1. Introduction
Germ cell tumors (GCTs) are curable, even in the setting of relapsed or refractory disease, but their treatment remains challenging [
1]. Approximately one-quarter of patients initially treated with cisplatin-based combination regimens will experience disease relapse or exhibit refractoriness, necessitating alternative therapeutic strategies [
2]. Despite this, durable remission or even cure can often be achieved through salvage treatments. The current therapeutic options for second-line treatment and beyond include conventional-dose chemotherapy (CDCT) and high-dose chemotherapy (HDCT), with both approaches widely utilized in clinical practice. Existing treatment guidelines indicate no definitive difference in efficacy between CDCT and HDCT [
3]. HDCT, when administered in conjunction with peripheral autologous stem cell transplantation (ASCT), has been demonstrated to improve recovery outcomes in salvage settings [
3]. Nonetheless, HDCT should be conducted in high-volume, experienced centers, presenting a significant limitation to its widespread implementation [
4]. Given the rarity of relapsed or refractory GCTs, conducting phase III randomized clinical trials remains a challenge; thus, treatment decisions are frequently guided by retrospective evidence. Notably, most phase III trials evaluating HDCT have not demonstrated a significant survival benefit [
3].
Emerging evidence indicates that body composition parameters—specifically skeletal muscle mass and adipose tissue distribution—are associated with treatment-related toxicities and the overall mortality in patients with cancer [
5].
These parameters are commonly evaluated using diagnostic imaging modalities, including computed tomography (CT), dual-energy X-ray absorptiometry, magnetic resonance imaging, and bioelectrical impedance analysis. Among these, CT imaging has become the predominant modality in oncology-related body composition research, primarily because cancer patients frequently undergo CT scans as part of routine care [
6,
7,
8,
9]. Most studies utilize axial CT images at the third lumbar vertebra (L3) level due to its consistent visualization of multiple muscle groups and visceral fat compartments, as well as its widespread availability in abdominal imaging. However, alternative anatomical landmarks such as the psoas muscle, temporalis muscle, and thoracic vertebral levels have also been explored in recent literature for body composition analysis [
10,
11,
12,
13]. CT-derived body composition analysis provides valuable quantitative metrics, including the skeletal muscle area (used to define sarcopenia), intramuscular fat infiltration (indicative of myosteatosis), subcutaneous and visceral adiposity, and fat density. To date, most oncology studies have focused on the clinical relevance of sarcopenia and myosteatosis. For example, a recent study involving 78 lymphoma patients undergoing ASCT demonstrated that those with sarcopenia exhibited significantly poorer progression-free survival, although the adiposity-related parameters were not evaluated in that cohort [
14]. Recently, research interest has expanded toward the prognostic value of adipose tissue metrics. Notably, some studies have reported paradoxical findings, such as an improved overall survival among patients with hematologic malignancies who exhibit higher levels of visceral adiposity [
15,
16].
In adults with lymphoma undergoing ASCT, sarcopenic obesity—defined by the coexistence of low muscle mass and an elevated body mass index (BMI > 25 kg/m
2)—has been associated with an increased incidence of early post-transplant complications, including prolonged hospitalization, intensive care unit admission, and 30-day unplanned readmissions. Similarly, in multiple myeloma patients undergoing an ASCT, a reduced pre-transplant high-density muscle mass (≤80%) was linked to a higher risk of cardiovascular toxicity within the first 100 days post-transplant [
17,
18].
Achieving a successful ASCT requires both effective eradication of the underlying malignancy and complete bone marrow engraftment. Timely hematologic recovery is particularly crucial, as delayed engraftment may lead to increased early transplant-related complications and higher healthcare costs. In this context, identifying clinical or morphological factors that influence engraftment kinetics—such as body composition and laboratory parameters—may provide valuable insights for optimizing outcomes in patients undergoing HDCT/ASCT [
19].
In addition to body composition metrics, we aimed to investigate the potential impact of other factors, such as post-transplant blood parameters (e.g., hemoglobin level) and patient age, on the kinetics of hematologic recovery following autologous transplantation.
We aimed to determine whether pre-transplant CT-derived body composition indices at L3 (SMI/PMI, TAMA, SFA, VFA, and VFA/SFA) and readily available clinical variables (age and pre-transplant hemoglobin) are associated with hematologic engraftment kinetics following HDCT/ASCT in relapsed/refractory GCT.
2. Materials and Methods
This retrospective study included 43 patients who were diagnosed with relapsed or refractory germ cell tumors, who underwent HDCT followed by ASCT at our institution. The demographic data, disease characteristics, treatment history, and clinical outcomes were extracted from electronic medical records.
2.1. CT-Based Body Composition Analysis and Image-Processing Protocol
Body composition analysis was conducted using non-contrast abdominopelvic CT scans obtained from two different multi-detector CT systems: the Toshiba Aquilion One 320 and Toshiba Aquilion 64 (Otawara, Japan). The scans were acquired with standardized technical parameters, including a tube voltage of 100–140 kVp, tube current of 200–500 mA, rotation time of 0.4 s, and field of view of 400 mm. The CT images were retrieved in Digital Imaging and Communications in Medicine format via the institutional Picture Archiving and Communication System. The muscle and adipose tissue areas were quantitatively analyzed by an experienced radiologist using the CoreSlicer 1.0 software package. Measurements were obtained at the level of the L3, which was anatomically verified using coronal and sagittal multiplanar reformatted images. A predefined attenuation range of −30 to +130 Hounsfield Units was applied to segment the skeletal muscle tissue. The anterior abdominal wall muscles and bilateral psoas muscles were manually outlined using the software’s cursor tool. Following initial segmentation, the software automatically calculated the tissue areas. Any discrepancies were manually corrected using a brush tool to ensure precision (
Figure 1).
2.2. Assessment of Body Composition Parameters and Index Calculations
The body composition parameters—including the subcutaneous fat area (SFA), visceral fat area (VFA), total muscle area (TAMA), and left and right psoas muscle areas—were evaluated using CT images obtained within 6 months before the HDCT/ASCT procedure. The images were acquired either from standard abdominal CT scans or from the CT component of the PET-CT examinations conducted within this period. Notably, TAMA also refers to the skeletal muscle area, as it represents the total cross-sectional area of the muscles at the level of the third lumbar vertebra (L3). Skeletal muscle measurements were obtained at the level of L3 using standardized imaging analysis techniques.
The skeletal muscle index (SMI) was calculated by dividing the cross-sectional area of skeletal muscle at the L3 level by the square of the patient’s height (cm
2/m
2):
The psoas muscle index (PMI) was calculated similarly by dividing the sum of the bilateral psoas muscle areas at the L3 level by the square of the patient’s height (cm
2/m
2):
The total fat area (TFA) was defined as the sum of the visceral and subcutaneous fat areas (TFA = VFA + SFA). In addition, the VFA/SFA ratio was calculated to evaluate the distribution pattern of abdominal fat. The body surface area (BSA) was computed using height and weight data recorded prior to HDCT.
2.3. Post-HDCT Laboratory Assessment and Engraftment Criteria
Laboratory data were obtained during the post-HDCT period. Specifically, the analysis used the lowest recorded values of hemoglobin, the platelet count, the neutrophil count, sodium, calcium, and albumin, and the highest recorded values of creatinine, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) during the transplant-related follow-up. These values were selected to reflect the peak hematologic and metabolic disturbances experienced during the engraftment phase.
Autologous stem cells (at least 2.5 million CD34+ cells per kilogram of body weight) harvested pre-HDCT were reinfused into patients two days after the last chemotherapy. GCSF therapy was then started the following day. All patients were administered antibiotics (levofloxacin), antivirals (valacyclovir), and antifungals (oral fluconazole) as a preventative measure against infections. In addition, prophylactic antiemetics were administered to manage nausea and vomiting, and oral care products were regularly included in the treatment protocol to address mucositis. Patients underwent daily monitoring of blood counts, biochemistry panels, C-reactive protein (CRP), and procalcitonin levels until engraftment. Platelet and erythrocyte transfusions were administered as needed to maintain platelet counts above 10,000/mm3 and hemoglobin levels above 8 g/dL, respectively.
In our cohort, the salvage chemotherapy was predominantly carboplatin–etoposide (CE); only a small subset received ifosfamide–carboplatin–etoposide (ICE), and no other salvage regimens were used.
Platelet engraftment was defined as achieving and maintaining a platelet count > 20,000/mm3 for at least three consecutive days without platelet transfusions, and neutrophil engraftment as an absolute neutrophil count ≥ 2000/mm3 maintained for at least three consecutive days without G-CSF. Engraftment was considered achieved when the predefined threshold for either neutrophils or platelets was met. Platelet and erythrocyte transfusions were administered as needed to maintain platelet counts above 10,000/mm3 and hemoglobin levels above 8 g/dL, respectively.
2.4. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics Version 27.0. pearman’s rank correlation coefficient was used to assess the associations between variables, as the data did not follow a normal distribution and included ordinal or non-parametric measurements, and the sample size was relatively small. Multiple linear regression analysis was conducted to identify independent predictors of engraftment duration. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
A total of 43 patients with primary gonadal germ cell tumors who underwent HDCT/ASCT were included in this study, with a median age of 29 years. The majority (93.0%) had non-seminomatous histology, with mixed germ cell tumors being the most common subtype (74.4%). Pure seminoma was observed in 7.0% of cases. At initial diagnosis, the distribution of disease stage was balanced, with 48.8% of patients presenting with stage < 3, and 51.2% with stage ≥ 3 disease. According to the IGCCCG risk classification, 58.1% of the patients were categorized as poor risk, while 27.9% and 14.0% were classified as good risk and intermediate risk, respectively. The metastatic involvement at the time of diagnosis included the lungs in 60.5% of patients, the liver in 20.9%, bone in 16.3%, and the brain in 7.0%. Lymph node involvement was nearly universal, affecting 97.7% of the cohort. Regarding the response to therapy prior to HDCT/ASCT, 55.8% of patients achieved a complete response (CR) or partial response with negative tumor markers, while 41.9% showed a partial response (PR) with positive markers or stable disease (SD). Only one patient (2.3%) demonstrated progressive disease (PD). HDCT/ASCT was administered as consolidation after two lines of chemotherapy in 79.1% of patients and after three lines in 20.9%. The most commonly used HDCT regimen was CE (carboplatin and etoposide), which was received by 93.0% of patients; ICE (ifosfamide + carboplatin + etoposide) was used in the remaining 7.0%. Following HDCT/ASCT, 65.1% of patients achieved a CR or marker-negative PR. However, 20.9% experienced stable disease or marker-positive PR, and 14.0% had progressive disease, indicating a subset of patients with treatment-resistant disease despite intensive therapy (
Table 1).
In the present study, the median hematologic engraftment duration was 12.0 days, the mean was 13.67 ± 3.60 days, and the range was 9 to 25 days. The 25th and 75th percentiles were 11.0 and 16.0 days, respectively.
3.2. Correlations Between Engraftment Duration and Pre-Transplant Morphological Parameters
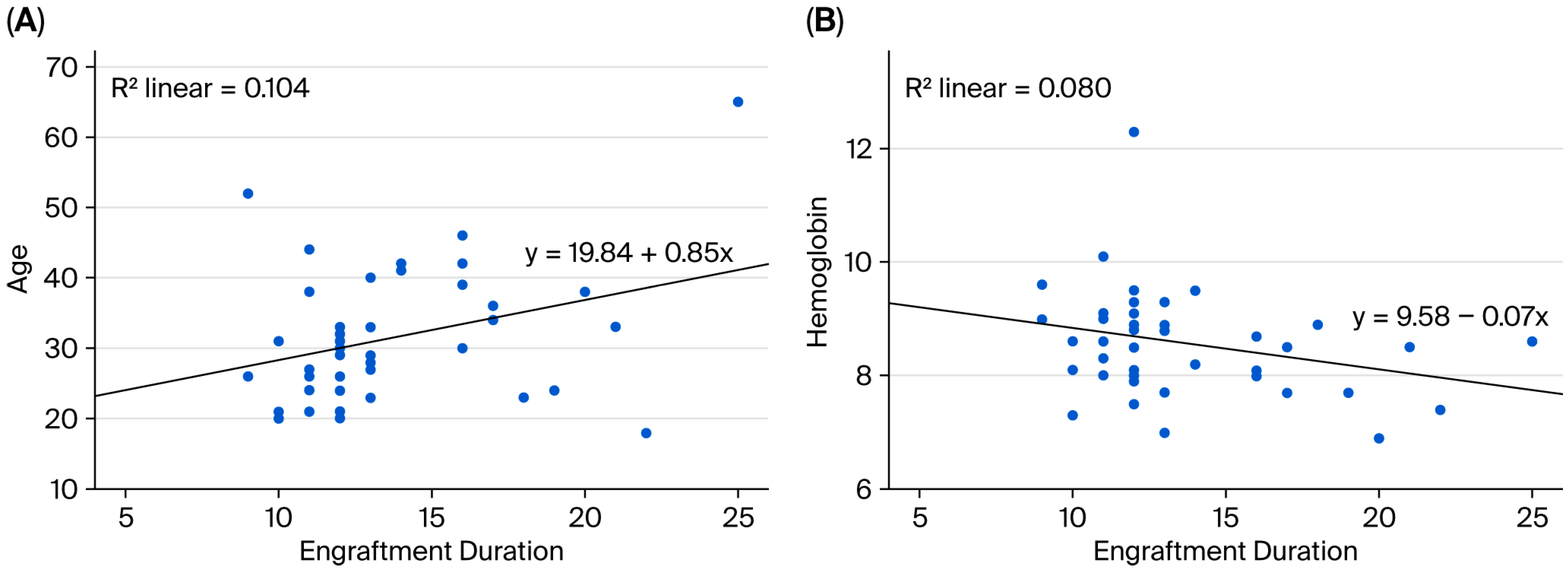
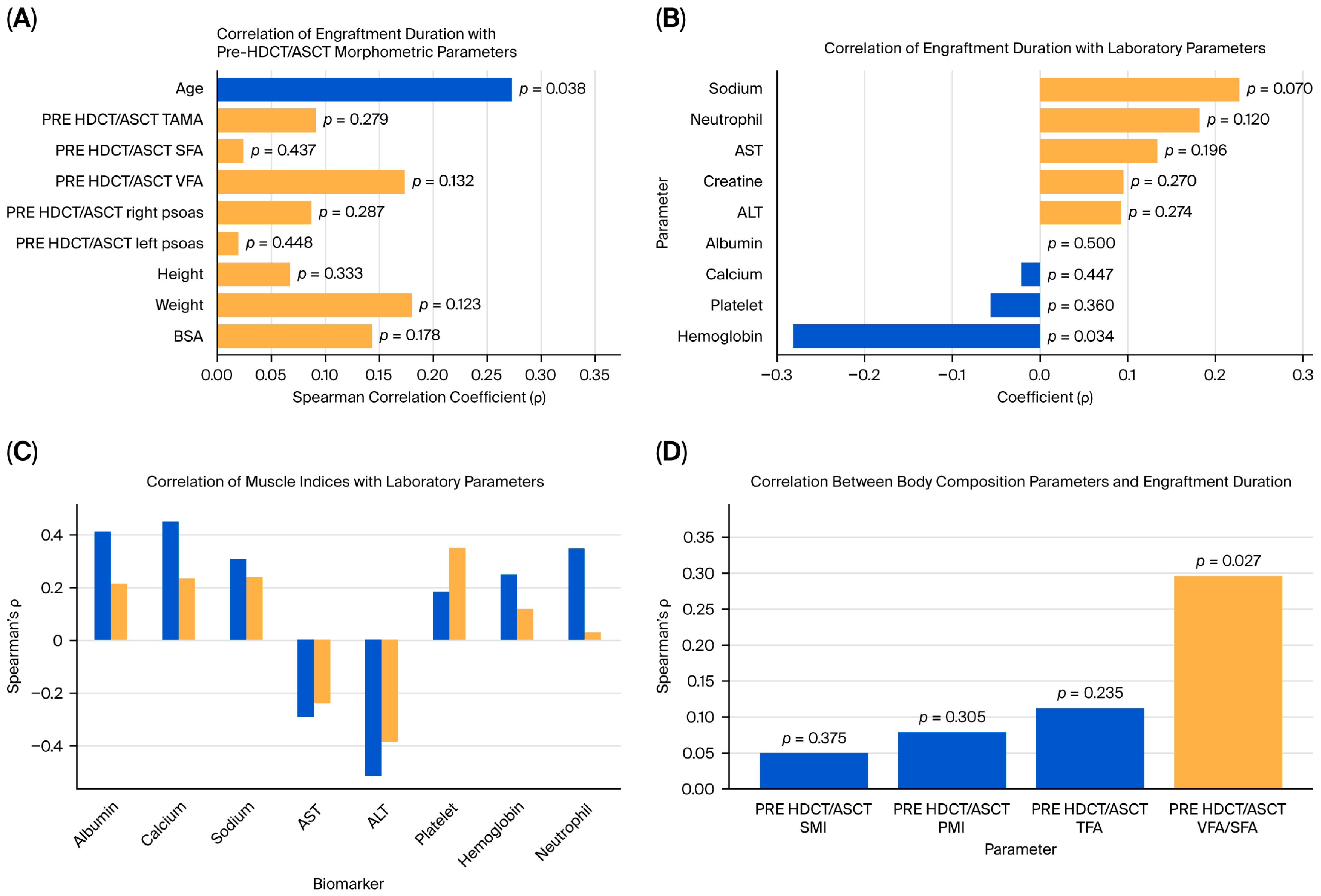
Spearman’s rank correlation analysis was performed to examine the association between engraftment duration and pre-HDCT/ASCT anthropometric and body composition metrics. A statistically significant positive correlation between engraftment duration and age was observed (ρ = 0.274,
p = 0.038). No statistically significant associations between the engraftment duration and pre-transplant area TAMA, SFA, VFA, or psoas muscle measurements were identified. However, weak positive but non-significant correlations with body weight (ρ = 0.181,
p = 0.123) and BSA (ρ = 0.144,
p = 0.178) were observed (
Figure 2A and
Figure 3A).
3.3. Correlations Between Engraftment Duration and Laboratory Parameters
Spearman’s correlation analysis was also conducted to assess the relationships between engraftment duration and various biochemical and hematologic markers. A significant negative correlation between the hemoglobin level and engraftment duration was identified (ρ = −0.281,
p = 0.034), indicating that lower baseline hemoglobin levels may be associated with delayed hematopoietic recovery. Other laboratory markers, including the neutrophil count (ρ = 0.183,
p = 0.120) and serum sodium levels (ρ = 0.229,
p = 0.070), exhibited weak positive correlations, but these were not statistically significant. No meaningful correlations between engraftment duration and creatinine, AST, ALT, calcium, albumin, or platelet count were observed (
Figure 2B and
Figure 3B).
3.4. Multiple Linear Regression Analysis of Engraftment Duration
A multiple linear regression analysis was conducted to identify predictors of engraftment duration. The dependent variable was engraftment duration, with age, hemoglobin level, body weight, and BSA included as independent variables. The model approached statistical significance (F(4, 38) = 2.588, p = 0.052), explaining approximately 21.4% of the variance in engraftment duration (R2 = 0.214), with an adjusted R2 of 0.131. Two variables were found to be statistically significant predictors of engraftment duration. Age was positively associated with engraftment duration (B = 0.136, p = 0.018), whereas hemoglobin levels were negatively associated with engraftment duration (B = −1.256, p = 0.036). Body weight and BSA were not statistically significant predictors (p = 0.866 and p = 0.907, respectively). The residual analysis indicated a standard error of ±3.19 days, reflecting a moderate prediction error.
3.5. Correlations Between Muscle Mass Indices and Laboratory Parameters
The associations between the pre-HDCT/ASCT SMI and PMI and selected laboratory parameters were explored. The SMI showed significant positive correlations with albumin (ρ = 0.414,
p = 0.003), calcium (ρ = 0.456,
p = 0.001), and the neutrophil count (ρ = 0.350,
p = 0.011). Additionally, it was significantly negatively correlated with ALT (ρ = −0.516,
p < 0.001) and AST (ρ = −0.295,
p = 0.028). The PMI was positively correlated with the platelet count (ρ = 0.353,
p = 0.010) (
Figure 3C,
Table 2).
3.6. Correlation of Pre-HDCT/ASCT Body Composition Indices with Engraftment Duration
In this study, the associations between the pre-HDCT/ASCT body composition parameters and engraftment duration were evaluated. Among the variables analyzed, only the VFA/SFA ratio exhibited a statistically significant correlation with engraftment duration (Spearman’s ρ = 0.297,
p = 0.027). No significant associations were observed between the SMI, PMI, or TFA and engraftment duration (
p > 0.05) (
Figure 3D,
Table 3).
3.7. Treatment-Related Hematologic and Biochemical Toxicities
Severe hematologic toxicities were universally observed among patients. All individuals (100%) experienced grade 4 neutropenia and thrombocytopenia, reflecting the expected profound myelosuppression following HDCT. Anemia was also prominent, with the majority of patients exhibiting grade 2 anemia (69.8%), and a small subset developing grade 3 anemia (7.0%).
Regarding biochemical toxicities, a mild-to-moderate creatinine elevation was detected in 27.9% of patients, although grade 3 toxicity was uncommon (4.7%), and no grade 4 renal toxicity was recorded. Hepatic enzyme elevations were relatively frequent; 76.7% of patients showed elevated ALT levels, and 74.4% had elevated AST levels, with grade 3 or higher elevations present in a notable proportion (ALT: 9.3%; AST: 16.3%).
Electrolyte imbalances were also prevalent. Hyponatremia affected over 80% of patients, predominantly grade 1–2, with only one case of grade 3. Hypocalcemia was observed in 55.8% of patients, most commonly at grade 1 severity. Additionally, hypoalbuminemia was frequent (93.0%), and over half of the cohort experienced grade 2 hypoalbuminemia (
Table 4).
4. Discussion
In this retrospective study involving patients with relapsed or refractory germ cell tumors undergoing HDCT/ASCT, we investigated the relationship between pre-transplant clinical, laboratory, and morphometric parameters and hematopoietic recovery kinetics. Our results indicate that age and post-transplant nadir hemoglobin levels were independently associated with prolonged hematologic engraftment, whereas classical body composition parameters, such as skeletal muscle and fat area, showed no significant correlation, with the exception of the VFA/SFA ratio.
We found that increasing age was significantly associated with a prolonged neutrophil engraftment time. This observation is in line with previous findings suggesting that hematopoietic recovery is affected by aging-related declines in bone marrow regenerative capacity. Specifically, Fedorov et al. demonstrated that patients over 75 years old experienced significantly longer WBC and platelet engraftment times compared to younger patients despite similar transplant-related mortality and hospitalization durations [
20]. Similarly, Elçin Erdoğan Yücel et al. observed a statistically significant difference in WBC engraftment between multiple myeloma patients aged above and below 65 years, with median engraftment durations of 12 and 10 days, respectively [
21]. These observations are in concordance with our current findings and support the consideration of age as a clinically relevant factor in post-transplant recovery trajectories.
Furthermore, our study revealed a significant association between lower post-transplant hemoglobin nadirs and prolonged neutrophil engraftment. Despite erythrocyte transfusions being administered during the post-transplant period, patients with lower nadir hemoglobin values exhibited delayed hematologic recovery. This suggests that not only symptomatic anemia but also optimal hemoglobin management may play a role in facilitating timely engraftment. As patients undergoing HSCT frequently require transfusional support until red blood cell and platelet engraftment is complete, a better understanding of transfusion needs may help to minimize complications due to overtransfusion. The American Association of Blood Banks recommends transfusion in asymptomatic patients when hemoglobin levels fall below 7–8 g/dL [
22]. Supporting this, Tabasi et al. reported that lower pre-transplant hemoglobin levels were associated with increased post-transplant transfusion requirements, highlighting the impact of pre-transplant hematologic status on recovery trajectory [
23]. Although their study focused on pre-transplant hemoglobin, our findings expand this perspective by highlighting the predictive significance of post-transplant hemoglobin nadirs on engraftment kinetics.
Consistent with prior reports, the association between older age and prolonged engraftment likely reflects diminished hematopoietic reserve, whereas a lower hemoglobin may serve as a surrogate for greater treatment-related toxicity or bleeding—both plausibly contributing to delayed hematologic recovery.
Interestingly, although the SMI, PMI, and TFA were not significantly associated with engraftment duration in our cohort, a significant positive correlation between the VFA/SFA ratio and prolonged neutrophil recovery was observed. It should not be overlooked that the lack of statistical significance observed for the SMI, PMI, and TFA may be at least partly attributable to the limited sample size. This observation aligns with the growing body of evidence suggesting that altered fat distribution—particularly increased visceral adiposity—may influence transplant-related outcomes. Notably, while sarcopenia and frailty have traditionally been linked to adverse post-transplant recovery trajectories, our findings highlight that visceral fat predominance, possibly through systemic inflammatory pathways or dysregulated metabolic signaling, may also play a detrimental role in delaying hematologic engraftment. It should be noted that unmeasured factors such as general physical fitness or cytokine profiles may also complicate this effect. In this context, the study by M. Pamukçuoğlu et al., which evaluated frailty in 98 patients (51 of whom underwent autologous transplantation), demonstrated that the neutrophil engraftment time was significantly prolonged in frail patients compared to non-frail counterparts [
24]. This finding supports the notion that sarcopenia and frailty may impair hematologic recovery, potentially through systemic inflammation, reduced physiological reserve, and altered bone marrow niche signaling. Taken together, our results reinforce the prognostic relevance of chronological age, visceral adiposity, and sarcopenia-related frailty in influencing post-transplant recovery trajectories. Therefore, integrating body composition profiling—encompassing both muscle and fat metrics—into the pre-transplant assessment may enhance risk stratification and personalized supportive strategies in patients undergoing HDCT-ASCT.
Moreover, we observed strong associations between muscle indices and several biochemical markers. The SMI was positively correlated with serum albumin and calcium levels and inversely correlated with liver transaminases (ALT and AST), reflecting a more favorable nutritional and metabolic profile. Similarly, the PMI was positively associated with the platelet count. These correlations reinforce the concept that muscle mass may serve as a surrogate for physiological reserve and systemic homeostasis.
Our findings extend and strengthen the existing literature in several ways. First, they derive from an understudied population—relapsed/refractory GCT patients undergoing HDCT/ASCT—where evidence on body composition and engraftment is scarce. Second, by evaluating a comprehensive panel of L3 CT-derived metrics (SMI, PMI, SFA, VFA, TFA, and VFA/SFA) within the same cohort and adjusting for key covariates (age, hemoglobin, weight, and BSA), we show that visceral fat predominance (VFA/SFA), rather than classical muscle or total fat areas, is related to delayed neutrophil recovery. Third, we corroborate age as a clinically meaningful correlate of engraftment and add novel evidence that lower post-transplant hemoglobin nadirs are independently associated with slower hematologic recovery, complementing prior work that focused on pre-transplant hemoglobin. Finally, by linking muscle indices with nutritional/metabolic markers (albumin, calcium, and transaminases) and conducting analyses under standardized supportive-care protocols, we provide biologically coherent and clinically actionable signals that can inform pre-transplant risk stratification and targeted optimization (e.g., metabolic and anemia management) in patients considered for HDCT/ASCT.
5. Conclusions
In conclusion, our study highlights the prognostic significance of both clinical and morphometric parameters in influencing hematopoietic recovery following HDCT-ASCT in patients with relapsed or refractory germ cell tumors. Increasing age and lower post-transplant nadir hemoglobin levels were independently associated with prolonged neutrophil engraftment, underlining the relevance of biological aging and hematologic reserve in post-transplant kinetics. While classical muscle and fat area indices did not predict engraftment duration, the visceral-to-subcutaneous fat area ratio emerged as a potential morphological marker that was associated with delayed recovery. These findings suggest that fat distribution, particularly visceral adiposity, alongside sarcopenia-related frailty, may affect hematologic regeneration, possibly through systemic inflammation or bone marrow niche disruption.
The strong correlation observed between muscle indices and biochemical parameters such as albumin, calcium, transaminases, and platelet count further supports the utility of body composition profiling as a surrogate for physiological resilience. The integration of comprehensive clinical, laboratory, and morphometric assessments into the pre-transplant evaluation may therefore enhance risk stratification and enable the development of individualized supportive care strategies aimed at optimizing outcomes in patients undergoing HDCT-ASCT.
6. Limitations
This retrospective, single-center study with a small cohort (n = 43) presents limited generalizability and statistical power, raising the risk of overfitting and false negatives (e.g., for SMI/PMI/TFA). Although salvage therapy was predominantly CE with standardized supportive care, residual heterogeneity (e.g., G-CSF timing and antimicrobial/transfusion practices) may confound the associations. Only pre-transplant CTs were analyzed, but variable imaging–ASCT intervals and incomplete historical imaging (selection bias) are possible. The multivariable adjustment was limited (no objective fitness/frailty or cytokine data), so residual confounding cannot be excluded; the association with post-transplant hemoglobin should be viewed as associative rather than causal. External validation in larger, and multicenter prospective cohorts is warranted.
7. Highlights
Older age and lower post-transplant hemoglobin are independently associated with longer engraftment after HDCT/ASCT. Among the morphometrics, the VFA/SFA (visceral adiposity predominance) shows a weak positive association with delayed engraftment; the SMI, PMI, and TFA are not predictive. The results support pre-transplant risk stratification using simple clinical and imaging metrics (
Figure 4).
8. Future Directions
Validation in prospective, multicenter cohorts with adequate power is warranted, using standardized conditioning/supportive-care protocols, harmonized imaging, and richer phenotyping (HU-based muscle quality, frailty/functional metrics, and cytokine–inflammation panels). Studies should extend follow-up to clinical outcomes (infection burden, readmissions, and PFS/OS) and test interventions—such as anemia/metabolic optimization and prehabilitation—for their effects on engraftment time. Finally, they should develop and externally validate predictive models that integrate clinical (age and hemoglobin), morphometric (VFA/SFA), and laboratory variables to guide individualized supportive care.
Author Contributions
All authors contributed to the study’s conception and design. methodology, Ö.F.K. and E.C.E.; software, A.G.A., N.B.K., U.B. and İ.E.; validation, M.B.A. and Ç.K.; formal analysis, N.K. and A.T.; investigation, U.B.; resources, D.B., A.G.A., N.B.K. and A.D.; data curation, E.C.E., N.B.K., A.G.A., Ç.K. and D.B.; writing—original draft preparation, Ö.F.K.; writing—review and editing, İ.E.; visualization, A.D. and M.B.A.; project administration, N.K.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Approval for the study was obtained from the Gulhane Education and Research Hospital Ethics Committee on 10 April 2025; approved number: 2025/72. This study was conducted in accordance with the guidelines approved by the ethics committee.
Informed Consent Statement
Due to the retrospective nature of the study, the Gulhane Education and Research Hospital Ethics Committee waived the need for obtaining informed consent.
Data Availability Statement
This manuscript does not report data generation or analysis. Therefore, there are no datasets available for public access.
Conflicts of Interest
The authors report no conflict of interest. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
| AFP | Alpha-fetoprotein |
| ANC | Absolute neutrophil count |
| AST | Aspartate aminotransferase |
| CDCT | Conventional-dose chemotherapy |
| CT | Computed tomography |
| Hb | Hemoglobin |
| HU | Hounsfield unit |
| IHC | Immunohistochemistry |
| OS | Overall survival |
| PFS | Progression-free survival |
| PR | Partial response |
| SALL4 | Spalt-like transcription factor 4 |
| SFA | Subcutaneous fat area (cm2) |
| TAMA | Total abdominal muscle area (cm2) |
| VFA | Visceral fat area (cm2) |
| ALT | Alanine aminotransferase |
| ASCT | Autologous stem cell transplantation |
| BMI | Body mass index |
| CE | Carboplatin–etoposide |
| GCT | Germ cell tumor |
| HDCT | High-dose chemotherapy |
| ICE | Ifosfamide–carboplatin–etoposide |
| L3 | Third lumbar vertebral level |
| PD | Progressive disease |
| PMI | Psoas muscle index (cm2/m2) |
| R2 | Coefficient of determination |
| SD | Stable disease |
| SMI | Skeletal muscle index at L3 (cm2/m2) |
| TFA | Total fat area (cm2) |
| VFA/SFA | Visceral-to-subcutaneous fat area ratio |
References
- Gillessen, S.; Sauvé, N.; Collette, L.; Daugaard, G.; de Wit, R.; Albany, C.; Tryakin, A.; Fizazi, K.; Stahl, O.; Gietema, J.A.; et al. Predicting Outcomes in Men with Metastatic Nonseminomatous Germ Cell Tumors (NSGCT): Results From the IGCCCG Update Consortium. J. Clin. Oncol. 2021, 39, 1563–1574. [Google Scholar] [CrossRef]
- Motzer, R.J. Paclitaxel (Taxol) combination therapy for resistant germ cell tumors. Semin. Oncol. 2000, 27 (Suppl. S1), 33–35. [Google Scholar]
- Chovanec, M.; Adra, N.; Abu Zaid, M.; Abonour, R.; Einhorn, L. High-dose chemotherapy for relapsed testicular germ cell tumours. Nat. Rev. Urol. 2023, 20, 217–225. [Google Scholar] [CrossRef]
- Connolly, E.A.; Weickhardt, A.; Grimison, P.; Asher, R.; Heller, G.Z.; Lewin, J.; Liow, E.; Toner, G.; Tung, I.L.Y.; Tran, B.; et al. High-dose chemotherapy for relapsed germ cell tumours: Outcomes in low-volume specialized centres. BJU Int. 2022, 130 (Suppl. S1), 5–16. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Popuri, K.; Cobzas, D.; Baracos, V.E.; Beg, M.F.; Khan, A.D.; Ma, C.; Chow, V.; Prado, C.M.; Xiao, J.; et al. Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J. Cachexia Sarcopenia Muscle 2020, 11, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Lee, V.; Albers, K.B.; Prado, C.M.; Alexeeff, S.; Xiao, J.; Shachar, S.S.; Caan, B.J. Body Composition, Adherence to Anthracycline and Taxane-Based Chemotherapy, and Survival After Nonmetastatic Breast Cancer. JAMA Oncol. 2020, 6, 264–270. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Prado, C.M.; Meyerhardt, J.A.; Weltzien, E.K.; Xiao, J.; Cespedes Feliciano, E.M.; Caan, B.J. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 2018, 124, 3008–3015. [Google Scholar] [CrossRef]
- Rollins, K.E.; Gopinath, A.; Awwad, A.; Macdonald, I.A.; Lobo, D.N. Computed tomography-based psoas skeletal muscle area and radiodensity are poor sentinels for whole L3 skeletal muscle values. Clin. Nutr. 2020, 39, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Alipour, O.; Lee, V.; Tejura, T.K.; Wilson, M.L.; Memel, Z.; Cho, J.; Cologne, K.; Hwang, C.; Shao, L. The assessment of sarcopenia using psoas muscle thickness per height is not predictive of post-operative complications in IBD. Scand. J. Gastroenterol. 2021, 56, 1175–1181. [Google Scholar] [CrossRef]
- Lee, B.; Bae, Y.J.; Jeong, W.-J.; Kim, H.; Choi, B.S.; Kim, J.H. Temporalis muscle thickness as an indicator of sarcopenia predicts progression-free survival in head and neck squamous cell carcinoma. Sci. Rep. 2021, 11, 19717. [Google Scholar] [CrossRef]
- Go, S.-I.; Park, M.J.; Song, H.-N.; Kim, H.-G.; Kang, M.H.; Kang, J.H.; Kim, H.R.; Lee, G.-W. A comparison of pectoralis versus lumbar skeletal muscle indices for defining sarcopenia in diffuse large B-cell lymphoma—Two are better than one. Oncotarget 2017, 8, 47007–47019. [Google Scholar] [CrossRef] [PubMed]
- Arayne, A.A.; Gartrell, R.; Qiao, J.; Baird, P.N.; Yeung, J.M. Comparison of CT derived body composition at the thoracic T4 and T12 with lumbar L3 vertebral levels and their utility in patients with rectal cancer. BMC Cancer 2023, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Sumransub, N.; Cao, Q.; Juckett, M.; Betts, B.; Holtan, S.; Jurdi, N.E.; Hu, M.; Allred, J.; Assi, R.; Maakaron, J.E. Sarcopenia Predicts Inferior Progression-Free Survival in Lymphoma Patients Treated with Autologous Hematopoietic Stem Cell Transplantation. Transplant. Cell. Ther. 2023, 29, 263.e1–263.e7. [Google Scholar] [CrossRef]
- Kapoor, N.D.; Twining, P.K.; Groot, O.Q.; Pielkenrood, B.J.; Bongers, M.E.R.; Newman, E.T.; Verlaan, J.J.; Schwab, J.H. Adipose tissue density on CT as a prognostic factor in patients with cancer: A systematic review. Acta Oncol. 2020, 59, 1488–1495. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Sheu, M.; Mirzai, S.; Majhail, N.S. Prognostic Impact of Adiposity in Hematological Malignancies: A Systematic Review and Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2022, 22, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Iukuridze, A.; Teh, J.B.; Mascarenhas, K.; Herrera, A.; McCune, J.S.; Zain, J.M.; Mostoufi-Moab, S.; McCormack, S.; Slavin, T.P.; et al. Abnormal body composition is a predictor of adverse outcomes after autologous haematopoietic cell transplantation. J. Cachexia Sarcopenia Muscle 2020, 11, 962–972. [Google Scholar] [CrossRef]
- Williams, A.; Baruah, D.; Patel, J.; Szabo, A.; Chhabra, S.; Dhakal, B.; Hari, P.; Janz, S.; Stolley, M.; D’Souza, A. Prevalence and significance of sarcopenia in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2021, 56, 225–231. [Google Scholar] [CrossRef]
- Henon, P.R.; Liang, H.; Beck-Wirth, G.; Eisenmann, J.C.; Lepers, M.; Wunder, E.; Kandel, G. Comparison of hematopoietic and immune recovery after autologous bone marrow or blood stem cell transplants. Bone Marrow Transplant. 1992, 9, 285–291. [Google Scholar]
- Autologous Stem Cell Transplantation in an Older Adult Population. Haematologica. Available online: https://haematologica.org/article/view/haematol.2022.281020 (accessed on 4 August 2025).
- Erdogan Yucel, E.; Kirmaz, A.T.; Kakci, M.; Yavuz, A.F.; Sencelikel, T.; Alacacioglu, I.; Ozsan, G.H. The Effect of Age on High-Dose Therapy with Autologous Stem Cell Support in Multiple Myeloma: A Single-Center Experience. J. Clin. Med. 2024, 13, 4142. [Google Scholar] [CrossRef]
- Carson, J.L.; Grossman, B.J.; Kleinman, S.; Tinmouth, A.T.; Marques, M.B.; Fung, M.K.; Holcomb, J.B.; Illoh, O.; Kaplan, L.J.; Katz, L.M.; et al. Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann. Intern. Med. 2012, 157, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, S.; Parkhideh, S.; Roshandel, E.; Karami, S.; Saeedi, A.; Jabbari, A.; Hajifathali, A. The association of disease type, pre-transplant hemoglobin level and platelet count with transfusion requirement after autologous hematopoietic stem cell transplantation. Caspian J. Intern. Med. 2021, 12, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Pamukcuoglu, M.; Bhatia, S.; Weisdorf, D.J.; DeFor, T.E.; Ustun, C.; Nayar, M.; Holtan, S.G.; Jurdi, N.-E.; Thyagarajan, B.; Brunstein, C.G.; et al. Hematopoietic Cell Transplant–Related Toxicities and Mortality in Frail Recipients. Biol. Blood Marrow Transplant. 2019, 25, 2454–2460. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).