implication of lymph nodes micrometastases and isolated tumor cells in patients with cervical cancer: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 241, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Marchiolé, P.; Buénerd, A.; Benchaib, M.; Nezhat, K.; Dargent, D.; Mathevet, P. Clinical Significance of Lympho Vascular Space Involvement and Lymph Node Micrometastases in Early-Stage Cervical Cancer: A Retrospective Case-Control SurgicoPathological Study. Gynecol. Oncol. 2005, 97, 727–732. [Google Scholar] [CrossRef]
- Zaal, A.; Zweemer, R.P.; Zikán, M.; Dusek, L.; Querleu, D.; Lécuru, F.; Bats, A.-S.; Jach, R.; Sevcik, L.; Graf, P.; et al. Pelvic Lymphadenectomy Improves Survival in Patients with Cervical Cancer with Low-Volume Disease in the Sentinel Node: A Retrospective Multicenter Cohort Study. Int. J. Gynecol. Cancer 2014, 24, 303–311. [Google Scholar] [CrossRef]
- Horn, L.C.; Hentschel, B.; Fischer, U.; Peter, D.; Bilek, K. Detection of micrometastases in pelvic lymph nodes in patients with carcinoma of the cervix uteri using step sectioning: Frequency, topographic distribution and prognostic impact. Gynecol. Oncol. 2008, 111, 276–281. [Google Scholar] [CrossRef]
- Guani, B.; Dorez, M.; Magaud, L.; Buenerd, A.; Lecuru, F.; Mathevet, P. Impact of Micrometastasis or Isolated Tumor Cells on Recurrence and Survival in Patients with Early Cervical Cancer: SENTICOL Trial. Int. J. Gynecol. Cancer 2019, 29, 447–452. [Google Scholar] [CrossRef]
- Guani, B.; Balaya, V.; Magaud, L.; Lecuru, F.; Mathevet, P. The Clinical Impact of Low-Volume Lymph Nodal Metastases in Early-Stage Cervical Cancer: The Senticol 1 and Senticol 2 Trials. Cancers 2020, 12, 1061. [Google Scholar] [CrossRef]
- Nica, A.; Gien, L.T.; Ferguson, S.E.; Covens, A. Does small volume metastatic lymph node disease affect long-term prognosis in early cervical cancer? Int. J. Gynecol. Cancer 2020, 30, 285–290. [Google Scholar] [CrossRef]
- Colpaert, C.; Jacomen, G.; Van de Vijver, K.; Baldewijns, M.; Van Rompuy, A.-S.; Bourgain, C.; Noël, J.-C. Ultrastaging of sentinel lymph nodes in gynecological cancer: Repeating the story of breast cancer? Letter to the editor, Reply to Cibula D, McCluggage WG. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol Oncol 2019;152:202–7. Gynecol. Oncol. Rep. 2019, 29, 130–131. [Google Scholar] [CrossRef]
- Mauro, J.; Viveros-Carreño, D.; Vizzielli, G.; De Ponti, E.; Fanfani, F.; Ramirez, P.T.; Buda, A. Survival after sentinel node biopsy alone in early-stage cervical cancer: A systematic review. Int. J. Gynecol. Cancer 2023, 33, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; De Franciscis, P.; Carotenuto, R.M.; Pasanisi, F.; Cobellis, L.; Colacurci, N. The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences. Medicina 2022, 58, 1539. [Google Scholar] [CrossRef]
- Bizzarri, N.; Querleu, D.; Ramirez, P.T.; Dostálek, L.; van Lonkhuijzen, L.R.W.; Giannarelli, D.; Lopez, A.; Salehi, S.; Ayhan, A.; Kim, S.H.; et al. Survival associated with the use of sentinel lymph node in addition to lymphadenectomy in early-stage cervical cancer treated with surgery alone: A sub-analysis of the Surveillance in Cervical CANcer (SCCAN) collaborative study. Eur. J. Cancer 2024, 211, 114310. [Google Scholar] [CrossRef]
- Favre, G.; Guani, B.; Balaya, V.; Magaud, L.; Lecuru, F.; Mathevet, P. Sentinel Lymph-Node Biopsy in Early-Stage Cervical Cancer: The 4-Year Follow-Up Results of the Senticol 2 Trial. Front. Oncol. 2021, 10, 621518. [Google Scholar] [CrossRef]
- Brar, H.; Hogen, L.; Covens, A. Cost-effectiveness of sentinel node biopsy and pathological ultrastaging in patients with early-stage cervical cancer. Cancer 2017, 123, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Suidan, R.S.; Sun, C.C.L.; Cantor, S.B.; Frumovitz, M.; Giordano, S.H.; Meyer, L.A. A cost-utility analysis of sentinel lymph node mapping versus complete lymphadenectomy in the management of early-stage cervical carcinoma. Gynecol. Oncol. 2019, 154, 191. [Google Scholar] [CrossRef]
- Vicus, D.; Covens, A. Role of sentinel lymph node biopsy in cervical cancer: Pro. Int. J. Gynecol. Cancer 2010, 20 (Suppl. S2), S34–S36. [Google Scholar] [CrossRef]
- Eiriksson, L.R.; Covens, A. Sentinel lymph node mapping in cervical cancer: The future? BJOG 2012, 119, 129–133. [Google Scholar] [CrossRef]
- Matsuura, Y.; Kawagoe, T.; Toki, N.; Tanaka, M.; Kashimura, M. Long-standing complications after treatment for cancer of the uterine cervix--clinical significance of medical examination at 5 years after treatment. Int. J. Gynecol. Cancer. 2006, 16, 294–297. [Google Scholar] [CrossRef]
- Biglia, N.; Librino, A.; Ottino, M.C.; Panuccio, E.; Daniele, A.; Chahin, A. Lower limb lymphedema and neurological complications after lymphadenectomy for gynecological cancer. Int. J. Gynecol. Cancer 2015, 25, 521–525. [Google Scholar] [CrossRef]
- Hareyama, H.; Hada, K.; Goto, K.; Watanabe, S.; Hakoyama, M.; Oku, K.; Hayakashi, Y.; Hirayama, E.; Okuyama, K. Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic malignancies: A retrospective study. Int. J. Gynecol. Cancer 2015, 25, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kitahara, Y.; Satoh, T.; Takei, Y.; Takano, M.; Nagao, S.; Sekiguchi, I.; Suzuki, M. Analysis of the effect of adjuvant radiotherapy on outcomes and complications after radical hysterectomy in FIGO stage IB1 cervical cancer patients with intermediate risk factors (GOTIC study). World J. Surg. Oncol. 2016, 14, 173. [Google Scholar] [CrossRef]
- Bona, A.F.; Ferreira, K.R.; Carvalho, R.B.M.; Thuler, L.C.S.; Bergmann, A. Incidence, prevalence, and factors associated with lymphedema after treatment for cervical cancer: A systematic review. Int. J. Gynecol. Cancer 2020, 30, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, Q.D.; Kenter, G.G.; Maas, C.P.; de Kroon, C.D.; Creutzberg, C.L.; Trimbos, J.B.M.Z.; Kuile, M.M.T. Self-reported sexual, bowel and bladder function in cervical cancer patients following different treatment modalities: Longitudinal prospective cohort study. Int. J. Gynecol. Cancer 2013, 23, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Sponholtz, S.E.; Ezendam, N.P.M.; de Rooij, B.H.; Parner, E.; Mogensen, O.; Hildebrandt, M.G.; Schledermann, D.; Markauskas, A.; Frøding, L.P.; Fuglsang, K.; et al. SENTIREC—The sentinel node mapping in women with cervical cancer study—Patient-reported early lymphedema and its impact on quality of life. Gynecol. Oncol. 2022, 164, 463–472. [Google Scholar] [CrossRef]
- Niikura, H.; Okamoto, S.; Otsuki, T.; Yoshinaga, K.; Utsunomiya, H.; Nagase, S.; Takano, T.; Ito, K.; Watanabe, M.; Yaegashi, N. Prospective study of sentinel lymph node biopsy without further pelvic lymphadenectomy in patients with sentinel lymph node-negative cervical cancer. Int. J. Gynecol. Cancer 2012, 22, 1244–1250. [Google Scholar] [CrossRef]
- Lennox, G.K.; Covens, A. Can sentinel lymph node biopsy replace pelvic lymphadenectomy for early cervical cancer? Gynecol. Oncol. 2017, 144, 16–20. [Google Scholar] [CrossRef]
- Gianoni, M.; Mathevet, P.; Uzan, C.; Bats, A.S.; Magaud, L.; Boutitie, F.; Lécuru, F. Does the Sentinel Lymph Node Sampling Alone Improve Quality of Life in Early Cervical Cancer Management? Front. Surg. 2020, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Borčinová, M.; Marnitz, S.; Jarkovský, J.; Klát, J.; Pilka, R.; Torné, A.; Zapardiel, I.; Petiz, A.; Lay, L.; et al. Lower-Limb Lymphedema after Sentinel Lymph Node Biopsy in Cervical Cancer Patients. Cancers 2021, 13, 2360. [Google Scholar] [CrossRef]
- Balaya, V.; Bresset, A.; Guani, B.; Magaud, L.; Montero Macias, R.; Delomenie, M.; Bonsang-Kitzis, H.; Ngô, C.; Bats, A.S.; Mathevet, P.; et al. Risk factors for failure of bilateral sentinel lymph node mapping in early-stage cervical cancer. Gynecol. Oncol. 2020, 156, 93–99. [Google Scholar] [CrossRef]
- Kiss, S.L.; Stanca, M.; Căpîlna, D.M.; Căpîlna, T.E.; Pop-Suciu, M.; Kiss, B.I.; Kiss, S.L., Sr.; Căpîlna, M.E. Sentinel Lymph Node Detection in Cervical Cancer: Challenges in Resource-Limited Settings with High Prevalence of Large Tumours. J. Clin. Med. 2025, 14, 1381. [Google Scholar] [CrossRef]
- Tu, H.; Wan, T.; Zhang, X.; Gu, H.; Feng, Y.; Huang, H.; Liu, J. Potential risks in sentinel lymph node biopsy for cervical cancer: A single-institution pilot study. World J. Surg. Oncol. 2020, 18, 133. [Google Scholar] [CrossRef]
- Kato, H.; Todo, Y.; Minobe, S.; Suzuki, Y.; Nakatani, M.; Ohba, Y.; Yamashiro, K.; Okamoto, K. Previous conization on patient eligibility of sentinel lymph node detection for early invasive cervical cancer. Int. J. Gynecol. Cancer 2011, 21, 1491–1494. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Wang, X.; Peng, X.; Chen, J.; Yang, H. Prediction of clinical stages of cervical cancer via machine learning integrated with clinical features and ultrasound-based radiomics. Sci. Rep. 2025, 15, 18862. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Sun, X.; Tian, M.; Jin, S.; Yu, J.; Song, J.; Wen, F.; Xu, H.; Yu, T.; Dong, Y. Prediction of lymph node metastasis in operable cervical cancer using clinical parameters and deep learning with MRI data: A multicentre study. Insights Imaging 2024, 15, 56. [Google Scholar] [CrossRef]
- Luo, S.; Guo, Y.; Ye, Y.; Mu, Q.; Huang, W.; Tang, G. Prediction of cervical cancer lymph node metastasis based on multisequence magnetic resonance imaging radiomics and deep learning features: A dual-center study. Sci. Rep. 2025, 15, 29259. [Google Scholar] [CrossRef] [PubMed]
- Baeten, I.G.T.; Hoogendam, J.P.; Stathonikos, N.; Gerestein, C.G.; Jonges, G.N.; van Diest, P.J.; Zweemer, R.P. Artificial Intelligence-Based Sentinel Lymph Node Metastasis Detection in Cervical Cancer. Cancers 2024, 16, 3619. [Google Scholar] [CrossRef] [PubMed]
| Study | Tracer Used | Detection Rate (Overall/Bilateral) | Sensitivity (%) | False Negative Rate (%) | NPV (%) | Surgical Approach |
|---|---|---|---|---|---|---|
| SENTICOL I [13] | Blue dye + 99 mTc | 97.8%/76.5% | 92 | 1.4 | 98.2 | Laparoscopy |
| SENTIREC [28] | ICG | 96.3%/82% | 96.3 | Not reported | 98.7 | Laparoscopy |
| Kim et al. [57] | ICG | 100%/85.4% | 71.4 (100% < 2 cm) | 23.1 | 92.4 | Robot-assisted laparoscopic |
| Papadia et al. [55] | Tc99 + dye/ICG | 91.7%/83.4% | 93 | Not reported | 97 | Laparoscopy |
| Dostalek et al. [58] | Blue dye + 99 mTc | 93%/80% | 93–96 | 0–1.6 | Not reported | Laparoscopy |
| SENTIX [59] | Blue dye + radiocolloid | Not reported/91% | Not reported | Not reported | Not reported | Laparoscopy |
| Chiyoda et al. (meta-analysis) [60] | ICG or 99 mTc ± dye | 95.7–100%/80.4–90% | Varies | Varies | Varies | Mixed (all approaches) |
| Anatomical Site | Detection Frequency (%) | Notes |
|---|---|---|
| External/interiliac area | 76–83% | Most common (“typical”) location |
| Obturator nodes | Often grouped with internal iliac nodes | Not always reported separately |
| Internal iliac area | 48–54% | May include presacral area |
| Common iliac area | 6.6–21.1% | Considered “atypical” |
| Parametrium | 3.9–4.3% | Challenging to access |
| Presacral area | 1.3–6% | Detected more frequently with ICG |
| Paraaortic area | 1.5–2.0% | Rare; detection often not clinically relevant |
| Hypogastric, inguinal, cardinal ligament | <0.5% | Extremely rare sites |
| Study | Population/FIGO Stage (Year of Classification) | n | Procedure | DFS (%) | OS (%) | Surgical Approach | Tracer Used |
|---|---|---|---|---|---|---|---|
| Chiyoda et al. [60] (meta-analysis 2019) | Early-stage cervical cancer ≤ 4 cm (FIGO 2009) | Not found | SLNB vs. pelvic LND | No significant difference | No significant difference | Laparoscopy, Laparotomy | Tc-99 m ± blue dye or ICG |
| Meta-analysis [94] (2022) | FIGO IA–IIA (FIGO 2018) | 2226 | SLNB vs. SLNB + LND | 93.1 vs. 92.5 (3-yr DFS) | Not reported | Laparoscopy, Laparotomy | Tc-99 m, blue dye, ICG |
| Casper Tax et al. [15] (2021) | FIGO IA–IB1 ≤ 4 cm (FIGO 2018) | 4130 | SLNB (bilateral negative) only | – | – | Laparoscopy, Laparotomy | ICG, Tc-99 m ± blue dye |
| Ronsini et al. [95] (2017) | FIGO IA–IB1 ≤ 4 cm (FIGO 2009) | 1952 | SLNB vs. LND | – | – | Laparoscopy, Laparotomy | Tc-99 m ± blue dye, ICG |
| SCCAN study [96] (2015) | Early-stage (FIGO 2009 IA–IB) | 1083 | SLNB + LND vs. LND only | 96 vs. 92 (5-yr DFS) | 96.8 vs. 98.4 (5-yr OS) | Laparoscopy, Laparotomy | ICG, Tc-99 m ± blue dye |
| SENTICOL II [97] (prospective 2021) | Early-stage (FIGO 2009 IA–IB) | 206 | SLNB vs. SLNB + LND | 89.5 vs. 93.1 (4-yr DFS) | Not reported | Laparoscopy, Robotic | ICG ± Tc-99 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yordanov, A.; Tsoneva, E.; Hasan, I.; Kostov, S. Sentinel Lymph Node Dissection—Novelty, Trend, or a Paradigm Shift in Surgical Decision-Making for Early Cervical Cancer? Medicina 2025, 61, 1660. https://doi.org/10.3390/medicina61091660
Yordanov A, Tsoneva E, Hasan I, Kostov S. Sentinel Lymph Node Dissection—Novelty, Trend, or a Paradigm Shift in Surgical Decision-Making for Early Cervical Cancer? Medicina. 2025; 61(9):1660. https://doi.org/10.3390/medicina61091660
Chicago/Turabian StyleYordanov, Angel, Eva Tsoneva, Ihsan Hasan, and Stoyan Kostov. 2025. "Sentinel Lymph Node Dissection—Novelty, Trend, or a Paradigm Shift in Surgical Decision-Making for Early Cervical Cancer?" Medicina 61, no. 9: 1660. https://doi.org/10.3390/medicina61091660
APA StyleYordanov, A., Tsoneva, E., Hasan, I., & Kostov, S. (2025). Sentinel Lymph Node Dissection—Novelty, Trend, or a Paradigm Shift in Surgical Decision-Making for Early Cervical Cancer? Medicina, 61(9), 1660. https://doi.org/10.3390/medicina61091660









