CT-Derived Aortic Valve Anatomy and Acute Complications After Self-Expanding and Balloon-Expandable TAVI
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar]
- Moradi, I.; Mustafa, M.S.; Sardar Sheikh, J.; Rahnama, B.S.; Fredericks, M.; Yennam, A.K.; Arain, M.; Saha, U.; Ma, A.R.; Nagendran, A.; et al. Comparative effectiveness of transcatheter vs surgical aortic valve replacement: A systematic review and meta-analysis. World J. Cardiol. 2025, 17, 104168. [Google Scholar] [CrossRef]
- Enezate, T.H.; Kumar, A.; Fadel, M.A.; Patel, M.; Al Dadah, A.; Omran, J. Transcatheter versus surgical aortic valve replacement in patients with non-high surgical risk severe aortic stenosis: A systematic review. Cardiovasc. Revascularization Med. 2017, 18, S40–S48. [Google Scholar] [CrossRef]
- Fassa, A.-A.; Himbert, D.; Vahanian, A. Mechanisms and management of TAVR-related complications. Nat. Rev. Cardiol. 2013, 10, 685–695. [Google Scholar] [CrossRef]
- Bianchini, F.; Bianchini, E.; Romagnoli, E.; Aurigemma, C.; Zito, A.; Busco, M.; Nesta, M.; Bruno, P.; Laezza, D.; Giambusso, N.; et al. Anatomical Annulus Predictors of New Permanent Pacemaker Implantation Risk After Balloon-Expandable Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2024, 224, 26–35. [Google Scholar] [CrossRef]
- Høydahl, M.P.; Kjønås, D.; Rösner, A.; Trones Antonsen, B.; Forsdahl, S.H.; Busund, R. Predictors of permanent pacemaker implantation after transcatheter aortic valve implantation. Scand. Cardiovasc. J. 2025, 59, 2481175. [Google Scholar] [CrossRef]
- Aurigemma, C.; Trani, C.; D’Errigo, P.; Barbanti, M.; Biancari, F.; Tarantini, G.; Ussia, G.P.; Ranucci, M.; Badoni, G.; Baglio, G.; et al. Long-Term Clinical Impact of Paravalvular Leak Following Transcatheter Aortic Valve Implantation. J. Clin. Med. 2025, 14, 605. [Google Scholar] [CrossRef]
- Ojeda, S.; González-Manzanares, R.; Jiménez-Quevedo, P.; Piñón, P.; Asmarats, L.; Amat-Santos, I.; Fernández-Nofrerias, E.; Valle, R.D.; Muñoz-García, E.; Ferrer-Gracia, M.C.; et al. Coronary Obstruction After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2023, 16, 1208–1217. [Google Scholar] [CrossRef]
- Sherwood, M.W.; Xiang, K.; Matsouaka, R.; Li, Z.; Vemulapalli, S.; Vora, A.N.; Fanaroff, A.; Harrison, J.K.; Thourani, V.H.; Holmes, D.; et al. Incidence, Temporal Trends, and Associated Outcomes of Vascular and Bleeding Complications in Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement: Insights From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry. Circ. Cardiovasc. Interv. 2020, 13, e008227. [Google Scholar]
- VARC-3 WRITING COMMITTEE:; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Cavanaugh, J.E.; Neath, A.A. The Akaike information criterion: Background, derivation, properties, application, interpretation, and refinements. WIREs Comput. Stat. 2019, 11, e1460. [Google Scholar] [CrossRef]
- Berkelmans, G.F.N.; Read, S.H.; Gudbjörnsdottir, S.; Wild, S.H.; Franzen, S.; van der Graaf, Y.; Eliasson, B.; Visseren, F.L.J.; Paynter, N.P.; Dorresteijn, J.A.N. Population median imputation was noninferior to complex approaches for imputing missing values in cardiovascular prediction models in clinical practice. J. Clin. Epidemiol. 2022, 145, 70–80. [Google Scholar] [CrossRef]
- Hokken, T.W.; Muhemin, M.; Okuno, T.; Veulemans, V.; Lopes, B.B.; Beneduce, A.; Vittorio, R.; Ooms, J.F.; Adrichem, R.; Neleman, T.; et al. Impact of membranous septum length on pacemaker need with different transcatheter aortic valve replacement systems: The INTERSECT registry. J. Cardiovasc. Comput. Tomogr. 2022, 16, 524–530. [Google Scholar] [CrossRef]
- Hokken, T.W.; van Wiechen, M.P.; Ooms, J.F.; El Azzouzi, I.; de Ronde, M.; Kardys, I.; Budde, R.; Daemen, J.; de Jaegere, P.P.; Van Mieghem, N.M. Impact of Interventricular membranous septum length on pacemaker need with different Transcatheter aortic valve implantation systems. Int. J. Cardiol. 2021, 333, 152–158. [Google Scholar] [CrossRef]
- Veulemans, V.; Frank, D.; Seoudy, H.; Wundram, S.; Piayda, K.; Maier, O.; Jung, C.; Polzin, A.; Frey, N.; Kelm, M.; et al. New insights on potential permanent pacemaker predictors in TAVR using the largest self-expandable device. Cardiovasc. Diagn. Ther. 2020, 10, 1816–1826. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Nombela-Franco, L.; Urena, M.; Mok, M.; Pasian, S.; Doyle, D.; DeLarochellière, R.; Côté, M.; Laflamme, L.; DeLarochellière, H.; et al. Coronary Obstruction Following Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2013, 6, 452–461. [Google Scholar] [CrossRef]
- Khan, J.M.; Kamioka, N.; Lisko, J.C.; Perdoncin, E.; Zhang, C.; Maini, A.; Chen, M.; Li, Y.; Ludwig, S.; Westermann, D.; et al. Coronary Obstruction From TAVR in Native Aortic Stenosis: Development and Validation of Multivariate Prediction Model. JACC Cardiovasc. Interv. 2023, 16, 415–425. [Google Scholar] [CrossRef]
- Primessnig, U.; Wiedenhofer, J.M.; Trippel, T.D.; Loddenkemper, C.M.; Schrader, H.; Brand, A.; Spethmann, S.; Stangl, K.; Haghikia, A.; Landmesser, U.; et al. Early clinical outcomes of Portico and Edwards Sapien 3 valve prosthesis in transcatheter aortic valve replacement: Propensity-matched analysis. Front. Cardiovasc. Med. 2024, 11, 1400626. [Google Scholar] [CrossRef]
- Costa, G.; Barbanti, M.; Rosato, S.; Seccareccia, F.; Tarantini, G.; Fineschi, M.; Salizzoni, S.; Valvo, R.; Tamburino, C.; Biancari, F.; et al. Real-World Multiple Comparison of Transcatheter Aortic Valves: Insights From the Multicenter OBSERVANT II Study. Circ. Cardiovasc. Interv. 2022, 15, e012294. [Google Scholar] [CrossRef]
- Eckel, C.E.; Kim, W.-K.; Grothusen, C.; Tiyerili, V.; Elsässer, A.; Sötemann, D.; Schlüter, J.; Choi, Y.H.; Charitos, E.I.; Renker, M.; et al. Comparison of the New-Generation Self-Expanding NAVITOR Transcatheter Heart Valve with Its Predecessor, the PORTICO, in Severe Native Aortic Valve Stenosis. J. Clin. Med. 2023, 12, 3999. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Tsao, T.-P.; Lee, K.-C.; Lin, H.-C.; Liu, C.-T.; Hsiung, M.-C.; Yin, W.-H.; Wei, J. Predictors of permanent pacemaker requirement in aortic stenosis patients undergoing self-expanding valve transcatheter aortic valve replacement using the cusp overlap technique. Front. Cardiovasc. Med. 2025, 12, 1486375. [Google Scholar] [CrossRef]
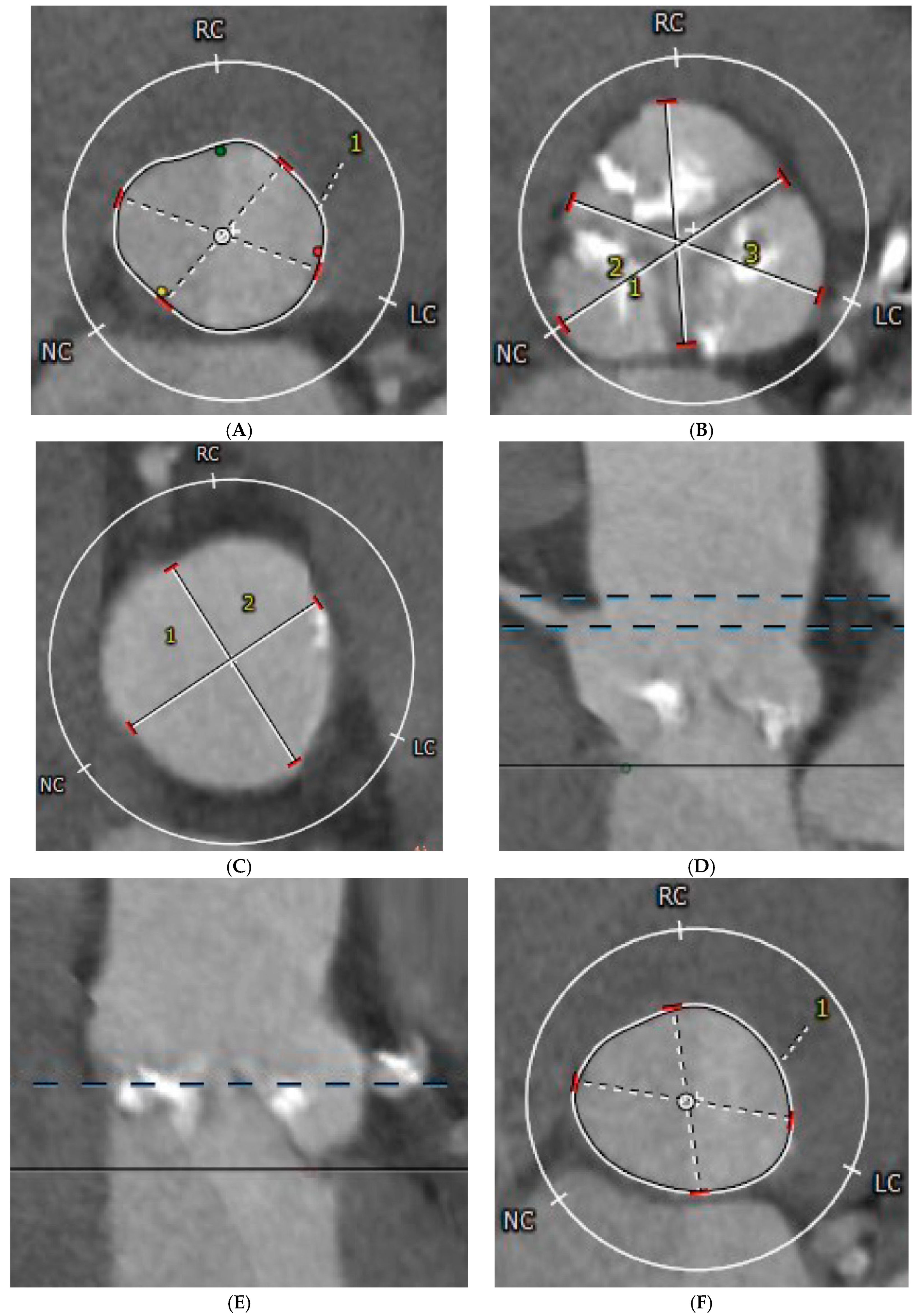
| Parameter | All Patients (n = 485) | Acute Complications | ||
|---|---|---|---|---|
| Without (n = 416) | With (n = 69) | p-Value | ||
| Age (years) | 78 (75–82) | 78 (75–82) | 76.94 ± 6.87 | 0.23 |
| Male sex | 276 (56.9%) | 245 (58.9%) | 31 (44.9%) | 0.03 |
| Hospitalization (days) | 6.8 (4.9–8.8) | 6.2 (4.9–7.9) | 10.9 (7.0–14) | 0.001 |
| Diabetes mellitus | 177 (36.5%) | 148 (35.6%) | 29 (42.0%) | 0.34 |
| Hypertension | 415 (85.6%) | 360 (86.5%) | 55 (79.7%) | 0.14 |
| Atrial fibrillation | 179 (36.9%) | 157 (37.7%) | 22 (31.9%) | 0.41 |
| Chronic kidney disease | 80 (16.5%) | 71 (17.1%) | 9 (13.0%) | 0.48 |
| Stroke | 30 (6.2%) | 26 (6.2%) | 4 (5.8%) | 1.00 |
| Prior MI | 49 (10.1%) | 38 (9.1%) | 11 (15.9%) | 0.08 |
| Prior CABG | 19 (3.9%) | 17 (4.1%) | 2 (2.9%) | 1.00 |
| LBBB | 62 (12.8%) | 48 (11.5%) | 14 (20.3%) | 0.05 |
| Active smoker | 12 (2.5%) | 11 (2.6%) | 1 (1.4%) | 1.00 |
| Dyslipidemia | 235 (48.5%) | 211 (50.7%) | 24 (34.8%) | 0.01 |
| COPD | 34 (7.0%) | 31 (7.5%) | 3 (4.3%) | 0.45 |
| CAD | 86 (17.7%) | 80 (19.2%) | 6 (8.7%) | 0.03 |
| DCM | 32 (6.6%) | 29 (7.0%) | 3 (4.3%) | 0.60 |
| Creatinine (mg/dl) | 1.08 (0.86–1.31) | 1.08 (0.87–1.33) | 1.06 (0.84–1.27) | 0.44 |
| Hemoglobin (g/dL) | 12.9 (11.7–14) | 12.8 ± 1.72 | 12.5 ± 1.73 | 0.16 |
| Leucocytes (×103/µL) | 7.04 (5.92–8.45) | 7.11 (5.96–8.56) | 6.65 (5.85–7.86) | 0.06 |
| Platelets (×103/µL) | 203 (169–247) | 203 (170–247) | 200 (164–244) | 0.46 |
| LVEF (%) | 50 (45–55) | 50 (45–55) | 50 (45–55) | 0.42 |
| LV diameter (mm) | 51 (45–56) | 50.50 (45–56) | 53.79 ± 8.70 | 0.05 |
| Maximum gradient (mmHg) | 79 (65–94) | 79 (66–93) | 78 (60–96) | 0.63 |
| Mean gradient (mmHg) | 47 (40–59) | 47 (40–59) | 50.5 ± 21.8 | 0.77 |
| AVA (cm2) | 0.66 ± 0.16 | 0.65 ± 0.17 | 0.68 ± 0.10 | 0.73 |
| Maximum transprosthetic gradient (mmHg) | 20 (13–26) | 21 (14–28) | 14.94 ± 6.84 | 0.001 |
| Mean transprosthetic gradient (mmHg) | 11 (8–15) | 11 (8–15) | 8.42 ± 3.56 | 0.008 |
| Valve implantation depth (mm) | 3.9 (3.5–4.5) | 3.8 (3.5–4.4) | 4.1 (3.4–4.8) | 0.12 |
| EuroSCORE I (%) | 8.1 (5.7–13.8) | 8.1 (5.6–13.3) | 8.6 (6.1–14.6) | 0.41 |
| EuroSCORE II (%) | 4.6 (2.3–15.1) | 4.2 (2.3–13.9) | 11.0 (3.1–19.7) | 0.39 |
| Parameter | All Patients (n = 485) | Acute Complications | ||
|---|---|---|---|---|
| Without (n = 416) | With (n = 69) | p-Value | ||
| Annulus area (mm2) | 463 (410–528) | 463 (409–527) | 472 ± 92 | 0.98 |
| Annulus perimeter (mm) | 77 (72–83) | 78 (72–83) | 78.24 ± 7.63 | 0.98 |
| Minimum annulus diameter (mm) | 21.4 (20.0–23.1) | 21.4 (20.0–23.1) | 21.61 ± 2.45 | 0.96 |
| Maximum annulus diameter (mm) | 27 (25–29) | 27 (25–29) | 27.7 ± 2.5 | 0.75 |
| Sinus of Valsalva diameter (mm) | 30.61 ± 3.55 | 30.63 ± 3.51 | 30.48 ± 3.89 | 0.79 |
| Sinotubular junction diameter(mm) | 28.6 (25.9–30.9) | 28.6 (26–31) | 28 (25–30) | 0.35 |
| LCA height (mm) | 14 (12–16) | 14 (12–16.32) | 12.0 (11–15) | 0.06 |
| RCA height (mm) | 17.9 (15.5–20) | 17.9 (15.5–20) | 17 (15–19) | 0.36 |
| Sinotubular junction height (mm) | 23.4 (21.5–25.5) | 23.4 (21.5–25.6) | 26.4 (22.5–28.0) | 0.07 |
| LVOT area (mm) | 462 (406–530) | 464 (404–530) | 475 ± 89 | 0.90 |
| Annulus ellipticity (%) | 22 (17–26) | 22 (17–26) | 22.06 ± 6.35 | 0.70 |
| LVOT ellipticity (%) | 24.11 ± 6.89 | 24.10 ± 6.76 | 24.21 ± 7.77 | 0.92 |
| Valve Type | Acute Complications | p-Value * | OR (95% CI) | p-Value ** | |
|---|---|---|---|---|---|
| Without (n = 416) | With (n = 69) | ||||
| EdwardsSapien3 | 334 (80.3%) | 47 (68.1%) | 0.02 | 0.52 (0.29–0.91) | 0.02 |
| Navitor/Portico | 17 (4.1%) | 9 (13.0%) | 0.006 | 3.52 (1.52–8.29) | 0.003 |
| Medtronic | 11 (2.6%) | 3 (4.3%) | 0.43 | 1.67 (0.45–6.15) | 0.43 |
| Accurate | 28 (6.7%) | 4 (5.8%) | 1.00 | 0.85 (0.28–2.51) | 0.77 |
| Boston | 26 (6.2%) | 6 (8.7%) | 0.43 | 1.42 (0.56–3.60) | 0.45 |
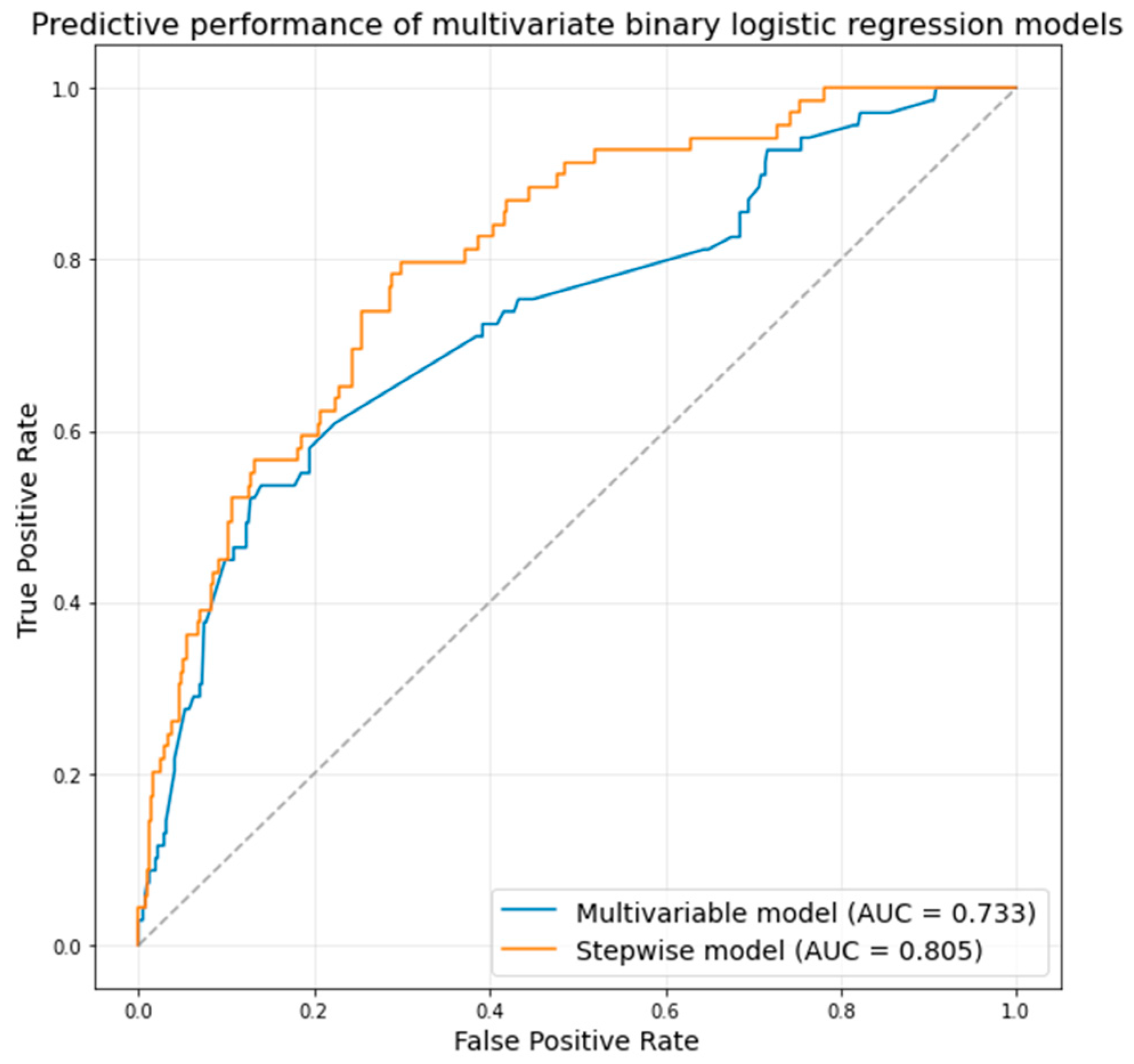
| Parameter | Multivariable Regression * | Stepwise Regression ** | ||
|---|---|---|---|---|
| OR | p-Value | OR (95% CI) | p-Value | |
| Male sex | 0.45 (0.25–0.81) | 0.01 | 0.31 (0.15–0.63) | 0.001 |
| Diabetes mellitus | - | - | 1.98 (1.08–3.64) | 0.03 |
| History of MI | - | - | 2.96 (1.24–7.04) | 0.01 |
| LBBB | 2.19 (1.06–4.49) | 0.03 | 2.94 (1.37–6.31) | 0.01 |
| LV diameter | 1.05 (1.00–1.09) | 0.04 | - | - |
| Mitral regurgitation | 1.76 (1.22–2.54) | 0.002 | 2.05 (1.38–3.04) | 0.004 |
| Transprosthetic gradient | 0.91 (0.86–0.97) | 0.001 | 0.90 (0.84–0.96) | 0.008 |
| Navitor/Portico valve | 2.58 (1.04–6.42) | 0.04 | 4.84 (1.13–20.71) | 0.03 |
| LCA height | - | - | 0.89 (0.8–0.99) | 0.03 |
| Sinotubular junction height | - | - | 1.16 (1.01–1.32) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stan, A.A.; Elkahlout, A.; Harpa, M.M.; Pop, M.; Veres, M.; Stan, A.D.; Călburean, P.-A.; Aniței, D.E.; Scurtu, A.-C.; Brînzaniuc, K.; et al. CT-Derived Aortic Valve Anatomy and Acute Complications After Self-Expanding and Balloon-Expandable TAVI. Medicina 2025, 61, 1650. https://doi.org/10.3390/medicina61091650
Stan AA, Elkahlout A, Harpa MM, Pop M, Veres M, Stan AD, Călburean P-A, Aniței DE, Scurtu A-C, Brînzaniuc K, et al. CT-Derived Aortic Valve Anatomy and Acute Complications After Self-Expanding and Balloon-Expandable TAVI. Medicina. 2025; 61(9):1650. https://doi.org/10.3390/medicina61091650
Chicago/Turabian StyleStan, Alexandru Antoniu, Ayman Elkahlout, Marius Mihai Harpa, Marian Pop, Mihaly Veres, Antonela Delia Stan, Paul-Adrian Călburean, David Emanuel Aniței, Anda-Cristina Scurtu, Klara Brînzaniuc, and et al. 2025. "CT-Derived Aortic Valve Anatomy and Acute Complications After Self-Expanding and Balloon-Expandable TAVI" Medicina 61, no. 9: 1650. https://doi.org/10.3390/medicina61091650
APA StyleStan, A. A., Elkahlout, A., Harpa, M. M., Pop, M., Veres, M., Stan, A. D., Călburean, P.-A., Aniței, D. E., Scurtu, A.-C., Brînzaniuc, K., & Suciu, H. (2025). CT-Derived Aortic Valve Anatomy and Acute Complications After Self-Expanding and Balloon-Expandable TAVI. Medicina, 61(9), 1650. https://doi.org/10.3390/medicina61091650







