Twelve-Month Health-Related Quality of Life Recovery Following COVID-19 Hospitalization: A Prospective Cohort Study from Lithuania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. COVID-19 Severity Classification
- Severe disease: Bilateral pneumonia with >50% lung involvement and either respiratory rate ≥ 30 breaths/min, oxygen saturation ≤ 93% on room air, or oxygen requirement ≤ 10 L/min, without ICU admission.
- Critical disease: Respiratory failure requiring high-flow oxygen (>10 L/min), mechanical ventilation, vasopressors, or intensive care unit admission due to hemodynamic instability or multi-organ dysfunction.
2.4. HRQoL Assessment
2.5. Statistical Analysis
2.6. Comparison with Population Norms
2.7. Rehabilitation Classification
- No rehabilitation: Patients discharged without structured rehabilitation services.
- Stage I rehabilitation: Inpatient rehabilitation during hospitalization (physiotherapy, occupational therapy, or respiratory therapy).
- Stage II rehabilitation: Post-discharge structured multidisciplinary programs (outpatient or sanatorium-based) lasting 2–4 weeks.
- Rehabilitation referrals during the study period were not standardized but were determined by individual clinicians’ judgment and local resource availability, which may have contributed to the low uptake of structured (Stage II) rehabilitation.
2.8. Ethics
3. Results
3.1. Cohort Characteristics
3.2. Changes in HRQoL over Time
- Bodily Pain: improved by +18.8 points (effect size r = 0.41, 95% CI: 0.25–0.57), surpassing the MCID (≥10 points).
- General Health: improved by +14.6 points (r = 0.42, 95% CI: 0.26–0.58), well above the MCID (≥5 points).
- Social Functioning: improved by +10.4 points (r = 0.38, 95% CI: 0.22–0.54), approaching the MCID (≥12.5 points).
- Physical Functioning: +10.2 points (r = 0.37, 95% CI: 0.21–0.53), meeting the MCID (≥10 points).
- Role Physical: +8.1 points (r = 0.34, 95% CI: 0.18–0.50), below the MCID (≥25 points).
- Mental Health: +7.0 points (r = 0.32, 95% CI: 0.16–0.48), exceeding the MCID (≥5 points).
- Vitality: +11.1 points (r = 0.30, 95% CI: 0.14–0.46), meeting the MCID (≥10 points).
- Role-Emotional: +3.6 points (r = 0.16, 95% CI: 0.02–0.30), well below the MCID (≥25 points) indicating limited recovery in emotional role functioning.
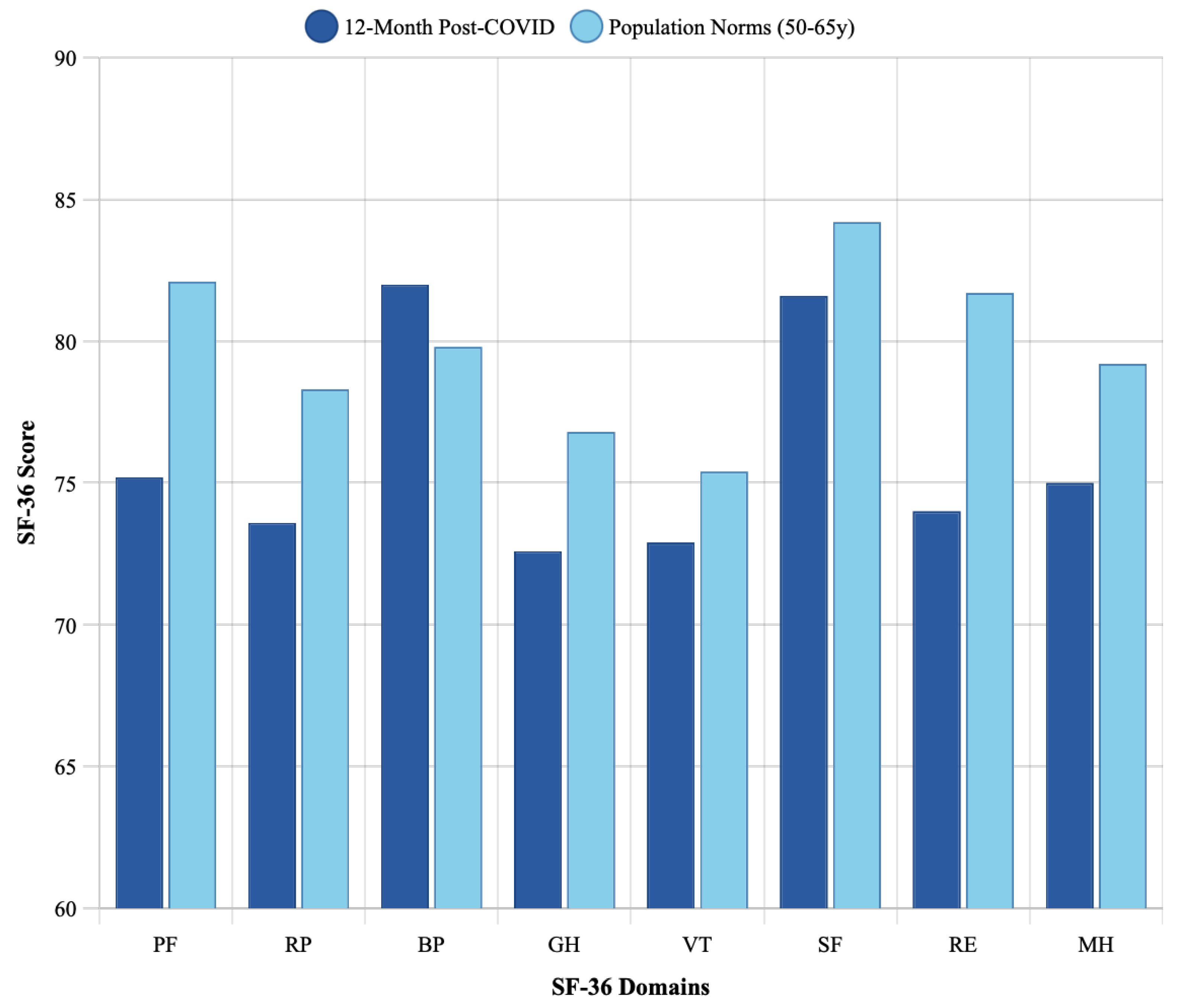
3.3. Comparison with Age-Matched Population Norms
3.4. Predictors of Recovery at 12 Months
- Male sex: β = 14.60, 95% CI: 6.50–22.70, p < 0.001;
- Fewer comorbidities: β = −10.76, 95% CI: −16.20 to −5.32, p < 0.001;
- Employment: β = 8.45, 95% CI: 2.10–14.80, p = 0.010.
- Male sex: β = 10.89, 95% CI: 3.62–18.16, p = 0.004;
- Employment: β = 6.73, 95% CI: 0.95–12.51, p = 0.023.
- More comorbidity: β = −7.46, 95% CI: −13.82 to −1.10, p = 0.021;
- Older age: β = −0.42, 95% CI: −0.78 to −0.06, p = 0.024.
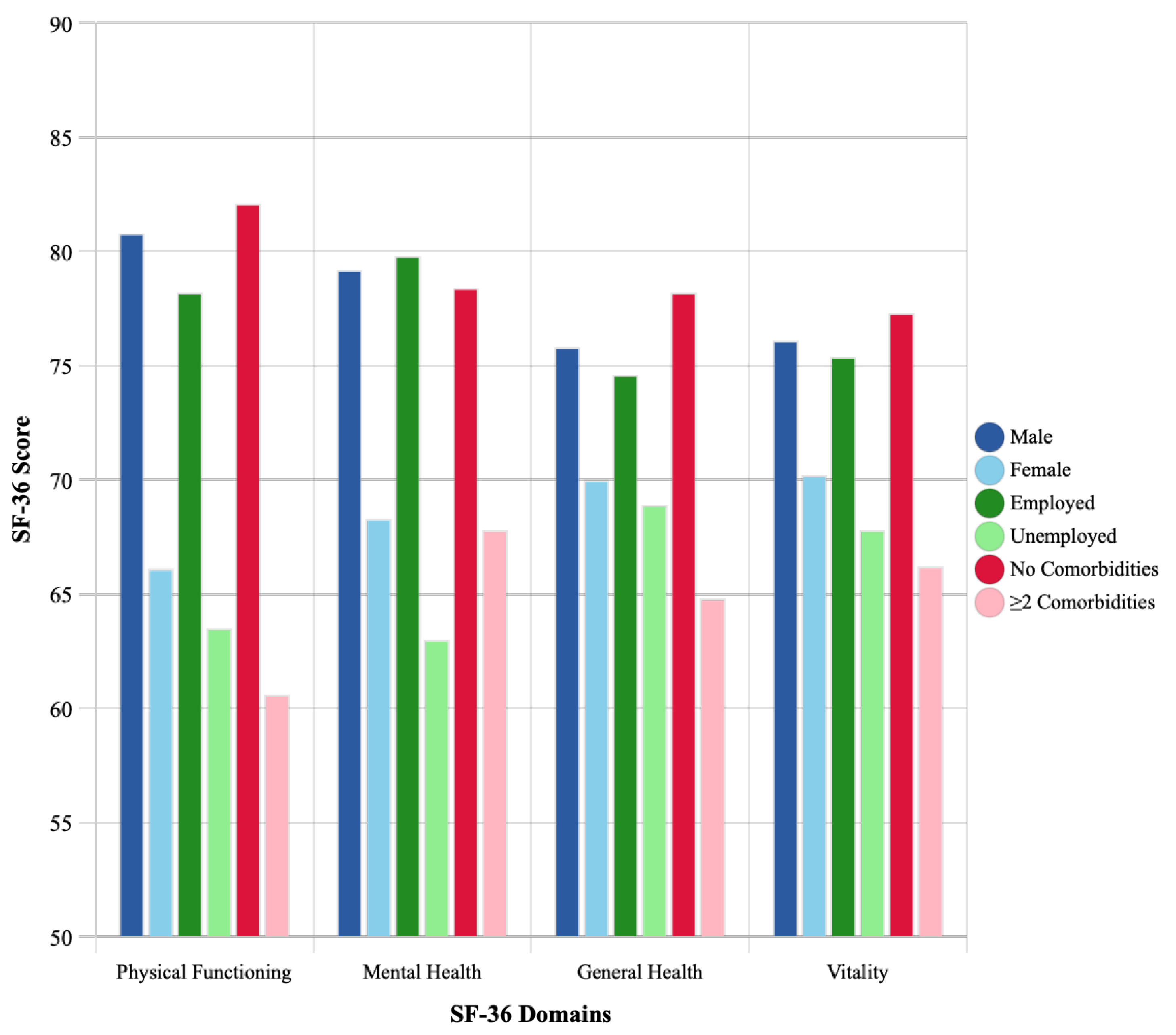
3.5. Disparities in Recovery by Demographic Characteristics
- Physical Functioning: males scored 80.8 ± 16.2 vs. females 66.1 ± 20.1 (p < 0.001);
- Mental Health: males 79.2 ± 15.4 vs. females 68.3 ± 18.7 (p = 0.002).
- Physical Functioning: employed 78.2 ± 17.1 vs. unemployed 63.5 ± 21.3 (p = 0.001);
- Mental Health: employed 79.8 ± 16.2 vs. unemployed 63.0 ± 19.4 (p < 0.001).
- Individuals with ≥2 comorbidities had significantly lower scores across most domains. For example, Physical Functioning obtained a score of 82.1± 14.6 with no comorbidities vs. 60.6± 22.8 with two or more (p < 0.001).
3.6. Healthcare Utilization and Access to Rehabilitation
4. Discussion
4.1. Key Findings
4.2. Clinical Relevance of Changes
4.3. Context in the Global Literature
4.4. Sociodemographic Disparities and Health Equity
4.5. Healthcare System and Rehabilitation Implications
5. Clinical Implications and Recommendations
6. Strengths and Limitations
7. Future Research Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’EM, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized survivors: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef]
- Harirchian, M.H.; Zahednasab, H.; Karampoor, S. Long-term outcomes of hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2022, 441, 120351. [Google Scholar] [CrossRef]
- Statistics Lithuania. COVID-19 Dashboard. 2023. Available online: https://osp.stat.gov.lt/covid-dashboards (accessed on 20 October 2023).
- Berentschot, J.C.; Bek, L.M.; Drost, M.; van den Berg-Emons, R.J.G.; Braunstahl, G.J.; Ribbers, G.M.; Aerts, J.G.J.V.; Hellemons, M.E.; Heijenbrok-Kal, M.H.; CO-FLOW collaboration Group. Health outcomes up to 3 years and post-exertional malaise in patients after hospitalization for COVID-19: A multicentre prospective cohort study (CO-FLOW). Lancet Reg. Health Eur. 2025, 53, 101290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berentschot, J.C.; Bek, L.M.; Heijenbrok-Kal, M.H.; van Bommel, J.; Ribbers, G.M.; Aerts, J.G.J.V.; Hellemons, M.E.; Berg-Emons, H.J.G.v.D. Long-term health outcomes of COVID-19 in ICU- and non-ICU-treated patients up to 2 years after hospitalization: A longitudinal cohort study (CO-FLOW). J. Intensive Care 2024, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Lemhöfer, C.; Sturm, C.; Loudovici-Krug, D.; Guntenbrunner, C.; Bülow, M.; Reuken, P.; Quickert, S.; Best, N. Quality of life and ability to work of patients with Post-COVID syndrome in relation to the number of existing symptoms and the duration since infection up to 12 months: A cross-sectional study. Qual Life Res. 2023, 32, 1991–2002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barker-Davies, R.M.; Osullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.J.C.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir. Med. 2021, 9, 1275–1287. [Google Scholar] [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Di Cristanziano, V.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Eurofound. Living, Working and COVID-19, COVID-19 Series. 2020. Available online: https://assets.eurofound.europa.eu/f/279033/5e54520687/ef20059en.pdf (accessed on 20 October 2023).
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 symptom burden: What is long-COVID and how should we manage it? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Fatima, S.; Ismail, M.; Ejaz, T.; Shah, Z.; Fatima, S.; Shahzaib, M.; Jafri, H.M. Association between long COVID and vaccination: A 12-month follow-up study in a low- to middle-income country. PLoS ONE 2023, 18, e0294780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagin, D.S. Group-based trajectory modeling: An overview. Ann. Nutr. Metab. 2014, 65, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: Boston, MA, USA, 2013. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 29.0; IBM Corp.: Armonk, NY, USA, 2022. [Google Scholar]
- EuroQol Research Foundation. EQ-5D-3L User Guide. 2018. Available online: https://euroqol.org/publications/user-guides (accessed on 18 October 2023).
- World Health Organization. WHO Global Health Observatory. Available online: https://www.who.int/data/gho (accessed on 20 October 2023).
- Ceravolo, M.G.; Anwar, F.; Andrenelli, E.; Udensi, C.; Qureshi, J.; Sivan, M.; Kiekens, C.; Zampolini, M. Evidence-based position paper on physical and rehabilitation medicine professional practice for persons with COVID-19, including post COVID-19 condition: The European PRM position (UEMS PRM Section). Eur. J. Phys. Rehabil. Med. 2023, 59, 789–799. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Wang, X.Q.; Iwashyna, T.J.; Prescott, H.C. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA 2021, 325, 304–306. [Google Scholar] [CrossRef]
- Lithuanian Ministry of Health. COVID-19 Vaccination Strategy. Available online: https://sam.lrv.lt/lt/veiklos-sritys/visuomenes-sveikatos-prieziura/uzkreciamuju-ligu-valdymas/koronavirusas/covid-19-vakcinos-ir-vakcinacija/ (accessed on 20 October 2023).
- World Health Organization. Post COVID-19 Condition (Long COVID). 2022. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 20 October 2023).
- Cuschieri, S.; Grech, V. COVID-19 and diabetes: The why, the what and the how. J. Diabetes Complicat. 2020, 34, 107637. [Google Scholar] [CrossRef]

| Characteristic | n (%) or Mean ± SD |
|---|---|
| Demographics | |
| Age (years) | 58.2 ± 10.2 |
| Female sex | 50 (53.8) |
| Employed | 62 (66.7) |
| COVID-19 Severity | |
| Severe | 37 (39.8) |
| Critical | 56 (60.2) |
| Vaccination Status | |
| Unvaccinated | 89 (95.7) |
| Vaccinated | 4 (4.3) |
| Comorbidities | |
| None | 32 (34.4) |
| One | 33 (35.5) |
| Two or more | 28 (30.1) |
| Hypertension | 45 (48.4) |
| Diabetes mellitus | 23 (24.7) |
| Cardiovascular disease | 19 (20.4) |
| Rehabilitation Status | |
| No rehabilitation | 54 (58.1) |
| Stage I only | 27 (29.0) |
| Stage II | 12 (12.9) |
| Domain | 3 Months Mean ± SD | 6 Months Mean ± SD | 12 Months Mean ± SD | Change (3–12 Months) | Effect Size r (95% CI) | MCID Threshold | Clinical Significance |
|---|---|---|---|---|---|---|---|
| Physical Functioning | 65.0 ± 22.1 | 72.1 ± 19.8 | 75.2 ± 18.5 | +10.2 | 0.37 (0.21–0.53) | ≥10 | Yes |
| Role Physical | 65.5 ± 28.3 | 70.8 ± 26.1 | 73.6 ± 25.4 | +8.1 | 0.34 (0.18–0.50) | ≥25 | No |
| Bodily Pain | 63.2 ± 24.6 | 76.8 ± 22.1 | 82.0 ± 20.3 | +18.8 | 0.41 (0.25–0.57) | ≥10 | Yes |
| General Health | 58.0 ± 18.9 | 68.2 ± 17.6 | 72.6 ± 16.8 | +14.6 | 0.42 (0.26–0.58) | ≥5 | Yes |
| Vitality | 61.8 ± 20.4 | 69.5 ± 18.7 | 72.9 ± 17.9 | +11.1 | 0.30 (0.14–0.46) | ≥10 | Yes |
| Social Functioning | 71.2 ± 25.8 | 78.6 ± 22.4 | 81.6 ± 21.1 | +10.4 | 0.38 (0.22–0.54) | ≥12.5 | Approaching |
| Role Emotional | 70.4 ± 29.2 | 72.8 ± 27.6 | 74.0 ± 26.8 | +3.6 | 0.16 (0.02–0.30) | ≥25 | No |
| Mental Health | 68.0 ± 18.5 | 73.2 ± 17.1 | 75.0 ± 16.9 | +7.0 | 0.32 (0.16–0.48) | ≥5 | Yes |
| Domain | Post-COVID 12-Month Mean ± SD | Population Norm (50–65 Years) | Gap | p-Value * |
|---|---|---|---|---|
| Physical Functioning | 75.2 ± 18.5 | 82.1 | 6.9 | 0.003 |
| Role-Physical | 73.6 ± 25.4 | 78.3 | 4.7 | 0.12 |
| Bodily Pain | 82.0 ± 20.3 | 79.8 | −2.2 | 0.38 |
| General Health | 72.6 ± 16.8 | 76.8 | 4.2 | 0.045 |
| Vitality | 72.9 ± 17.9 | 75.4 | 2.5 | 0.26 |
| Social Functioning | 81.6 ± 21.1 | 84.2 | 2.6 | 0.33 |
| Role-Emotional | 74.0 ± 26.8 | 81.7 | 7.7 | 0.021 |
| Mental Health | 75.0 ± 16.9 | 79.2 | 4.2 | 0.052 |
| Variable | Physical Functioning β (95% CI) | Mental Health β (95% CI) | General Health β (95% CI) |
|---|---|---|---|
| Male gender | 14.60 (6.50–22.70) * | 10.89 (3.62–18.16) | 5.23 (−1.84–12.30) |
| Age (years) | −0.21 (−0.58–0.16) | −0.15 (−0.49–0.19) | −0.42 (−0.78 to −0.06) |
| Employment | 8.45 (2.10–14.80) | 6.73 (0.95–12.51) | 3.82 (−1.95–9.59) |
| Comorbidity burden | −10.76 (−16.20 to −5.32) * | −5.14 (−10.08 to −0.20) | −7.46 (−13.82 to −1.10) |
| Critical COVID-19 | −3.22 (−9.87–3.43) | −2.15 (−8.12–3.82) | −1.88 (−7.85–4.09) |
| Stage II rehabilitation | 5.42 (−3.78–14.62) | 2.31 (−6.11–10.73) | 4.17 (−3.25–11.59) |
| Model Statistics | |||
| R2 | 0.42 | 0.31 | 0.28 |
| F-statistic | 10.2 *** | 6.4 ** | 5.5 ** |
| RMSE | 14.1 | 14.8 | 14.2 |
| Domain | Gender | p-Value | Employment | p-Value | Comorbidities | p-Value † | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 43) | Female (n = 50) | Employed (n = 62) | Unemployed (n = 31) | None (n = 32) | One (n = 33) | ≥Two (n = 28) | ||||
| Physical Functioning | 80.8 ± 16.2 | 66.1 ± 20.1 | <0.001 | 78.2 ± 17.1 | 63.5 ± 21.3 | 0.001 | 82.1 ± 14.6 | 74.8 ± 18.2 | 60.6 ± 22.8 | <0.001 |
| Role Physical | 78.4 ± 23.8 | 69.6 ± 26.2 | 0.089 | 77.1 ± 24.2 | 65.8 ± 27.1 | 0.041 | 79.2 ± 22.1 | 73.6 ± 26.4 | 65.4 ± 28.9 | 0.098 |
| Bodily Pain | 85.2 ± 18.4 | 79.3 ± 21.6 | 0.142 | 83.8 ± 19.1 | 78.4 ± 22.8 | 0.223 | 87.1 ± 16.8 | 81.2 ± 20.8 | 76.8 ± 23.1 | 0.124 |
| General Health | 75.8 ± 15.9 | 70.0 ± 17.2 | 0.095 | 74.6 ± 16.1 | 68.9 ± 18.2 | 0.126 | 78.2 ± 14.5 | 72.1 ± 17.2 | 64.8 ± 18.4 | 0.008 |
| Vitality | 76.1 ± 16.8 | 70.2 ± 18.6 | 0.111 | 75.4 ± 17.2 | 67.8 ± 19.1 | 0.052 | 77.3 ± 15.9 | 72.8 ± 18.4 | 66.2 ± 19.8 | 0.045 |
| Social Functioning | 84.8 ± 19.2 | 78.9 ± 22.4 | 0.172 | 83.6 ± 20.1 | 77.4 ± 23.1 | 0.184 | 86.2 ± 17.8 | 81.4 ± 21.8 | 75.9 ± 24.2 | 0.156 |
| Role Emotional | 77.2 ± 25.1 | 71.3 ± 28.1 | 0.283 | 76.8 ± 25.9 | 68.4 ± 28.4 | 0.158 | 78.1 ± 24.2 | 74.2 ± 27.8 | 68.6 ± 29.1 | 0.345 |
| Mental Health | 79.2 ± 15.4 | 68.3 ± 18.7 | 0.002 | 79.8 ± 16.2 | 63.0 ± 19.4 | <0.001 | 78.4 ± 15.8 | 75.2 ± 17.1 | 67.8 ± 18.9 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strumiliene, E.; Malinauskiene, L.; Zablockiene, B.; Jancoriene, L. Twelve-Month Health-Related Quality of Life Recovery Following COVID-19 Hospitalization: A Prospective Cohort Study from Lithuania. Medicina 2025, 61, 1657. https://doi.org/10.3390/medicina61091657
Strumiliene E, Malinauskiene L, Zablockiene B, Jancoriene L. Twelve-Month Health-Related Quality of Life Recovery Following COVID-19 Hospitalization: A Prospective Cohort Study from Lithuania. Medicina. 2025; 61(9):1657. https://doi.org/10.3390/medicina61091657
Chicago/Turabian StyleStrumiliene, Edita, Laura Malinauskiene, Birute Zablockiene, and Ligita Jancoriene. 2025. "Twelve-Month Health-Related Quality of Life Recovery Following COVID-19 Hospitalization: A Prospective Cohort Study from Lithuania" Medicina 61, no. 9: 1657. https://doi.org/10.3390/medicina61091657
APA StyleStrumiliene, E., Malinauskiene, L., Zablockiene, B., & Jancoriene, L. (2025). Twelve-Month Health-Related Quality of Life Recovery Following COVID-19 Hospitalization: A Prospective Cohort Study from Lithuania. Medicina, 61(9), 1657. https://doi.org/10.3390/medicina61091657





