Effects of Low-Level Laser Therapy on Oral Mucosal Wound Healing and Systemic Oxidative Stress in Diabetic Rats: An In Vivo Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Protocol
2.3. Biochemical Measurements
2.4. Statistical Analysis
2.5. Blinding
3. Results
3.1. Body Weight and Glycemia
3.2. The Percentage of Wound Healing
3.3. Biochemical Parameters
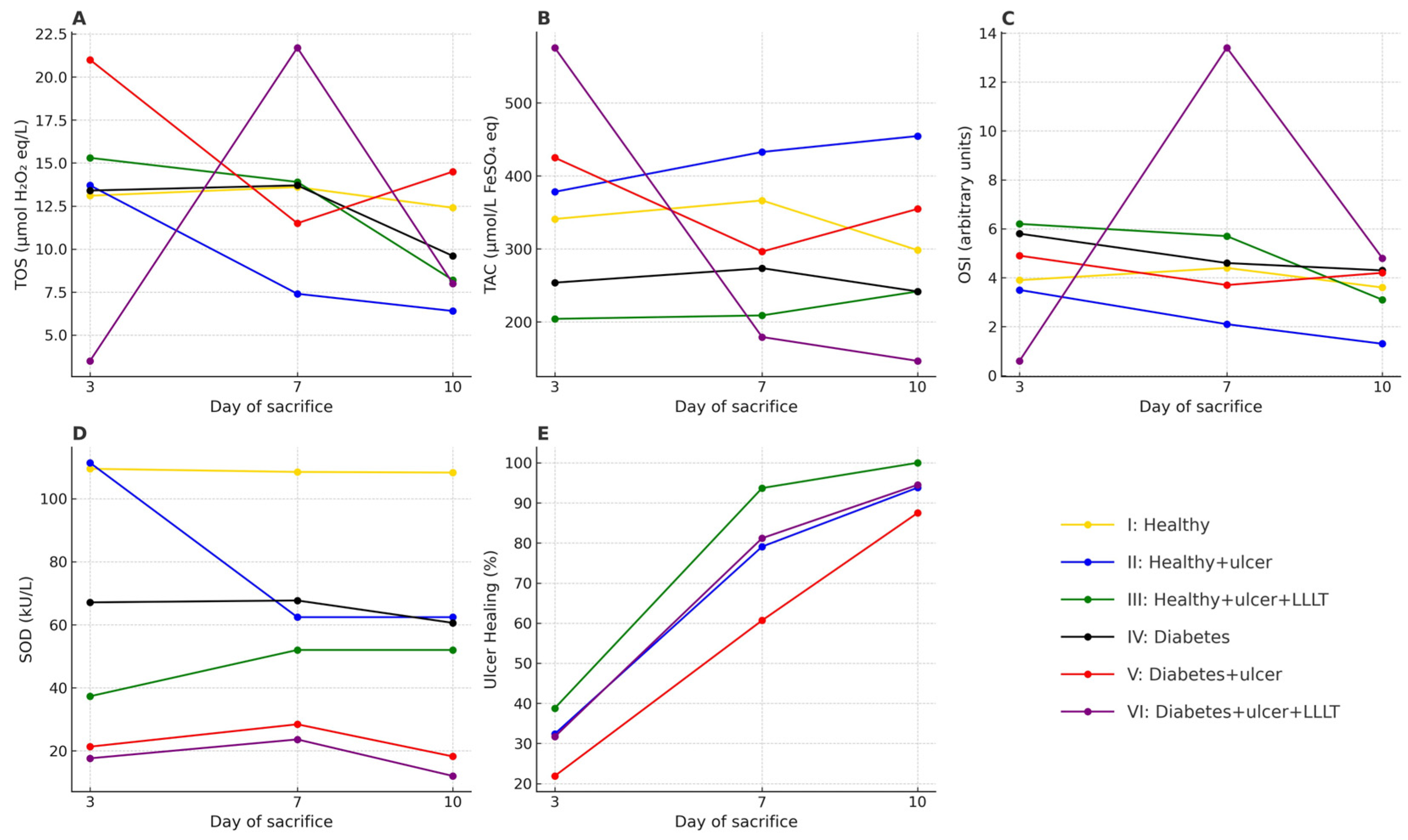
3.3.1. TOS
3.3.2. TAC
3.3.3. OSI
3.3.4. SOD Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas 10th edition scientific committee. In IDF DIABETES ATLAS, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581934/ (accessed on 29 August 2025).
- Miyazawa, I.; Morino, K.; Harada, K.; Ishikado, A.; Kume, S. The Relationship Among Obesity, Diabetes, and Oral Health: A Narrative Review of Real-World Evidence. Curr. Oral Health Rep. 2025, 12, 1–6. [Google Scholar] [CrossRef]
- Ahmad, R.; Haque, M. Oral Health Messiers: Diabetes Mellitus Relevance. Diabetes Metab. Syndr. Obes. 2021, 14, 3001–3015. [Google Scholar] [CrossRef]
- Verhulst, M.J.L.; Loos, B.G.; Gerdes, V.E.A.; Teeuw, W.J. Evaluating All Potential Oral Complications of Diabetes Mellitus. Front. Endocrinol. 2019, 10, 56. [Google Scholar] [CrossRef]
- Madathil, J.; Salim, H.P.; Balan, A.; Radhakrishnan, C.; Kumar, N.R. Prevalence of Oral Lesions in Patients with type 2 Diabetes in North Kerala Population. J. Diabetol. 2020, 11, 32–38. [Google Scholar] [CrossRef]
- Ranbhise, J.S.; Ju, S.; Singh, M.K.; Han, S.; Akter, S.; Ha, J.; Choe, W.; Kim, S.S.; Kang, I. Chronic Inflammation and Glycemic Control: Exploring the Bidirectional Link Between Periodontitis and Diabetes. Dent. J. 2025, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Mirea, A.A.; Ștefan, A.G.; Maria, M.; Clenciu, D.; Mitrea, A.; Efrem, I.C.; Roșu, M.M.; Protasiewicz-Timofticiuc, D.C.; Vladu, B.E.; Gheonea, T.C.; et al. The Associations of Dental and Periodontal Lesions with the Microvascular Complications in Patients with Type 2 Diabetes Mellitus: A Case–Control Study. Life 2024, 14, 1585. [Google Scholar] [CrossRef]
- Cristina de Lima, D.; Nakata, G.C.; Balducci, I.; Almeida, J.D. Oral manifestations of diabetes mellitus in complete denture wearers. J. Prosthet. Dent. 2008, 99, 60–65. [Google Scholar] [CrossRef]
- Perić, M.; Miličić, B.; Kuzmanović Pfićer, J.; Živković, R.; Arsić Arsenijević, V. A Systematic Review of Denture Stomatitis: Predisposing Factors, Clinical Features, Etiology, and Global Candida spp. Distribution. J. Fungi 2024, 10, 328. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, J.S.; Han, K.; Lee, S.Y. Association between Diabetes and the Use of Removable Dental Prostheses among the Korean Population. J. Korean Med. Sci. 2019, 34, e262. [Google Scholar] [CrossRef]
- Devadiga, T.J.; Godil, A.Z.; Wadwan, S.A.; Kazi, A.I.; Dugal, R.J.; Khan, M.A.A. Diabetes and Edentulism: A Survey on Oral Health-Related Quality of Life in Indian Sub-Population. Indian J. Endocrinol. Metab. 2022, 26, 594–600. [Google Scholar] [CrossRef]
- Xiong, Y.; Knoedler, S.; Alfertshofer, M.; Kim, B.S.; Jiang, D.; Liu, G.; Rinkevich, Y.; Mi, B. Mechanisms and therapeutic opportunities in metabolic aberrations of diabetic wounds: A narrative review. Cell Death Dis. 2025, 16, 341. [Google Scholar] [CrossRef]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021, 236, 72–86. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, P.; Sheikh, P.A.; Kumar, A.; Koul, V.; Bhattacharyya, J. Simultaneous regulation of AGE/RAGE signaling and MMP-9 expression by an immunomodulating hydrogel accelerates healing in diabetic wounds. Biomater. Adv. 2024, 163, 213937. [Google Scholar] [CrossRef]
- Radziszewski, M.; Galus, R.; Łuszczyński, K.; Winiarski, S.; Wąsowski, D.; Malejczyk, J.; Włodarski, P.; Ścieżyńska, A. The RAGE Pathway in Skin Pathology Development: A Comprehensive Review of Its Role and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 13570. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.C.; Silva, L.A.; Freitas, T.P.; Latini, A.; Pinho, R.A. Effects of low-power laser irradiation (LPLI) at different wavelengths and doses on oxidative stress and fibrogenesis parameters in an animal model of wound healing. Lasers Med. Sci. 2011, 26, 125–131. [Google Scholar] [CrossRef]
- Abesi, F.; Derikvand, N. Efficacy of Low-Level Laser Therapy in Wound Healing and Pain Reduction After Gingivectomy: A Systematic Review and Meta-analysis. J. Lasers Med. Sci. 2023, 14, e17. [Google Scholar] [CrossRef]
- Duarte de Oliveira, F.J.; Brasil, G.M.L.C.; Araújo Soares, G.P.; Fernandes Paiva, D.F.; de Assis de Souza Júnior, F. Use of low-level laser therapy to reduce postoperative pain, edema, and trismus following third molar surgery: A systematic review and meta-analysis. J. Craniomaxillofac. Surg. 2021, 49, 1088–1096. [Google Scholar] [CrossRef]
- Kwaśna, M.; Cłapińska, P.; Piosik, Z.; Barysz, K.; Dubiec, I.; Bęben, A.; Ordyniec-Kwaśnica, I. Intraoral Applications of Lasers in the Prosthetic Rehabilitation with Fixed Partial Dentures-A Narrative Review. Dent. J. 2024, 12, 164. [Google Scholar] [CrossRef]
- Rathod, A.; Jaiswal, P.; Bajaj, P.; Kale, B.; Masurkar, D. Implementation of Low-Level Laser Therapy in Dentistry: A Review. Cureus 2022, 14, e28799. [Google Scholar] [CrossRef]
- Tamimi, R.; Benisi, S.Z.; Boroujeni, M.E.; Torkamani, M.J. Review on the molecular mechanisms of low-level laser therapy: Gene expression and signaling pathways. Lasers Med. Sci. 2025, 40, 160. [Google Scholar] [CrossRef]
- Mathioudaki, E.; Rallis, M.; Politopoulos, K.; Alexandratou, E. Photobiomodulation and Wound Healing: Low-Level Laser Therapy at 661 nm in a Scratch Assay Keratinocyte Model. Ann. Biomed. Eng. 2024, 52, 376–385. [Google Scholar] [CrossRef]
- Giannakopoulos, E.; Katopodi, A.; Rallis, M.; Politopoulos, K.; Alexandratou, E. The effects of low power laser light at 661 nm on wound healing in a scratch assay fibroblast model. Lasers Med. Sci. 2022, 38, 27. [Google Scholar] [CrossRef]
- Gagnon, D.; Gibson, T.W.; Singh, A.; zur Linden, A.R.; Kazienko, J.E.; LaMarre, J. An in vitro method to test the safety and efficacy of low-level laser therapy (LLLT) in the healing of a canine skin model. BMC Vet. Res. 2016, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Gopal, L.; Palwankar, P.; Dhalla, N. The Efficacy of Low-Level Laser Therapy on the Healing of Oral Wounds: A Systematic Review. Cureus 2024, 16, e70832. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.M.; Sousa de Paula, R.J.; Souza, L.P.; Sousa, F.B.; Mota, M.R.; Alves, A.P. Experimental model of traumatic ulcer in the cheek mucosa of rats. Acta Cir. Bras. 2011, 26, 227–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wagner, V.P.; Meurer, L.; Martins, M.A.; Danilevicz, C.K.; Magnusson, A.S.; Marques, M.M.; Filho, M.S.; Squarize, C.H.; Martins, M.D. Influence of different energy densities of laser phototherapy on oral wound healing. J. Biomed. Opt. 2013, 18, 128002. [Google Scholar] [CrossRef]
- Maia, L.G.; Alves, A.V.; Bastos, T.S.; Moromizato, L.S.; Lima-Verde, I.B.; Ribeiro, M.A.; Gandini Júnior, L.G.; de Albuquerque-Júnior, R.L. Histological analysis of the periodontal ligament and alveolar bone during dental movement in diabetic rats subjected to low-level laser therapy. J. Photochem. Photobiol. B 2014, 135, 65–74. [Google Scholar] [CrossRef]
- Fahimipour, F.; Nouruzian, M.; Anvari, M.; Tafti, M.A.; Yazdi, M.; Khosravi, M.; Dehghannayeri, Z.; Sabounchi, S.S.; Bayat, M. Effect of low-level laser therapy on experimental wounds of hard palate mucosa in mice. Indian J. Exp. Biol. 2011, 49, 357–361. [Google Scholar] [PubMed]
- Fahimipour, F.; Mahdian, M.; Houshmand, B.; Asnaashari, M.; Sadrabadi, A.N.; Farashah, S.E.; Mousavifard, S.M.; Khojasteh, A. The effect of He-Ne and Ga-Al-As laser light on the healing of hard palate mucosa of mice. Lasers Med. Sci. 2013, 28, 93–100. [Google Scholar] [CrossRef]
- França, C.M.; França, C.M.; Núñez, S.C.; Prates, R.A.; Noborikawa, E.; Faria, M.R.; Ribeiro, M.S. Low-intensity red laser on the prevention and treatment of induced-oral mucositis in hamsters. J. Photochem. Photobiol. B 2009, 94, 25–31. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; Lanzafame, R.J. Effects of photostimulation on wound healing in diabetic mice. Lasers Surg. Med. 1997, 20, 56–63. [Google Scholar] [CrossRef]
- Ma, H.; Li, Y.; Chen, H.; Kang, M.; Liu, T.C. Effects of Low-Intensity Laser Irradiation on Wound Healing in Diabetic Rats. Int. J. Photoenergy 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Chaves, M.E.; Araújo, A.R.; Piancastelli, A.C.; Pinotti, M. Effects of low-power light therapy on wound healing: LASER x LED. An. Bras. Dermatol. 2014, 89, 616–623. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.; Daoud, H.; Malik, T.; Shettysowkoor, J.; Rahman, S. The Effects of Low-Level Laser Therapy on Wound Healing and Pain Management in Skin Wounds: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e72542. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Włodarczak, S.; Lesiak, M.; Doroszko, A.; Włodarczak, A. Changes in Cell Biology under the Influence of Low-Level Laser Therapy. Photonics 2022, 9, 502. [Google Scholar] [CrossRef]
- Fahimipour, F.; Houshmand, B.; Alemi, P.; Asnaashari, M.; Tafti, M.A.; Akhoundikharanagh, F.; Farashah, S.E.; Aminisharifabad, M.; Korani, A.S.; Mahdian, M.; et al. The effect of He-Ne and Ga-Al-As lasers on the healing of oral mucosa in diabetic mice. J. Photochem. Photobiol. B 2016, 159, 149–154. [Google Scholar] [CrossRef]
- Muthuraman, P.; Senthilkumar, R.; Srikumar, K. Alterations in beta-islets of Langerhans in alloxan-induced diabetic rats by marine Spirulina platensis. J. Enzym. Inhib. Med. Chem. 2009, 24, 1253–1256. [Google Scholar] [CrossRef]
- Kim, J.M. Induction of Diabetes Mellitus Using Alloxan in Sprague Dawley Rats. Cureus 2024, 16, e63359. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Elajaili, H.; Lyttle, B.D.; Lewis, C.V.; Bardill, J.R.; Dee, N.; Seal, S.; Nozik, E.S.; Liechty, K.W.; Zgheib, C. Increased ROS and Persistent Pro-Inflammatory Responses in a Diabetic Wound Healing Model (db/db): Implications for Delayed Wound Healing. Int. J. Mol. Sci. 2025, 26, 4884. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Uckun, F.M.; Orhan, C.; Tuzcu, M.; Durmus, A.S.; Ozercan, I.H.; Volk, M.; Sahin, K. RJX Improves Wound Healing in Diabetic Rats. Front. Endocrinol. 2022, 13, 874291. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, J.; Xiong, S.; Huang, J.; Liu, Z. The effect of low-level laser therapy on diabetic foot ulcers: A meta-analysis of randomised controlled trials. Int. Wound J. 2021, 18, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Nurwulandari, R.; Sari, Y. The Role of Low-Level Laser Therapy As a Complementary Approach in Promoting Wound Healing Among Patients With Diabetic Foot Ulcers: A Systematic Review. Int. J. 2025, 8, 182–192. [Google Scholar] [CrossRef]
- Karkada, G.; Maiya, G.A.; Arany, P.; Kg, M.R.; Adiga, S.; Kamath, S.U. Dose-response relationship of photobiomodulation therapy and oxidative stress markers in healing dynamics of diabetic neuropathic ulcers in Wistar rats. J. Diabetes Metab. Disord. 2022, 22, 393–400. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Photobiomodulation reduces oxidative stress in diabetic wounded fibroblast cells by inhibiting the FOXO1 signaling pathway. J. Cell Commun. Signal. 2021, 15, 195–206. [Google Scholar] [CrossRef]
- Moheghi, A.; Noori Mougehi, S.M.H.; Amini, A.; Mostafavinia, A.; Rezaei, F.; Bagheri Tadi, F.; Chien, S.; Bayat, M. Anti-inflammatory, Antioxidant, and Wound-Healing Effects of Photobiomodulation on Type-2 Diabetic Rats. J. Lasers Med. Sci. 2023, 14, e45. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. In Vitro Wound Healing Potential of Photobiomodulation Is Possibly Mediated by Its Stimulatory Effect on AKT Expression in Adipose-Derived Stem Cells. Oxid. Med. Cell Longev. 2021, 2021, 6664627. [Google Scholar] [CrossRef] [PubMed]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Jere, S.W.; Houreld, N.N. PBM stimulates fibroblast survival and regeneration in diabetic wounds. Front. Photonics 2024, 5, 1423280. [Google Scholar] [CrossRef]
- Oyebode, O.; Houreld, N.N.; Abrahamse, H. Photobiomodulation in diabetic wound healing: A review of red and near-infrared wavelength applications. Cell Biochem. Funct. 2021, 39, 596–612. [Google Scholar] [CrossRef]
- Woodruff, L.D.; Bounkeo, J.M.; Brannon, W.M.; Dawes, K.S.; Barham, C.D.; Waddell, D.L.; Enwemeka, C.S. The efficacy of laser therapy in wound repair: A meta-analysis of the literature. Photomed. Laser Surg. 2004, 22, 241–247. [Google Scholar] [CrossRef]
- Prado, T.P.; Zanchetta, F.C.; Barbieri, B.; Aparecido, C.; Melo Lima, M.H.; Araujo, E.P. Photobiomodulation with Blue Light on Wound Healing: A Scoping Review. Life 2023, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Chaple Gil, A.; Díaz, L.; Von Marttens, A.; Sotomayor, C.; Basualdo, J.; Beltrán, V.; Jorquera, G.; Caviedes, R.; Fernández, E. The efficacy of low-level laser therapy in oral surgery: A systematic review of randomized controlled trials. Photodiagn. Photodyn. Ther. 2025, 53, 104594. [Google Scholar] [CrossRef] [PubMed]


| Group | Number of Animals | General Condition | Ulceration | Treatment |
|---|---|---|---|---|
| I | 18 | Healthy | No | None (control) |
| II | 18 | Healthy | Yes | No treatment (spontaneous healing) |
| III | 18 | Healthy | Yes | LLLT |
| IV | 18 | DM | No | None (control) |
| V | 18 | DM | Yes | No treatment (spontaneous healing) |
| VI | 18 | DM | Yes | LLLT |
| Measurement Time | Groups | Mean ± SD | p Value |
|---|---|---|---|
| Body Weight (g) | |||
| First | Healthy | 300.7 ± 24.3 | 0.179 |
| DM | 306.8 ± 22.6 | ||
| Second | Healthy | 319.6 ± 24.4 | <0.001 |
| DM | 287.2 ± 34.8 | ||
| Third | Healthy | 444.1 ± 35.4 | <0.001 |
| DM | 272.2 ± 51.8 | ||
| Glycemia (mmol/L) | |||
| First | Healthy | 5.7 ± 0.4 | 0.530 |
| DM | 5.6 ± 0.5 | ||
| Second | Healthy | 5.7 ± 0.3 | <0.001 |
| DM | 28.3 ± 3.1 | ||
| Third | Healthy | 5.7 ± 0.4 | <0.001 |
| DM | 28.2 ± 3.5 | ||
| Groups | Ulcer Healing (%) | p Value | |
|---|---|---|---|
| n | Mean ± SD | ||
| 3rd Day of Sacrifice | |||
| II | 6 | 32.4 ± 4.3 | |
| III | 6 | 38.8 ± 6.2 | p = 0.001 |
| V | 6 | 21.9 ± 7.3 | |
| VI | 6 | 31.7 ± 4.2 | |
| 7th Day of Sacrifice | |||
| II | 6 | 79.1 ± 7.8 | |
| III | 6 | 93.7 ± 3.8 | p < 0.001 |
| V | 6 | 60.7 ± 10.7 | |
| VI | 6 | 81.2 ± 7.0 | |
| 10th Day of Sacrifice | |||
| II | 6 | 93.8 ± 7.6 | |
| III | 6 | 100.0 ± 0.0 | |
| V | 6 | 87.5 ± 4.7 | p = 0.002 |
| VI | 6 | 94.5 ± 3.3 | |
| Groups | Day of Sacrifice | ||
|---|---|---|---|
| 3rd | 7th | 10th | |
| TOS (μmol H2O2 equivalents/L) | |||
| I | 13.1 (10.0–14.7) | 13.6 (10.0–17.4) | 12.4 (8.6–17.4) |
| II | 13.7 (9.4–17.9) | 7.4 (5.4–12.8) | 6.4 (3.5–7.5) |
| III | 15.3 (12.6–17.7) | 13.9 (11.8–16.1) | 8.2 (5.6–13.7) |
| IV | 13.4 (6.2–20.6) | 13.7 (6.2–20.6) | 9.6 (6.3–17.2) |
| V | 21.0 (16.9–28.3) | 11.5 (9.4–12.3) | 14.5 (10.7–21.2) |
| VI | 3.5 (1.3–3.5) | 21.7 (17.4–30.8) | 8.0 (5.4–8.3) |
| p value | p = 0.001 | p = 0.004 | p = 0.011 |
| TAC (μmol/L FeSO4 equivalents) | |||
| I | 341.1 (201.2–469.3) | 366.5 (201.2–430.9) | 298.4 (201.2–472.7) |
| II | 378.3 (329.9–405.2) | 432.9 (299.5–535.0) | 454.7 (316.2–496.0) |
| III | 204.3 (187.2–340.8) | 209.0 (160.6–343.6) | 241.8 (179.4–449.2) |
| IV | 253.9 (206.7–318.2) | 273.7 (180.2–356.8) | 241.8 (195.2–424.2) |
| V | 425.1 (314.3–535.0) | 296.4 (232.8–433.6) | 354.9 (346.3–523.8) |
| VI | 575.7 (541.0–756.6) | 179.4 (107.6–258.9) | 146.7 (138.9–288.6) |
| p value | p = 0.004 | p = 0.016 | p = 0.011 |
| OSI | |||
| I | 3.9 (2.7–6.4) | 4.4 (2.1–6.7) | 3.6 (2.7–5.4) |
| II | 3.5 (2.7–4.7) | 2.1 (1.0–3.3) | 1.3 (1.0–1.9) |
| III | 6.2 (5.2–7.9) | 5.7 (4.7–8.0) | 3.1 (1.5–7.2) |
| IV | 5.8 (2.0–10.0) | 4.6 (2.3–8.3) | 4.3 (2.6–5.8) |
| V | 4.9 (4.3–8.7) | 3.7 (2.5–5.2) | 4.2 (2.0–5.9) |
| VI | 0.6 (0.2–0.6) | 13.4 (8.1–23.8) | 4.8 (2.6–5.7) |
| p value | p = 0.003 | p = 0.002 | p = 0.047 |
| SOD (kU/L) | |||
| I | 109.5 (103.6–115.3) | 108.5 (103.4–117.0) | 108.3 (94.9–116.8) |
| II | 111.4 (108.0–114.8) | 62.4 (52.0–72.8) | 62.4 (46.8–83.2) |
| III | 37.3 (30.2–47.5) | 52.0 (52.0–72.8) | 52.0 (45.8–72.8) |
| IV | 67.1 (53.3–80.8) | 67.7 (41.0–91.6) | 60.6 (53.2–78.1) |
| V | 21.3 (14.2–28.4) | 28.4 (14. 2- 28.4) | 18.2 (14.2–27.1) |
| VI | 17.6 (11.1–22.3) | 23.6 (14.9–28.4) | 12.0 (11.1–19.5) |
| p value | p < 0.001 | p < 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đorđević, N.S.; Dragojević, I.M.; Ilić, A.N.; Stojanović, N.M.; Todić, J.T.; Puhalo Sladoje, D.; Stošović Kalezić, I.; Đorđević, A.M.; Jovanović, R.; Šubarić, L.; et al. Effects of Low-Level Laser Therapy on Oral Mucosal Wound Healing and Systemic Oxidative Stress in Diabetic Rats: An In Vivo Experimental Study. Medicina 2025, 61, 1651. https://doi.org/10.3390/medicina61091651
Đorđević NS, Dragojević IM, Ilić AN, Stojanović NM, Todić JT, Puhalo Sladoje D, Stošović Kalezić I, Đorđević AM, Jovanović R, Šubarić L, et al. Effects of Low-Level Laser Therapy on Oral Mucosal Wound Healing and Systemic Oxidative Stress in Diabetic Rats: An In Vivo Experimental Study. Medicina. 2025; 61(9):1651. https://doi.org/10.3390/medicina61091651
Chicago/Turabian StyleĐorđević, Nadica S., Ilija M. Dragojević, Aleksandra N. Ilić, Nikola M. Stojanović, Jelena T. Todić, Dragana Puhalo Sladoje, Ivana Stošović Kalezić, Aleksandar M. Đorđević, Radovan Jovanović, Ljiljana Šubarić, and et al. 2025. "Effects of Low-Level Laser Therapy on Oral Mucosal Wound Healing and Systemic Oxidative Stress in Diabetic Rats: An In Vivo Experimental Study" Medicina 61, no. 9: 1651. https://doi.org/10.3390/medicina61091651
APA StyleĐorđević, N. S., Dragojević, I. M., Ilić, A. N., Stojanović, N. M., Todić, J. T., Puhalo Sladoje, D., Stošović Kalezić, I., Đorđević, A. M., Jovanović, R., Šubarić, L., Filipović, G., Stojanović, Z., & Kostić, M. (2025). Effects of Low-Level Laser Therapy on Oral Mucosal Wound Healing and Systemic Oxidative Stress in Diabetic Rats: An In Vivo Experimental Study. Medicina, 61(9), 1651. https://doi.org/10.3390/medicina61091651







