Evidence for the Pathogenicity of a CFH Variant in a Multigenerational Family with Cuticular Drusen
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioethics and Samples
2.2. Ophthalmic Investigation
2.3. DNA Extraction
2.4. Whole-Exome Sequencing
2.5. Sanger Sequencing
2.6. In Silico Protein Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
2.9. Data Management
3. Results
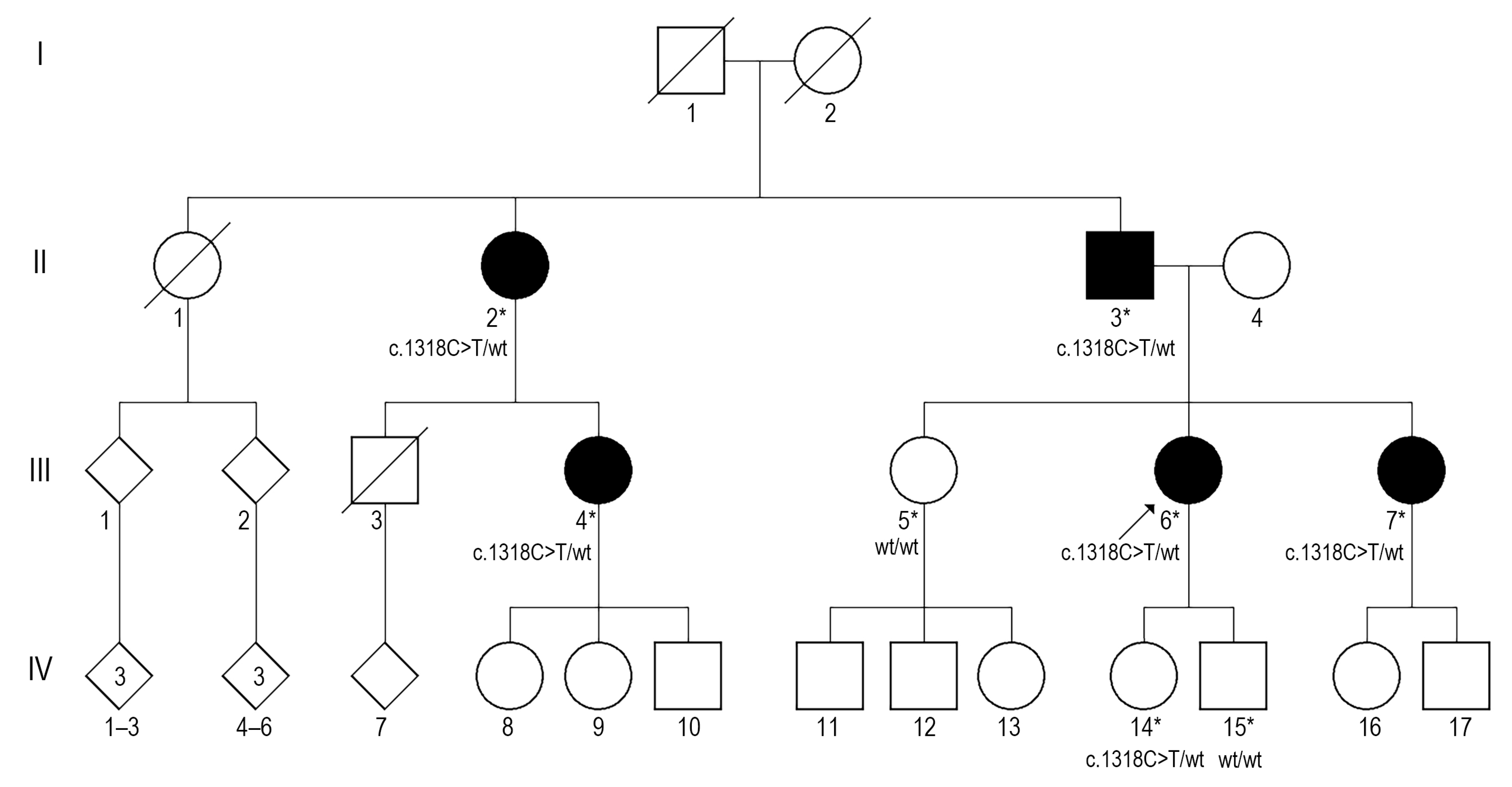
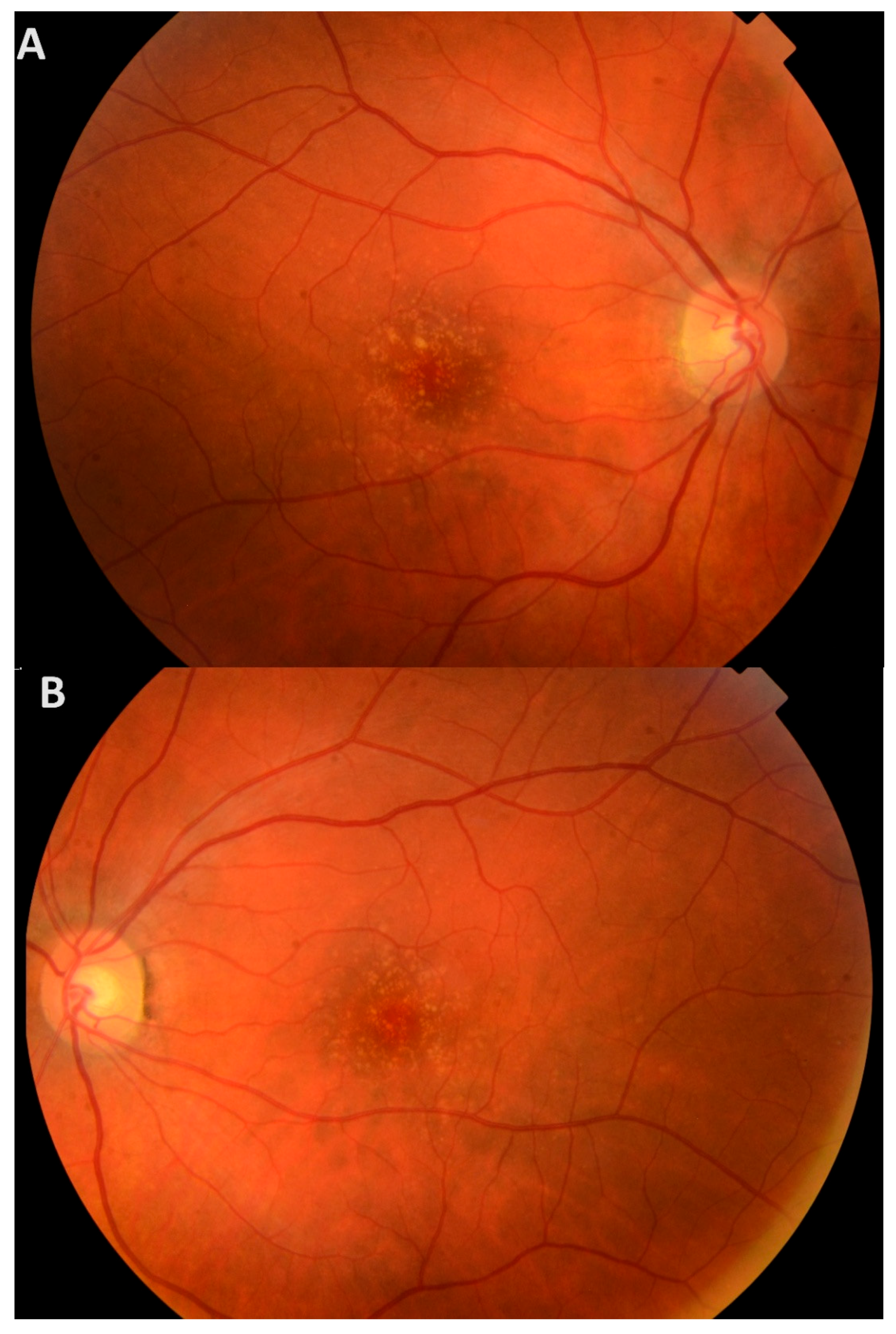
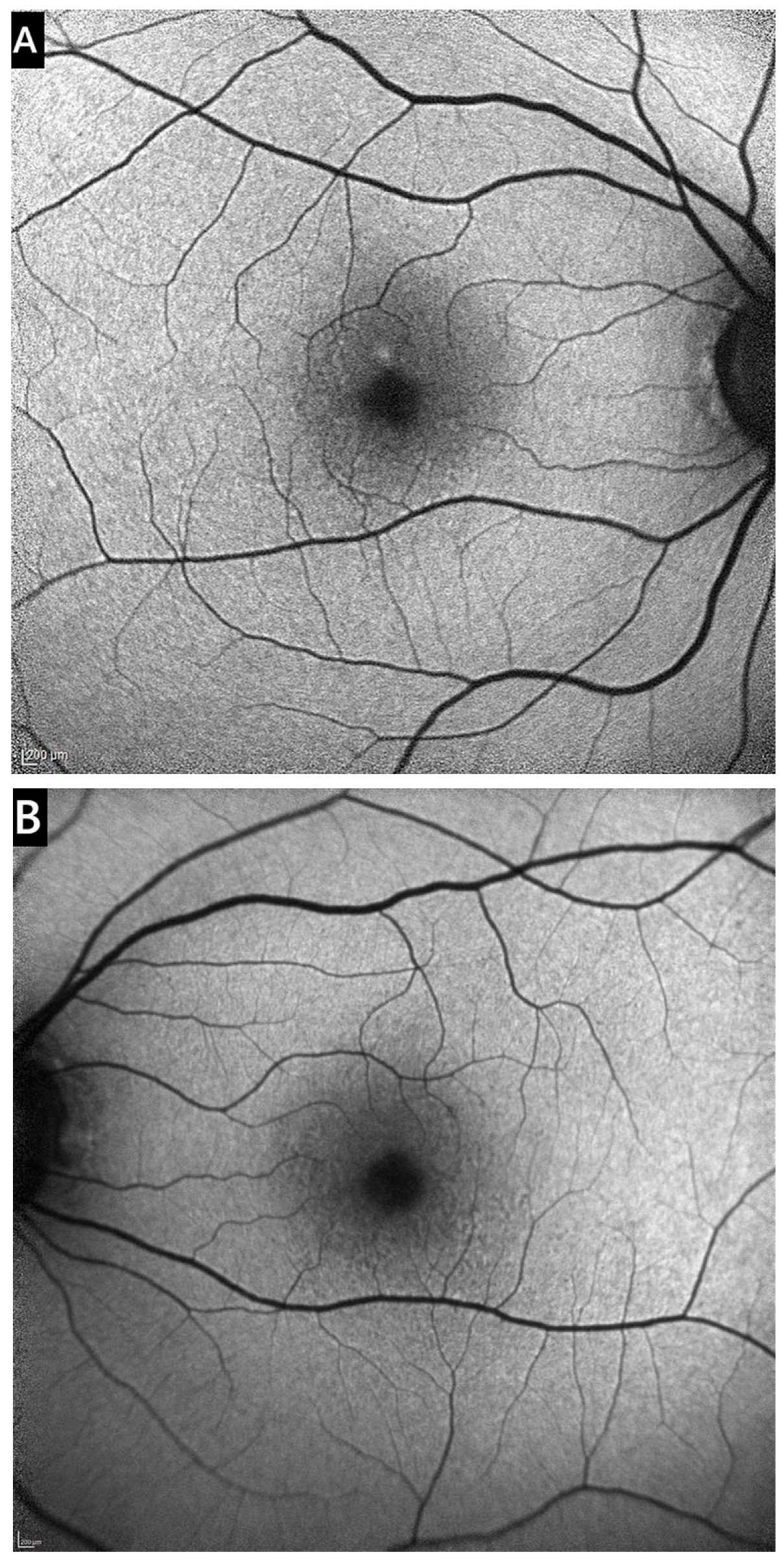
3.1. Clinical Presentation
3.2. WES and Sanger Sequencing
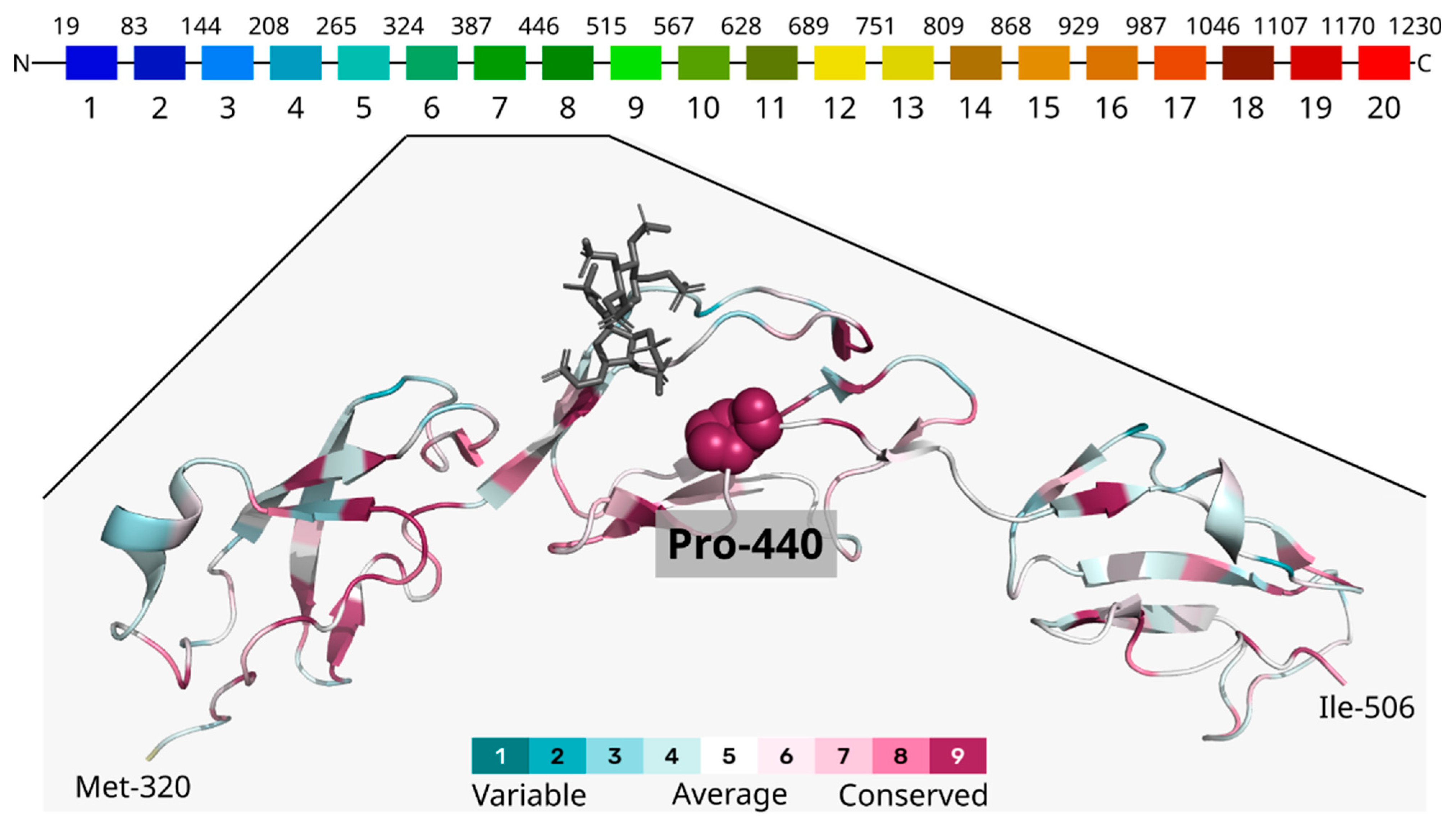
3.3. Computational Analysis
3.4. CFH Concentration in Plasma
3.5. sC5b-9 Concentration in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| anti-VEGF | Vascular endothelial growth factor inhibitors |
| BL | Basal lamina |
| BCVA | Best corrected visual acuity |
| CD | Cuticular drusen |
| CNV | Choroidal neovascularization |
| DFE | Dilated fundus examination |
| EODM | Early-onset drusen maculopathy |
| FDD | Familial dominant drusen |
| GA | Geographic atrophy |
| IRF | Intraretinal fluid |
| gDNA | Genomic DNA |
| LCD | Large colloid drusen |
| LE | Left eye |
| MAC | Membrane attack complex |
| nAMD | Neovascular AMD |
| OCT | Optical coherence tomography |
| OD | Optical density |
| PCR | Polymerase chain reaction |
| RE | Right eye |
| RPE | Retinal pigment epithelium |
| SMD | Sorsby macular dystrophy |
| SRF | Subretinal fluid |
| VUHSK | Vilnius University Hospital Santaros Klinikos |
| WES | Whole-exome sequencing |
References
- Sergejeva, O.; Botov, R.; Liutkevičienė, R.; Kriaučiūnienė, L. Genetic factors associated with the development of age-related macular degeneration. Medicina 2016, 52, 79–88. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Armento, A.; Schmidt, T.L.; Sonntag, I.; Merle, D.A.; Jarboui, M.A.; Kilger, E.; Clark, S.J.; Ueffing, M. CFH Loss in Human RPE Cells Leads to Inflammation and Complement System Dysregulation via the NF-κB Pathway. Int. J. Mol. Sci. 2021, 22, 8727. [Google Scholar] [CrossRef]
- Taylor, R.L.; Poulter, J.A.; Downes, S.M.; McKibbin, M.; Khan, K.N.; Inglehearn, C.F.; Webster, A.R.; Hardcastle, A.J.; Michaelides, M.; Bishop, P.N.; et al. Loss-of-Function Mutations in the CFH Gene Affecting Alternatively Encoded Factor H-like 1 Protein Cause Dominant Early-Onset Macular Drusen. Ophthalmology 2019, 126, 1410–1421. [Google Scholar] [CrossRef]
- Fragiotta, S.; Fernández-Avellaneda, P.; Breazzano, M.P.; Scuderi, G. Clinical Manifestations of Cuticular Drusen: Current Perspectives. Clin. Ophthalmol. 2021, 15, 3877–3887. [Google Scholar] [CrossRef] [PubMed]
- Guigui, B.; Leveziel, N.; Martinet, V.; Massamba, N.; Sterkers, M.; Coscas, G.; Souied, E.H. Angiography features of early onset drusen. Br. J. Ophthalmol. 2011, 95, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.J.; van de Ven, J.P.; Hoyng, C.B.; Hollander, A.I.D.; Klevering, B.J. Cuticular drusen: Stars in the sky. Prog. Retin. Eye Res. 2013, 37, 90–113. [Google Scholar] [CrossRef]
- van de Ven, J.P.; Boon, C.J.; Smailhodzic, D.; Lechanteur, Y.T.; Hollander, A.I.D.; Hoyng, C.B.; Theelen, T. Short-Term Changes of Basal Laminar Drusen on Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2012, 154, 560–567. [Google Scholar] [CrossRef][Green Version]
- Shin, D.H.; Kong, M.; Han, G.; Han, J.C.; Ham, D.I. Clinical manifestations of cuticular drusen in Korean patients. Sci. Rep. 2020, 10, 11469. [Google Scholar] [CrossRef]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef]
- Boon, C.J.; Klevering, B.J.; Hoyng, C.B.; Zonneveld-Vrieling, M.N.; Nabuurs, S.B.; Blokland, E.; Cremers, F.P.; Hollander, A.I.D. Basal Laminar Drusen Caused by Compound Heterozygous Variants in the CFH Gene. Am. J. Hum. Genet. 2008, 82, 516–523. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.C.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.A.; Folk, J.C.; Scheetz, T.E.; Taylor, C.M.; Sheffield, V.C.; Stone, E.M. Complement Factor H Polymorphism p.Tyr402His and Cuticular Drusen. Arch. Ophthalmol. 2007, 125, 93–97. [Google Scholar] [CrossRef]
- Duvvari, M.R.; van de Ven, J.P.H.; Geerlings, M.J.; Saksens, N.T.M.; Bakker, B.; Henkes, A.; Neveling, K.; del Rosario, M.; Westra, D.; Heuvel, L.P.W.J.v.D.; et al. Whole Exome Sequencing in Patients with the Cuticular Drusen Subtype of Age-Related Macular Degeneration. PLoS ONE 2016, 11, e0152047. [Google Scholar] [CrossRef]
- Merle, D.A.; Sen, M.; Armento, A.; Stanton, C.M.; Thee, E.F.; Meester-Smoor, M.A.; Kaiser, M.; Clark, S.J.; Klaver, C.C.; Keane, P.A.; et al. 10q26—The enigma in age-related macular degeneration. Prog. Retin. Eye Res. 2023, 96, 101154. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Purification of nucleic acids by extraction with phenol:chloroform. CSH Protoc. 2006, 2006, pdb.prot4455. [Google Scholar] [CrossRef]
- Jiang, H.; Lei, R.; Ding, S.W.; Zhu, S. Skewer: A fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 2014, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic. Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic. Acids Res. 2023, 52, D368–D375. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Olechnovič, K.; Venclovas, Č. VoroMQA web server for assessing three-dimensional structures of proteins and protein complexes. Nucleic. Acids Res. 2019, 47, W437–W442. [Google Scholar] [CrossRef]
- Olechnovič, K.; Venclovas, Č. VoroContacts: A Tool for the Analysis of Interatomic Contacts in Macromolecular Structures. Bioinformatics 2021, 37, 4873–4875. [Google Scholar] [CrossRef]
- Dapkūnas, J.; Timinskas, A.; Olechnovič, K.; Tomkuvienė, M.; Venclovas, Č. PPI3D: A web server for searching, analyzing and modeling protein-protein, protein-peptide and protein-nucleic acid interactions. Nucleic. Acids Res. 2024, 52, W264–W271. [Google Scholar] [CrossRef]
- Ben Chorin, A.; Masrati, G.; Kessel, A.; Narunsky, A.; Sprinzak, J.; Lahav, S.; Ashkenazy, H.; Ben-Tal, N. ConSurf-DB: An accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Sci. 2020, 29, 258–267. [Google Scholar] [CrossRef]
- Yariv, B.; Yariv, E.; Kessel, A.; Masrati, G.; Ben Chorin, A.; Martz, E.; Mayrose, I.; Pupko, T.; Ben-Tal, N. Using evolutionary data to make sense of macromolecules with a “face-lifted” ConSurf. Protein Sci. 2023, 32, e4582. [Google Scholar] [CrossRef]
- Cheng, J.; Novati, G.; Pan, J.; Bycroft, C.; Žemgulytė, A.; Applebaum, T.; Pritzel, A.; Wong, L.H.; Zielinski, M.; Sargeant, T.; et al. Accurate proteome-wide missense variant effect prediction with AlphaMissense. Science 2023, 381, eadg7492. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 24 June 2025).
- Balaratnasingam, C.; Cherepanoff, S.; Dolz-Marco, R.; Killingsworth, M.; Chen, F.K.; Mendis, R.; Mrejen, S.; Too, L.K.; Gal-Or, O.; Curcio, C.A.; et al. Cuticular Drusen. Ophthalmology 2018, 125, 100–118. [Google Scholar] [CrossRef]
- Goh, K.L.; Chen, F.K.; Balaratnasingam, C.; Abbott, C.J.; Hodgson, L.A.; Guymer, R.H.; Wu, Z. Cuticular Drusen in Age-Related Macular Degeneration. Ophthalmology 2022, 129, 653–660. [Google Scholar] [CrossRef]
- Roberti, N.C.; Dias JRde, O.; Novais, E.A.; Regatieri, C.S.; Belfort, R. Large colloid drusen analyzed with structural en face optical coherence tomography. Arq. Bras. Oftalmol. 2017, 80, 122–124. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, X.; Chen, Y.; Zhong, Q.; Lin, L.; Gao, Y.; Hong, F. Doyne honeycomb retinal dystrophy/malattia leventinese induced by EFEMP1 mutation in a Chinese family. BMC Ophthalmol. 2018, 18, 318. [Google Scholar] [CrossRef] [PubMed]
- Anand-Apte, B.; Chao, J.R.; Singh, R.; Stöhr, H. Sorsby Fundus Dystrophy: Insights from the past and looking to the future. J. Neurosci. Res. 2019, 97, 88–97. [Google Scholar] [CrossRef]
- Fu, L.; Garland, D.; Yang, Z.; Shukla, D.; Rajendran, A.; Pearson, E.; Stone, E.M.; Zhang, K.; Pierce, E.A. The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice. Hum. Mol. Genetics. 2007, 16, 2411–2422. [Google Scholar] [CrossRef]
- Khan, K.N.; Mahroo, O.A.; Khan, R.S.; Mohamed, M.D.; McKibbin, M.; Bird, A.; Michaelides, M.; Tufail, A.; Moore, A.T. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog. Retin. Eye Res. 2016, 53, 70–106. [Google Scholar] [CrossRef]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.P.; Deakin, J.A.; Schmidt, C.Q.; Blaum, B.S.; Egan, C.; Ferreira, V.P.; Pangburn, M.K.; Lyon, M.; Uhrín, D.; Barlow, P.N. Structure shows that a glycosaminoglycan and protein recognition site in factor H is perturbed by age-related macular degeneration-linked single nucleotide polymorphism. J. Biol. Chem. 2007, 282, 18960–18968. [Google Scholar] [CrossRef]
- Prosser, B.E.; Johnson, S.; Roversi, P.; Herbert, A.P.; Blaum, B.S.; Tyrrell, J.; Jowitt, T.A.; Clark, S.J.; Tarelli, E.; Uhriín, D.; et al. Structural basis for complement factor H–linked age-related macular degeneration. J. Exp. Med. 2007, 204, 2277–2283. [Google Scholar] [CrossRef]
- Schneider, M.C.; Prosser, B.E.; Caesar, J.J.E.; Kugelberg, E.; Li, S.; Zhang, Q.; Quoraishi, S.; Lovett, J.E.; Deane, J.E.; Sim, R.B.; et al. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 2009, 458, 890–893. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man, OMIM®. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD). Available online: https://omim.org/ (accessed on 18 September 2024).
- Geerlings, M.J.; Volokhina, E.B.; de Jong, E.K.; Van De Kar, N.; Pauper, M.; Hoyng, C.B.; van den Heuvel, L.P.; den Hollander, A.I. Genotype-Phenotype Correlations of Low-Frequency Variants in the Complement SYSTEM in renal Disease and Age-Related Macular Degeneration. Clin. Genet. 2018, 94, 330–338. [Google Scholar] [CrossRef]
- CFH Haploinsufficiency and Complement Alterations in Early-Onset Macular Degeneration IOVS ARVO Journals. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2793613 (accessed on 23 May 2025).
- Geerlings, M.J.; Kremlitzka, M.; Bakker, B.; Nilsson, S.C.; Saksens, N.T.; Lechanteur, Y.T.; Pauper, M.; Corominas, J.; Fauser, S.; Hoyng, C.B.; et al. The Functional Effect of Rare Variants in Complement Genes on C3b Degradation in Patients with Age-Related Macular Degeneration. JAMA Ophthalmol. 2017, 135, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Schmidt, C.Q.; White, A.M.; Hakobyan, S.; Morgan, B.P.; Bishop, P.N. Identification of Factor H–like Protein 1 as the Predominant Complement Regulator in Bruch’s Membrane: Implications for Age-Related Macular Degeneration. J. Immunol. 2014, 193, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- McHarg, S.; Clark, S.J.; Day, A.J.; Bishop, P.N. Age-related macular degeneration and the role of the complement system. Mol. Immunol. 2015, 67, 43–50. [Google Scholar] [CrossRef]
- Giannakis, E.; Jokiranta, T.S.; Male, D.A.; Ranganathan, S.; Ormsby, R.J.; Fischetti, V.A.; Mold, C.; Gordon, D.L. A common site within factor H SCR 7 responsible for binding heparin, C-reactive protein and streptococcal M protein. Eur. J. Immunol. 2003, 33, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, T.K.; Sadlon, T.A.; Ward, H.M.; Lublin, D.M.; Gordon, D.L. Identification of a heparin binding domain in the seventh short consensus repeat of complement factor H. J. Immunol. 1996, 157, 5422–5427. [Google Scholar] [CrossRef]
- Toomey, C.B.; Pflugmacher, S.; Park, K.; Pihl, J.; Novak, S.W.; Rodriguez, J.; Jalali, M.; Jung, J.; Mozafari, M.; Omran, S.P.; et al. Bruch’s membrane heparan sulfate retains lipoproteins in the early stages of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2025, 122, e2500727122. [Google Scholar] [CrossRef]
- Rodriguez, E.; Rallapalli, P.M.; Osborne, A.J.; Perkins, S.J. New functional and structural insights from updated mutational databases for complement factor H, Factor I, membrane cofactor protein and C3. Biosci. Rep. 2014, 34, e00146. [Google Scholar] [CrossRef]
- Saunders, R.E.; Abarrategui-Garrido, C.; Frémeaux-Bacchi, V.; de Jorge, E.G.; Goodship, T.H.; Trascasa, M.L.; Noris, M.; Castro, I.M.P.; Remuzzi, G.; de Córdoba, S.R.; et al. The interactive Factor H-atypical hemolytic uremic syndrome mutation database and website: Update and integration of membrane cofactor protein and Factor I mutations with structural models. Hum. Mutat. 2007, 28, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Warwick, A.; Khandhadia, S.; Ennis, S.; Lotery, A. Age-Related Macular Degeneration: A Disease of Systemic or Local Complement Dysregulation? J. Clin. Med. 2014, 3, 1234–1257. [Google Scholar] [CrossRef]
- Lynch, A.M.; Mandava, N.; Patnaik, J.L.; A Frazer-Abel, A.; Wagner, B.D.; Palestine, A.G.; Mathias, M.T.; Siringo, F.S.; Cathcart, J.N.; Holers, V.M. Systemic activation of the complement system in patients with advanced age-related macular degeneration. Eur. J. Ophthalmol. 2020, 30, 1061–1068. [Google Scholar] [CrossRef]
- Kijlstra, A.; Berendschot, T.T.J.M. Age-related macular degeneration: A complementopathy? Ophthalmic Res. 2015, 54, 64–73. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preiksaitiene, E.; Gurskytė, V.; Mikštienė, V.; Strupaitė-Šileikienė, R.; Dzindzalieta, R.; Petraitytė, G.; Dapkūnas, J.; Vyčaitė, E.; Karčiauskaitė, D.; Černiauskas, L.; et al. Evidence for the Pathogenicity of a CFH Variant in a Multigenerational Family with Cuticular Drusen. Medicina 2025, 61, 1649. https://doi.org/10.3390/medicina61091649
Preiksaitiene E, Gurskytė V, Mikštienė V, Strupaitė-Šileikienė R, Dzindzalieta R, Petraitytė G, Dapkūnas J, Vyčaitė E, Karčiauskaitė D, Černiauskas L, et al. Evidence for the Pathogenicity of a CFH Variant in a Multigenerational Family with Cuticular Drusen. Medicina. 2025; 61(9):1649. https://doi.org/10.3390/medicina61091649
Chicago/Turabian StylePreiksaitiene, Egle, Viktorija Gurskytė, Violeta Mikštienė, Rasa Strupaitė-Šileikienė, Ramūnas Dzindzalieta, Gunda Petraitytė, Justas Dapkūnas, Enrika Vyčaitė, Dovilė Karčiauskaitė, Linas Černiauskas, and et al. 2025. "Evidence for the Pathogenicity of a CFH Variant in a Multigenerational Family with Cuticular Drusen" Medicina 61, no. 9: 1649. https://doi.org/10.3390/medicina61091649
APA StylePreiksaitiene, E., Gurskytė, V., Mikštienė, V., Strupaitė-Šileikienė, R., Dzindzalieta, R., Petraitytė, G., Dapkūnas, J., Vyčaitė, E., Karčiauskaitė, D., Černiauskas, L., & Utkus, A. (2025). Evidence for the Pathogenicity of a CFH Variant in a Multigenerational Family with Cuticular Drusen. Medicina, 61(9), 1649. https://doi.org/10.3390/medicina61091649




