The Prevalence of Dental Caries Among Children Aged 6–11: A Cross-Sectional Study from Mureș County, Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethical Consideration
2.3. Data Collection
2.4. Variables Followed in the Study
- Untreated caries (dt/DT): the number of teeth with untreated decay (primary dentition: dt; permanent dentition: DT). We derived two outcomes: (a) a binary prevalence of untreated decay (coded 1 if dt > 0 or DT > 0; 0 otherwise) and (b) a count outcome (the number of untreated decayed teeth: dt for ages 6–8; DT for ages 9–11).
- Caries experience (dmft/DMFT): the sum of decayed (treated or untreated), missing due to caries, and filled teeth (primary dentition: dmft; permanent dentition: DMFT). We report descriptively the prevalence of caries experience (dmft > 0/DMFT > 0), the mean caries experience (mean dmft/DMFT), and the Significant Caries Index (SiC).
2.5. Statistical Analysis
- Logistic regression estimated associations with the presence of untreated caries (binary outcome: dt > 0 or DT > 0 vs. 0).
- Negative binomial regression modeled the severity of untreated caries (count outcome: number of untreated decayed teeth; dt for ages 6–8 and DT for ages 9–11), accounting for overdispersion.
- School location (urban/rural);
- Parental education;
- Parental employment status;
- Oral hygiene behaviors (tooth brushing frequency and duration);
- Dietary habits (consumption frequency of sweets, soda, natural fruit juice);
- Dental care history (past dental visit, treatment, or emergency).
3. Results
The Socioeconomic and Oral Health Behavior Factors Associated with Dental Caries
4. Discussion
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of dental caries and dental caries management: Consensus report of a workshop organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, W.; Xu, T.; Zheng, S. Risk factors of early childhood caries among children in Beijing: A case-control study. BMC Oral Health 2016, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Nanda, J.; Sachdev, V.; Sandhu, M.; Deep-Singh-Nanda, K. Correlation between dental caries experience and mutans streptococci counts using saliva and plaque as microbial risk indicators in 3–8 year old children. A cross-sectional study. J. Clin. Exp. Dent. 2015, 7, e114–e118. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Luo, A.H.; Yang, D.Q.; Xin, B.C.; Paster, B.J.; Qin, J. Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Dis. 2012, 18, 595–601. [Google Scholar] [CrossRef]
- Ozgocmen, E.; Yigit, T.; Kutlu, H.H. Presence of Candida in the dental plaque and saliva of patients with severe early childhood caries and early childhood caries: A pilot study. Eur. Oral Res. 2024, 58, 102–107. [Google Scholar]
- Jiang, S.; Gao, X.; Jin, L.; Lo, E.C. Salivary Microbiome Diversity in Caries-Free and Caries-Affected Children. Int. J. Mol. Sci. 2016, 17, 1978. [Google Scholar] [CrossRef]
- Gokhale, N.; Nuvvula, S. Influence of socioeconomic and working status of the parents on the incidence of their children’s dental caries. J. Nat. Sci. Biol. Med. 2016, 7, 127–129. [Google Scholar] [CrossRef]
- Jindal, L.; Dua, P.; Mangla, R.; Gupta, K.; Vyas, D.; Gupta, P. Dental Caries in Relation to Socioeconomic Factors of 6 and 12-year-old Schoolchildren of Paonta Sahib, Himachal Pradesh, India: An Epidemiological Study. Int. J. Clin. Pediatr. Dent. 2020, 13, 395–398. [Google Scholar] [CrossRef]
- Moraes, R.B.; Menegazzo, G.R.; Knorst, J.K.; Ardenghi, T.M. Availability of public dental care service and dental caries increment in children: A cohort study. J. Public Health Dent. 2021, 81, 57–64. [Google Scholar] [CrossRef]
- Kato, H.; Tanaka, K.; Shimizu, K.; Nagata, C.; Furukawa, S.; Arakawa, M.; Miyake, Y. Parental occupations, educational levels, and income and prevalence of dental caries in 3-year-old Japanese children. Environ. Health Prev. Med. 2017, 22, 80. [Google Scholar] [CrossRef]
- Javed, F.; Feng, C.; Kopycka-Kedzierawski, D.T. Incidence of early childhood caries: A systematic review and meta-analysis. J. Investig. Clin. Dent. 2017, 8, e12238. [Google Scholar] [CrossRef]
- Moynihan, P.J.; Kelly, S.A. Effect on caries of restricting sugars intake: Systematic review to inform WHO guidelines. J. Dent. Res. 2014, 93, 8–18. [Google Scholar] [CrossRef]
- Mahboobi, Z.; Pakdaman, A.; Yazdani, R.; Azadbakht, L.; Montazeri, A. Dietary free sugar and dental caries in children: A systematic review on longitudinal studies. Health Promot. Perspect. 2021, 11, 271–280. [Google Scholar] [CrossRef]
- Badarch, J.; Batbaatar, S.; Paulik, E. Prevalence and Correlates of Poor Oral Hygiene among School-Going Students in Mongolia. Dent. J. 2021, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Doichinova, L.; Kirilova, J.; Kirov, D. Study of the prevalence of dental caries in adults. J. Med. Dent. Pract. 2022, 9, 1584–1589. [Google Scholar] [CrossRef]
- Kaushik, M.; Sood, S. A Systematic Review of Parents’ Knowledge of Children’s Oral Health. Cureus 2023, 15, e41485. [Google Scholar] [CrossRef]
- Olak, J.; Nguyen, M.S.; Nguyen, T.T.; Nguyen, B.B.T.; Saag, M. The influence of mothers’ oral health behaviour and perception thereof on the dental health of their children. EPMA J. 2018, 9, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.S.; Baker, S.R.; Deery, C.; Vettore, M.V. The relationship of children’s dental clinical status with school performance and school attendance in the Kingdom of Bahrain: A life-course approach. Community Dent. Oral Epidemiol. 2024, 52, 93–100. [Google Scholar] [CrossRef]
- Rebelo, M.A.B.; Rebelo Vieira, J.M.; Pereira, J.V.; Quadros, L.N.; Vettore, M.V. Does oral health influence school performance and school attendance? A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2018, 29, 138–148. [Google Scholar] [CrossRef]
- Scarpelli, A.C.; Paiva, S.M.; Viegas, C.M.; Carvalho, A.C.; Ferreira, F.M.; Pordeus, I.A. Oral health-related quality of life among Brazilian preschool children. Community Dent. Oral Epidemiol. 2013, 41, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Elfitrozy, A.; Handayani, A.T.W.; Yani, R.W.E. The Association Between Dental and Oral Care Behavior Towards Quality of Life in Stunting Toddlers. Int. J. Integr. Med. Res. 2023, 10, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Van Chuyen, N.; Van Du, V.; Van Ba, N.; Long, D.D.; Son, H.A. The prevalence of dental caries and associated factors among secondary school children in rural highland Vietnam. BMC Oral Health 2021, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Sapkota, D.; Poudel, L.; Poudel, S.; Khatri, E.; Sapkota, K. Prevalence and Associated Factors of Dental Caries among School Children in Bharatpur, Chitwan. Kathmandu Univ. Med. J. 2024, 22, 191–196. [Google Scholar]
- Giri, M.; Pandit, S.; Oli, H.; Giri, S. Prevalence and associated factors of dental caries among basic school children in Kathmandu Metropolitan City, Nepal: A cross-sectional study. Meds Alliance J. Med. Med. Sci. 2021, 1, 89–94. [Google Scholar] [CrossRef]
- PL, S.K.; Bharathi, S.; Christopher, A.; Pandian, P.; Raj, M.; Prabhu, M. Prevalence of dental caries among primary school students in Madurai City, Tamil Nadu, India. Int. J. Contemp. Dent. Res. 2023, 1, 21–28. [Google Scholar] [CrossRef]
- Pandey, P.; Nandkeoliar, T.; Tikku, A.P.; Singh, D.; Singh, M.K. Prevalence of Dental Caries in the Indian Population: A Systematic Review and Meta-analysis. J. Int. Soc. Prev. Community Dent. 2021, 11, 256–265. [Google Scholar] [CrossRef]
- Altaş, Z.M.; Sezerol, M.A. Prevalence and Associated Factors of Dental Caries in Syrian Immigrant Children Aged 6–12 Years. Children 2023, 10, 1000. [Google Scholar] [CrossRef]
- Aalemi, A.K.; Yaqubi, B. Prevalence of dental caries among school-going children aged 7–13 years in Kabul City. BMC Oral Health 2024, 24, 1092. [Google Scholar] [CrossRef]
- Adhikari, S.; Tamrakar, L.; Humagain, M.; Bhattarai, R. Dental caries prevalence among 3–14 year old school children of Chitwan. J. Nepal. Assoc. Pediatr. Dent. 2021, 2, 19–23. [Google Scholar] [CrossRef]
- Mahanta, S.; Prajapati, D.; Khadka, A.; Ghosh, S.; Ghimire, U.; Shahi, S.; Khimbaja, A. Prevalence of dental caries among school-going children in Dolakha, Nepal. J. Coll. Med. Sci.-Nepal 2023, 19, 282–287. [Google Scholar] [CrossRef]
- Peltzer, K.; Mongkolchati, A.; Satchaiyan, G.; Rajchagool, S.; Pimpak, T. Sociobehavioral factors associated with caries increment: A longitudinal study from 24 to 36-month-old children in Thailand. Int. J. Environ. Res. Public Health 2014, 11, 10838–10850. [Google Scholar] [CrossRef]
- Sa Lira, A.L.; Vasconcelos Fontenele, M.K.; de Sousa, F.J.; Carvalho de Sousa, F.D.; Campos Ribeiro, C.K.; Gomes Ferreira, L.E. The prevalence of caries in the primary dentition and associated factors in children in the city of parnaíba-piauí. Rev. Odontológica Do Bras. Cent. 2022, 31, 147–165. [Google Scholar] [CrossRef]
- Sfeatcu, R.; Cărămidă, M.; Sava-Rosianu, R.; Matichescu, M.L.; Galuscan, A.; Dumitrache, M.A. Carious status and socio-behavioral risk factors among 12 year-old children in the South-Central region in Romania. BMC Oral Health 2023, 23, 644. [Google Scholar] [CrossRef]
- Tudoroniu, C.; Popa, M.; Iacob, S.M.; Pop, A.L.; Năsui, B.A. Correlation of Caries Prevalence, Oral Health Behavior and Sweets Nutritional Habits among 10 to 19-Year-Old Cluj-Napoca Romanian Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 6923. [Google Scholar] [CrossRef] [PubMed]
- Funieru, C.; Twetman, S.; Funieru, E.; Dumitrache, A.M.; Sfeatcu, R.I.; Baicus, C. Caries experience in schoolchildren in Bucharest, Romania: The PAROGIM study. J. Public Health Dent. 2014, 74, 153–158. [Google Scholar] [CrossRef]
- Baciu, D.; Danila, I.; Balcos, C.; Gallagher, J.E.; Bernabé, E. Caries experience among Romanian schoolchildren: Prevalence and trends 1992–2011. Community Dent. Health 2015, 32, 93–97. [Google Scholar]
- Hobdell, M.; Petersen, P.E.; Clarkson, J.; Johnson, N. Global goals for oral health 2020. Int. Dent. J. 2003, 53, 285–288. [Google Scholar] [CrossRef]
- Obregón, N.-R.; Fernández-Riveiro, P.; Piñeiro-Lamas, M.; Smyth, E.-C.; Montes, A.-M.; Suarez, M.M.-C. Prevalence and caries-related risk factors in schoolchildren of 12- and 15-year-old: A cross-sectional study. BMC Oral Health 2019, 19, 120. [Google Scholar]
- Zaborskis, A.; Milciuviene, S.; Narbutaite, J.; Bendoraitiene, E.; Kavaliauskiene, A. Caries Experience and Oral Health Behaviour among 11–13-Year-Olds: An Ecological Study of Data from 27 European Countries, Israel, Canada and USA. Community Dent. Health 2010, 27, 102–108. [Google Scholar] [PubMed]
- Dumitrescu, R.; Sava-Rosianu, R.; Jumanca, D.; Balean, O.; Damian, L.-R.; Campus, G.G.; Maricutoiu, L.; Alexa, V.T.; Sfeatcu, R.; Daguci, C.; et al. Dental Caries, Oral Health Behavior, and Living Conditions in 6–8-Year-Old Romanian School Children. Children 2022, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, R.; Sava-Rosianu, R.; Jumanca, D.; Balean, O.; Damian, L.-R.; Fratila, A.D.; Maricutoiu, L.; Hajdu, A.I.; Focht, R.; Dumitrache, M.A.; et al. The Impact of Parental Education on Schoolchildren’s Oral Health-A Multicenter Cross-Sectional Study in Romania. Int. J. Environ. Res. Public Health 2022, 19, 11102. [Google Scholar] [CrossRef]
- Moca, A.E.; Iurcov, R.; Ciavoi, G.; Moca, R.T.; Sipoş, L.R. Pediatric Dental Emergencies during the COVID-19 Pandemic in Romania: A Retrospective Study. Children 2023, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Armencia, A.; Bobu, L.; Bosînceanu, D.; Bolat, M.; Stupu, A.; Nicolau, A.; Hurjui, L.; Bârlean, M.; Roșu, S.; Balcoș, C.; et al. Study on the association between caries experience and social and behavioural factors in young adults and adults in Iasi, Romania. Rom. J. Med. Dent. Educ. 2019, 8, 39–46. [Google Scholar]
- Cernega, A.; Mincă, D.G.; Furtunescu, F.L.; Radu, C.-P.; Pârvu, S.; Pițuru, S.-M. The Predictability of the Dental Practitioner in a Volatile Healthcare System: A 25-Year Study of Dental Care Policies in Romania (1999–2023). Healthcare 2025, 13, 249. [Google Scholar] [CrossRef]
- Populaţie Judeţul Mureş. Available online: https://populatia.ro/populatie-judetul-mures/ (accessed on 2 July 2025).
- Recensământul Populaţiei şi Locuinţelor. Available online: https://www.recensamantromania.ro/rezultate-rpl-2021/rezultate-definitive/ (accessed on 2 July 2025).
- Institutul Naţional de Statistică. Available online: https://insse.ro/cms/ro/ (accessed on 2 July 2025).
- World Health Organization. Oral Health Surveys. Basic Methods, 4th ed.; World Health Organization: Geneva, Switzerland, 1997; Available online: https://apps.who.int/iris/handle/10665/41905 (accessed on 5 July 2025).
- Nishi, M.; Bratthall, D.; Stjernsward, J. How to Calculate the Significant Caries Index (SiC Index); WHO Collaborating Center, Faculty of Odontology, Malmö University: Malmö, Sweden, 2001. [Google Scholar]
- Heinze, G.; Dunkler, D. Five myths about variable selection. Transpl. Int. 2017, 30, 6–10. [Google Scholar] [CrossRef]
- Hiremath, A.; Murugaboopathy, V.; Ankola, A.V.; Hebbal, M.; Mohandoss, S.; Pastay, P. Prevalence of Dental Caries Among Primary School Children of India—A Cross-Sectional Study. J. Clin. Diagn. Res. 2016, 10, ZC47–ZC50. [Google Scholar] [CrossRef]
- Ashoori, F.; Karimi, M.; Seif, M. Comparison of the effect of mothers’ and students’ education on the promotion of oral health behaviours in female students, using the health belief model. Int. J. Dent. Hyg. 2022, 20, 601–608. [Google Scholar] [CrossRef]
- Shirahmadi, S.; Bashirian, S.; Soltanian, A.R.; Karimi-Shahanjarini, A.; Vahdatinia, F. Effectiveness of theory-based educational interventions of promoting oral health among elementary school students. BMC Public Health 2024, 24, 130. [Google Scholar] [CrossRef]
- Pargaputri, A.; Maharani, A.; Patrika, F. Dental and oral health education in parents of Taam Avicenna playgroup students. J. Pemberdaya. Publ. Has. Pengabdi. Kpd. Masy. 2023, 6, 83–88. [Google Scholar] [CrossRef]
- Perpelea, A.-C.; Sfeatcu, R.; Tănase, M.; Meleșcanu Imre, M.; Ripszky Totan, A.; Cernega, A.; Funieru, C.; Pițuru, S.-M. A STEPwise Approach for Oral Hygiene Behavior of Schoolchildren in Romania. Healthcare 2024, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Bratthall, D. Introducing the Significant Caries Index together with a proposal for a new global oral health goal for 12-year-olds. Int. Dent. J. 2000, 50, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Kaur, K.; Narang, S.; Yadav, S.; Kaur, S.; Singh, N.V. Assessment of Prevalence of Dental Caries among School-going Children: A Cross-sectional Study. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. 1), S333–S335. [Google Scholar] [CrossRef]
- Ferizi, L.; Dragidella, F.; Staka, G.; Bimbashi, V.; Mrasori, S. Oral Health Status Related to Social Behaviors among 6–11 Year Old Schoolchildren in Kosovo. Acta Stomatol. Croat. 2017, 51, 122–132. [Google Scholar] [CrossRef]
- Basha, S.; Swamy, H.S. Dental caries experience, tooth surface distribution and associated factors in 6- and 13- year- old school children from Davangere, India. J. Clin. Exp. Dent. 2012, 4, e210-6. [Google Scholar] [CrossRef]
- Kim, H.N.; Han, D.H.; Jun, E.J.; Kim, S.Y.; Jeong, S.H.; Kim, J.B. The decline in dental caries among Korean children aged 8 and 12 years from 2000 to 2012 focusing SiC Index and DMFT. BMC Oral Health 2016, 16, 38. [Google Scholar] [CrossRef]
- Alraqiq, H.; Eddali, A.; Boufis, R. Prevalence of dental caries and associated factors among school-aged children in Tripoli, Libya: A cross-sectional study. BMC Oral Health 2021, 21, 224. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health Surveys: Basic Methods, 5th ed.; World Health Organization: Geneva, Switzerland, 2013; Available online: https://iris.who.int/bitstream/handle/10665/97035/9789241548649_eng.pdf (accessed on 19 August 2025).
- Cianetti, S.; Lombardo, G.; Lupatelli, E.; Rossi, G.; Abraha, I.; Pagano, S.; Paglia, L. Dental caries, parents’ educational level, family income, and dental service attendance among children in Italy. Eur. J. Paediatr. Dent. 2017, 18, 15–18. [Google Scholar]
- Moimaz, S.A.S.; Fadel, C.B.; Lolli, L.F.; Garbin, C.A.S.; Garbin, A.J.I.; Saliba, N.A. Social aspects of dental caries in the context of mother-child pairs. J. Appl. Oral. Sci. 2014, 22, 73–78. [Google Scholar] [CrossRef]
- Feldens, C.A.; Kramer, P.F.; Sequeira, M.C.; Rodrigues, P.H.; Vitolo, M.R. Maternal education is an independent determinant of cariogenic feeding practices in the first year of life. Eur. Arch. Paediatr. Dent. 2012, 13, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Cakar, T.; Harrison-Barry, L.; Pukallus, M.L.; Kazoullis, S.; Seow, W.K. Caries experience of children in primary schools with long-term tooth brushing programs: A pilot Australian study. Int. J. Dent. Hyg. 2018, 16, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kowash, M.B.; Alkhabuli, J.O.; Dafaalla, S.A.; Shah, A.; Khamis, A.H. Early childhood caries and associated risk factors among preschool children in Ras Al-Khaimah, United Arab Emirates. Eur. Arch. Paediatr. Dent. 2017, 18, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Children’s Oral Health; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024. Available online: https://archive.cdc.gov/www_cdc_gov/healthyschools/bam/child-development/child-oral-health.htm (accessed on 21 June 2025).
- Elidrissi, S.M.; Naidoo, S. Prevalence of dental caries and toothbrushing habits among preschool children in Khartoum State. Sudan. Int. Dent. J. 2016, 66, 215–220. [Google Scholar] [CrossRef]
- Toumba, K.J.; Twetman, S.; Splieth, C.; Parnell, C.; van Loveren, C.; Lygidakis, N. Guidelines on the use of fluoride for caries prevention in children: An updated EAPD policy document. Eur. Arch. Paediatr. Dent. 2019, 20, 507–516. [Google Scholar] [CrossRef]
- Hong, J.; Whelton, H.; Douglas, G.; Kang, J. Consumption frequency of added sugars and UK children’s dental caries. Community Dent. Oral Epidemiol. 2018, 46, 457–464. [Google Scholar] [CrossRef]
- Taqi, M.; Razak, I.A.; Ab-Murat, N. Sugar consumption and caries occurrence among Pakistani school children. J. Pak. Med. Assoc. 2018, 68, 1483–1487. [Google Scholar]
- American Academy of Pediatric Dentistry. Policy on dietary recommendations for infants, children, and adolescents. Pediatr. Dent. 2018, 40, 65–67. [Google Scholar]
- Moynihan, P. Sugars and dental caries: Evidence for setting a recommended threshold for intake. Adv. Nutr. 2016, 7, 149–156. [Google Scholar] [CrossRef]
- Huew, R.; Waterhouse, P.; Moynihan, P.; Kometa, S.; Maguire, A. Dental caries and its association with diet and dental erosion in Libyan schoolchildren. Int. J. Paediatr. Dent. 2012, 22, 68–76. [Google Scholar] [CrossRef]
- Kiwanuka, S.N.; Astrom, A.N.; Trovik, T.A. Dental caries experience and its relationship to social and behavioural factors among 3–5-year–old children in Uganda. Int. J. Paediatr. Dent. 2004, 14, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Ndagire, B.; Kutesa, A.; Ssenyonga, R.; Kiiza, H.M.; Nakanjako, D.; Rwenyonyi, C.M. Prevalence, severity and factors associated with dental caries among school adolescents in Uganda: A cross-sectional study. Braz. Dent. J. 2020, 31, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, C.; Ardenghi, T.M.; Mendes, F.M.; Agostini, B.A.; Michel-Crosato, E. Individual and contextual factors influencing dental health care utilization by preschool children: A multilevel analysis. Braz. Oral Res. 2017, 31, e27. [Google Scholar] [CrossRef] [PubMed]

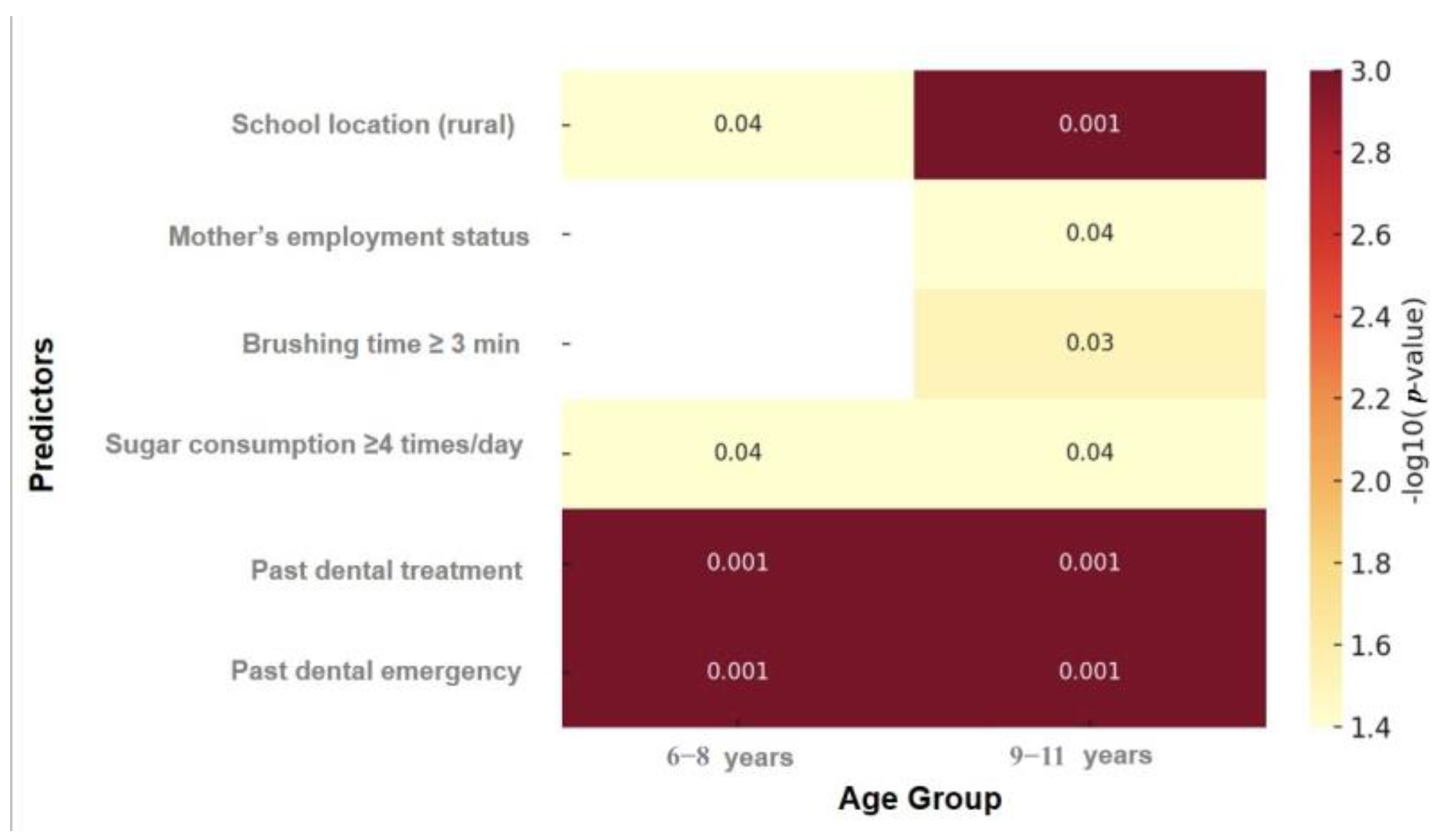
| Characteristic | Children Aged 6–8 Years (N = 524) | Children Aged 9–11 Years (N = 600) |
|---|---|---|
| Gender | ||
| 258 (49.2%) | 269 (44.8%) |
| 266 (50.8%) | 331 (55.2%) |
| School location | ||
| 262 (50%) | 276 (46%) |
| 262 (50%) | 324 (54%) |
| Mother’s educational level | ||
| 168 (32.1%) | 181 (30.2%) |
| 356 (67.9%) | 419 (69.8%) |
| Father’s educational level | ||
| 206 (39.3%) | 209 (34.8%) |
| 318 (60.7%) | 391 (65.2%) |
| Mother’s employment status | ||
| 249 (47.5%) | 290 (48.3%) |
| 275 (52.5%) | 310 (51.7%) |
| Father’s employment status | ||
| 329 (62.8%) | 381 (63.5%) |
| 195 (37.2%) | 219 (36.5%) |
| Variables | N (%) | Caries Experience 3, Mean ± SD | SiC Score 4, Mean ± SD | |||
|---|---|---|---|---|---|---|
| Decayed 1 | Missing | Filled | Caries Experience 2 | |||
| Children aged 6–8 years (N = 524) | dmft | dmft | ||||
| Total | 401 (76.5%) | 28 (5.3%) | 33 (6.3%) | 407 (77.7%) | 3.9 ± 3.5 | 7.7 ± 2.5 |
| Urban schools | 200 (76.3%) | 10 (3.8%) | 19 (7.3%) | 202 (77.1%) | 3.9 ± 3.5 | 7.6 ± 2.5 |
| Rural schools | 201 (76.7%) | 18 (6.9%) | 14 (5.3%) | 205 (78.2%) | 3.9 ± 3.5 | 8.0 ± 2.6 |
| Children aged 9–11 years (N = 600) | DMFT | DMFT | ||||
| Total | 261 (43.5%) | 41 (7.8%) | 38 (6.3%) | 289 (48.2%) | 1.9 ± 1.8 | 3.3 ± 3.8 |
| Urban schools | 125 (45.3%) | 18 (6.5%) | 15 (5.4%) | 135 (48.9%) | 1.6 ± 2.1 | 3.3 ± 2.0 |
| Rural schools | 136 (42.0%) | 23 (7.1%) | 23 (7.1%) | 154 (47.5%) | 1.2 ± 1.6 | 3.2 ± 1.5 |
| Characteristic | Children Aged 6–8 Years (N = 524) | Children Aged 9–11 Years (N = 600) |
|---|---|---|
| Sugar consumption | ||
| 95 (18.1%) | 269 (44.8%) |
| 167 (31.9%) | 111 (18.5%) |
| 262 (50%) | 220 (36.7%) |
| Toothbrushing habits | ||
| 252 (48.1%) | 259 (43.2%) |
| 272 (51.9%) | 341 (56.8%) |
| Dental consult | ||
| 109 (20.8%) | 119 (19.8%) |
| 158 (30.2%) | 177 (29.5%) |
| 257 (49.0%) | 304 (50.7%) |
| Children Aged 6–8 Years Old | Children Aged 9–11 Years Old | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| OR (CI 95%) | p | OR (CI 95%) | p | OR (CI 95%) | p | OR (CI 95%) | p | |
| Socioeconomic characteristics | ||||||||
| Gender | ||||||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Female | 0.83 (0.62–1.10) | 0.18 | 0.79 (0.52–1.21) | 0.26 | 1.14 (0.88–1.47) | 0.42 | 1.41 (1.01–1.98) | 0.08 |
| School location | ||||||||
| Urban | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Rural | 0.99 (0.75–1.35) | 0.96 | 0.53 (0.31–0.92) | 0.04 | 0.82 (0.55–1.22) | 0.29 | 0.50 (0.34–0.75) | <0.001 |
| Mother’s educational level | ||||||||
| High school | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| University | 0.81 (0.57–1.17) | 0.24 | 0.93 (0.53–1.65) | 0.77 | 0.90 (0.68–1.19) | 0.40 | 1.15 (0.76–1.74) | 0.58 |
| Father’s educational level | ||||||||
| High school | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| University | 0.78 (0.57–1.09) | 0.14 | 0.85 (0.51–1.44) | 0.53 | 1.07 (0.82–1.40) | 0.74 | 1.09 (0.73–1.62) | 0.77 |
| Mother’s employment status | ||||||||
| Unemployed | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Employed | 1.1 (0.80–1.52) | 0.66 | 0.97 (0.63–1.49) | 0.84 | 0.74 (0.58–0.96) | 0.04 | 0.64 (0.45–0.90) | 0.04 |
| Father’s employment status | ||||||||
| Unemployed | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Employed | 0.95 (0.68–1.33) | 0.69 | 0.91 (0.57–1.45) | 0.64 | 1.33 (1.01–1.74) | 0.08 | 1.34 (0.94–1.91) | 0.15 |
| Sugar consumption | ||||||||
| Drinking soda | <0.001 | 0.44 | 0.88 | 0.34 | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Several times/week | 1.81 (1.29–2.55) | <0.001 | 1.63 (1.0–2.69) | 0.35 | 0.99 (0.72–1.37) | 0.89 | 0.73 (0.49–1.1) | 0.13 |
| Once/day | 1.95 (1.21–3.17) | 0.03 | 1.45 (0.70–3.04) | 0.57 | 1.28 (0.83–1.96) | 0.32 | 0.82 (0.46–1.46) | 0.48 |
| 2–3 times/day | 1.99 (1.08–3.68) | 0.04 | 1.89 (0.75–4.81) | 0.52 | 1.66 (1.08–2.56) | 0.04 | 1.13 (0.61–2.13) | 0.76 |
| ≥4 times/day | 2.28 (0.67–7.94) | 0.22 | 3.47 (0.30–42.7) | 0.66 | 1.73 (0.53–5.77) | 0.41 | 1.90 (0.28–13.5) | 0.55 |
| Drinking natural juice | <0.001 | 0.24 | 0.39 | 0.58 | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Several times/week | 2.80 (1.81–4.36) | <0.001 | 2.03 (0.99–4.16) | 0.34 | 1.19 (0.76–1.87) | 0.52 | 1.48 (0.81–2.72) | 0.24 |
| Once/day | 2.78 (1.72–4.50) | <0.001 | 1.54 (0.70–3.42) | 0.97 | 1.36 (0.84–2.20) | 0.24 | 1.56 (0.81–3.01) | 0.22 |
| 2–3 times/day | 3.36 (2.03–5.55) | <0.001 | 1.83 (0.77–4.38) | 0.66 | 1.55 (0.95–2.54) | 0.11 | 1.89 (0.92–3.91) | 0.12 |
| ≥4 times/day | 2.82 (1.25–6.40) | 0.03 | 2.11 (0.51–9.02) | 0.84 | 1.67 (0.67–4.20) | 0.32 | 1.33 (0.42–4.27) | 0.67 |
| Eating sweets | <0.001 | 0.04 | 0.04 | 0.35 | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Several times/week | 2.31 (1.40–3.81) | <0.001 | 1.85 (0.78–4.43) | 0.90 | 1.01 (0.64–1.60) | 0.98 | 0.79 (0.43–1.48) | 0.44 |
| Once/day | 2.62 (1.54–4.45) | <0.001 | 2.73 (1.07–6.97) | 0.35 | 0.93 (0.56–1.55) | 0.74 | 0.70 (0.35–1.41) | 0.31 |
| 2–3 times/day | 3.72 (2.1–6.60) | <0.001 | 3.51 (1.27–9.72) | 0.11 | 1.31 (0.78–2.21) | 0.37 | 0.87 (0.41–1.86) | 0.69 |
| ≥4 times/day | 2.03 (0.96–4.28) | 0.09 | 1.13 (0.33–3.93) | 0.04 | 2.24 (1.12–4.51) | 0.04 | 1.60 (0.61–4.25) | 0.39 |
| Toothbrushing habits | ||||||||
| Frequency of brushing | 0.03 | 0.93 | 0.39 | 0.61 | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Once/day | 0.96 (0.61–1.52) | 0.81 | 1.38 (0.37–5.27) | 0.76 | 0.82 (0.53–1.25) | 0.33 | 0.78 (0.51–1.18) | 0.22 |
| 2–3 times/day | 0.92 (0.58–1.47) | 0.69 | 1.60 (0.42–6.22) | 0.54 | 0.94 (0.61–1.46) | 0.74 | 0.89 (0.57–1.38) | 0.55 |
| ≥4 times/day | 0.41 (0.23–0.77) | 0.03 | 1.89 (0.41–8.97) | 0.74 | 0.78 (0.43–1.42) | 0.39 | 0.73 (0.38–1.45) | 0.36 |
| Brushing time | <0.001 | 0.04 | <0.001 | <0.001 | ||||
| < 1 min | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 1 min | 0.66 (0.42–1.05) | 0.09 | 1.55 (0.85–2.82) | 0.12 | 1.30 (0.87–1.94) | 0.25 | 1.36 (0.85–2.17) | 0.25 |
| 2 min | 0.48 (0.30–0.78) | <0.001 | 0.98 (0.53–1.83) | 0.92 | 0.83 (0.55–1.26) | 0.34 | 0.78 (0.49–1.27) | 0.31 |
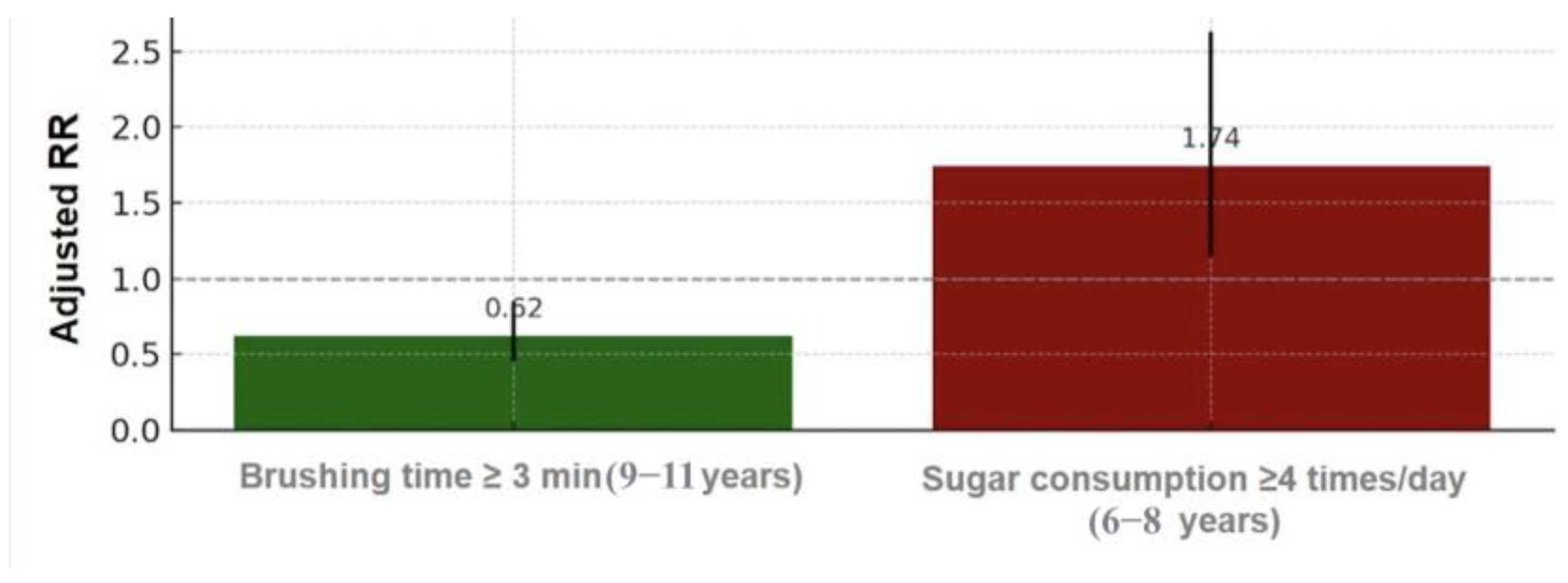
| ≥3 min | 0.44 (0.26–0.74) | <0.001 | 0.55 (0.27–1.13) | 0.12 | 0.55 (0.31–0.98) | 0.04 | 0.41 (0.21–0.81) | 0.03 |
| Dental consult | ||||||||
| Past consult | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Regularly | 1.10 (0.73–1.66) | 0.75 | 0.80 (0.41–1.57) | 0.50 | 1.82 (1.17–2.72) | <0.001 | 1.73 (0.94–3.18) | 0.11 |
| Treatment | 2.76 (1.62–4.72) | <0.001 | 3.83 (1.84–7.97) | <0.001 | 1.96 (1.43–2.69) | <0.001 | 2.25 (1.51–3.35) | <0.001 |
| Emergency | 14.3 (5.17–36.2) | <0.001 | 9.93 (3.49–25.3) | <0.001 | 2.37 (1.63–3.45) | <0.001 | 2.78 (1.77–4.36) | <0.001 |
| Children Aged 6–8 Years Old | Children Aged 9–11 Years Old | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| RR (CI 95%) | p | RR (CI 95%) | p | RR (CI 95%) | p | RR (CI 95%) | p | |
| Socioeconomic characteristics | ||||||||
| Gender | ||||||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Female | 0.95 (0.83–1.09) | 0.35 | 0.96 (0.79–1.16) | 0.54 | 1.02 (0.87–1.23) | 0.90 | 1.18 (0.94–1.48) | 0.24 |
| School location | ||||||||
| Urban | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Rural | 0.94 (0.80–1.10) | 0.34 | 1.02 (0.81–1.29) | 0.20 | 0.75 (0.64–0.89) | <0.001 | 0.61 (0.47–0.81) | <0.001 |
| Mother’s educational level | ||||||||
| High school | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| University | 0.88 (0.75–1.05) | 1.02 | 0.86 (0.67–1.10) | 0.18 | 1.09 (0.90–1.31) | 0.53 | 1.07 (0.80–1.42) | 0.78 |
| Father’s educational level | ||||||||
| High school | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| University | 0.93 (0.80–1.09) | 0.14 | 0.99 (0.79–1.24) | 0.79 | 1.22 (1.01–1.47) | 0.06 | 1.30 (0.98–1.73) | 0.12 |
| Mother’s employment status | ||||||||
| Unemployed | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Employed | 1.01 (0.86–1.18) | 0.86 | 1.02 (0.84–1.25) | 0.99 | 0.91 (0.76–1.08) | 0.21 | 0.75 (0.59–0.95) | 0.04 |
| Father’s employment status | ||||||||
| Unemployed | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Employed | 1.0 (0.85–1.18) | 0.86 | 1.0 (0.81–1.24) | 0.86 | 1.32 (1.1–1.60) | 0.03 | 1.27 (0.99–1.63) | 0.10 |
| Sugar consumption | ||||||||
| Drinking soda | <0.001 | 0.59 | 0.14 | 0.34 | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Several times/week | 1.46 (1.24–1.72) | <0.001 | 1.52 (0.60–3.93) | 0.43 | 0.80 (0.65–1.0) | 0.04 | 0.76 (0.57–1.0) | 0.04 |
| Once/day | 1.27 (1.02–1.58) | 0.05 | 1.05 (0.71–1.56) | 0.90 | 1.01 (0.76–1.33) | 0.94 | 0.78 (0.52–1.17) | 0.21 |
| 2–3 times/day | 1.39 (1.06–1.82) | 0.04 | 1.07 (0.77–1.48) | 0.82 | 1.04 (0.79–1.39) | 0.90 | 0.85 (0.56–1.30) | 0.42 |
| ≥4 times/day | 1.69 (1.02–2.79) | 0.05 | 1.20 (0.97–1.52) | 0.15 | 1.1 (0.51–2.41) | 0.87 | 1.15 (0.34–4.06) | 0.87 |
| Drinking natural juice | <0.001 | 0.24 | 0.89 | 0.54 | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Several times/week | 1.99 (1.55–2.55) | <0.001 | 1.44 (0.99–2.1) | 0.16 | 0.97 (0.73–1.31) | 0.79 | 0.99 (0.45–2.20) | 0.95 |
| Once/day | 1.91 (1.46–2.49) | <0.001 | 1.28 (0.85–1.92) | 0.30 | 0.93 (0.68–1.28) | 0.60 | 1.46 (0.91–2.37) | 0.16 |
| 2–3 times/day | 2.11 (1.62–2.76) | <0.001 | 1.41 (0.92–2.18) | 0.09 | 1.04 (0.75–1.44) | 0.93 | 1.27 (0.82–1.96) | 0.35 |
| ≥4 times/day | 2.60 (1.75–3.86) | <0.001 | 1.99 (1.05–3.81) | 0.04 | 0.81 (0.43–1.55) | 0.50 | 1.15 (0.78–1.71) | 0.56 |
| Eating sweets | <0.001 | 0.04 | 0.80 | 0.33 | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Several times/week | 1.95 (1.45–2.61) | <0.001 | 1.36 (0.84–2.20) | 0.26 | 0.98 (0.73–1.34) | 0.84 | 1.0 (0.53–1.93) | 0.98 |
| Once/day | 2.11 (1.56–2.85) | <0.001 | 1.53 (0.93–2.51) | 0.13 | 0.89 (0.64–1.26) | 0.50 | 0.88 (0.53–1.46) | 0.59 |
| 2–3 times/day | 2.36 (1.81–3.34) | <0.001 | 1.81 (1.08–3.05) | 0.04 | 1.08 (0.76–1.52) | 0.78 | 0.84 (0.52–1.35) | 0.44 |
| ≥4 times/day | 2.24 (1.50–3.35) | <0.001 | 1.03 (0.54–1.95) | 1.01 | 1.01 (0.63–1.60) | 0.98 | 0.90 (0.60–1.36) | 0.58 |
| Toothbrushing habits | ||||||||
| Frequency of brushing | 0.03 | 0.79 | 0.22 | 0.53 | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Once/day | 0.99 (0.80–1.23) | 0.76 | 1.16 (0.64–2.12) | 0.70 | 0.80 (0.59–1.07) | 0.12 | 1.18 (0.75–1.87) | 0.55 |
| 2–3 times/day | 0.98 (0.79–1.23) | 0.82 | 1.02 (0.57–1.85) | 1.02 | 1.21 (0.90–1.62) | 0.25 | 0.98 (0.72–1.33) | 0.81 |
| ≥4 times/day | 0.59 (0.42–0.84) | 0.01 | 1.17 (0.65–2.15) | 0.67 | 1.19 (0.80–1.77) | 0.48 | 0.87 (0.65–1.17) | 0.32 |
| Brushing time | 0.03 | 0.23 | <0.001 | <0.001 | ||||
| < 1 min | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 1 min | 0.79 (0.65–0.96) | 0.03 | 0.84 (0.60–1.19) | 0.29 | 1.48 (1.12–1.95) | 0.01 | 1.49(1.07–2.07) | 0.03 |
| 2 min | 0.71 (0.57–0.88) | <0.001 | 0.83 (0.63–1.10) | 0.18 | 1.11 (0.83–1.49) | 0.56 | 1.09(0.77–1.54) | 0.74 |
| ≥3 min | 0.78 (0.61–1.01) | 0.04 | 1.04 (0.80–1.36) | 0.91 | 0.82 (0.54–1.23) | 0.27 | 0.67(0.41–1.09) | 0.08 |
| Dental consult | ||||||||
| Past consult | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Regularly | 1.04 (0.84–1.28) | 0.89 | 1.06 (0.75–1.51) | 0.85 | 1.65 (1.22–2.24) | <0.001 | 1.47 (0.96–2.24) | 0.11 |
| Treatment | 1.61 (1.31–1.98) | <0.001 | 1.93 (1.46–2.54) | <0.001 | 2.12 (1.71–2.64) | <0.001 | 2.27 (1.72–3.01) | <0.001 |
| Emergency | 1.98 (1.62–2.41) | <0.001 | 2.03 (1.57–2.63) | <0.001 | 2.15 (1.67–2.77) | <0.001 | 2.63 (1.93–3.59) | <0.001 |
| Age of Children (Years) | 6 | 8 | 11 | |
|---|---|---|---|---|
| Number of children | 182 | 159 | 218 | |
| dmft ± SD | dmft ± SD | DMFT ± SD | p | |
| Sugar consumption | ||||
| Drinking soda | 1.95±1.87 | 1.81±1.71 | 4.59 ± 4.33 | 0.04 |
| Drinking natural juice | 2.15 ± 2.01 | 4.33 ± 3.72 | 1.95 ± 1.59 | 0.56 |
| Eating sweets | 4.05 ± 3.11 | 2.56 ± 2.67 | 2.06 ± 1.94 | 0.03 |
| Oral health behaviors | ||||
| Brushing frequency | 1.90 ± 1.79 | 1.85 ± 1.71 | 2.24 ± 1.91 | 0.57 |
| Brushing time | 3.95 ± 1.18 | 3.38 ± 2.10 | 4.10 ± 3.60 | <0.001 |
| Dental consult | ||||
| Treatment | 5.38 ± 2.92 | 5.20 ± 4.43 | 4.26 ± 2.93 | <0.001 |
| Emergency | 6.30 ± 3.38 | 6.27 ± 3.74 | 5.31 ± 2.43 | <0.001 |
| Age Children (Years) | 6 | 8 | 11 | |
|---|---|---|---|---|
| Number of children | 182 | 159 | 218 | |
| SiC index ± SD | SiC index ± SD | SiC index ± SD | p | |
| Sugar consumption | ||||
| Drinking soda | 3.85 ± 1.34 | 3.57 ± 1.22 | 7.97 ± 9.14 | 0.04 |
| Drinking natural juice | 4.24 ± 1.44 | 8.55 ± 2.66 | 3.39 ± 3.36 | 0.03 |
| Eating sweets | 8.00 ± 2.22 | 5.05 ± 1.91 | 3.58 ± 4.10 | <0.001 |
| Oral health behaviors | ||||
| Brushing frequency | 3.75 ± 1.28 | 3.65 ± 1.22 | 3.89 ± 4.03 | 0.85 |
| Brushing time | 7.80 ± 0.84 | 6.67 ± 1.50 | 7.12 ± 7.60 | 0.53 |
| Dental consult | ||||
| Treatment | 10.62 ± 2.09 | 10.27 ± 3.16 | 7.40 ± 6.19 | <0.001 |
| Emergency | 12.44 ± 2.41 | 12.38 ± 2.67 | 9.22 ± 5.13 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seni, A.-G.; Sălcudean, A.; Popovici, R.A.; Olariu, I.; Cincu, M.-G.; Jinga, V.; Trusculescu, L.-M.; Pitic, D.E.; Cosoroabă, R.M.; Kis, A.; et al. The Prevalence of Dental Caries Among Children Aged 6–11: A Cross-Sectional Study from Mureș County, Romania. Medicina 2025, 61, 1648. https://doi.org/10.3390/medicina61091648
Seni A-G, Sălcudean A, Popovici RA, Olariu I, Cincu M-G, Jinga V, Trusculescu L-M, Pitic DE, Cosoroabă RM, Kis A, et al. The Prevalence of Dental Caries Among Children Aged 6–11: A Cross-Sectional Study from Mureș County, Romania. Medicina. 2025; 61(9):1648. https://doi.org/10.3390/medicina61091648
Chicago/Turabian StyleSeni, Ana-Gabriela, Andreea Sălcudean, Ramona Amina Popovici, Iustin Olariu, Mădălina-Gabriela Cincu, Viorel Jinga, Laria-Maria Trusculescu, Dana Emanuela Pitic, Raluca Mioara Cosoroabă, Andreea Kis, and et al. 2025. "The Prevalence of Dental Caries Among Children Aged 6–11: A Cross-Sectional Study from Mureș County, Romania" Medicina 61, no. 9: 1648. https://doi.org/10.3390/medicina61091648
APA StyleSeni, A.-G., Sălcudean, A., Popovici, R. A., Olariu, I., Cincu, M.-G., Jinga, V., Trusculescu, L.-M., Pitic, D. E., Cosoroabă, R. M., Kis, A., Talpos-Niculescu, C. I., Todor, L., & Tarcea, M. (2025). The Prevalence of Dental Caries Among Children Aged 6–11: A Cross-Sectional Study from Mureș County, Romania. Medicina, 61(9), 1648. https://doi.org/10.3390/medicina61091648








