Intravenous Iron Infusion in the Treatment of Iron Deficiency Anaemia Following Bariatric and Metabolic Surgery and Correlation with Gynaecological Disorders: Retrospective Review of Experience from a Tertiary Centre
Abstract
1. Introduction
2. Materials and Methods
2.1. Operative Technique
2.2. Investigation of Iron Deficiency
2.3. Data Collection and Definitions
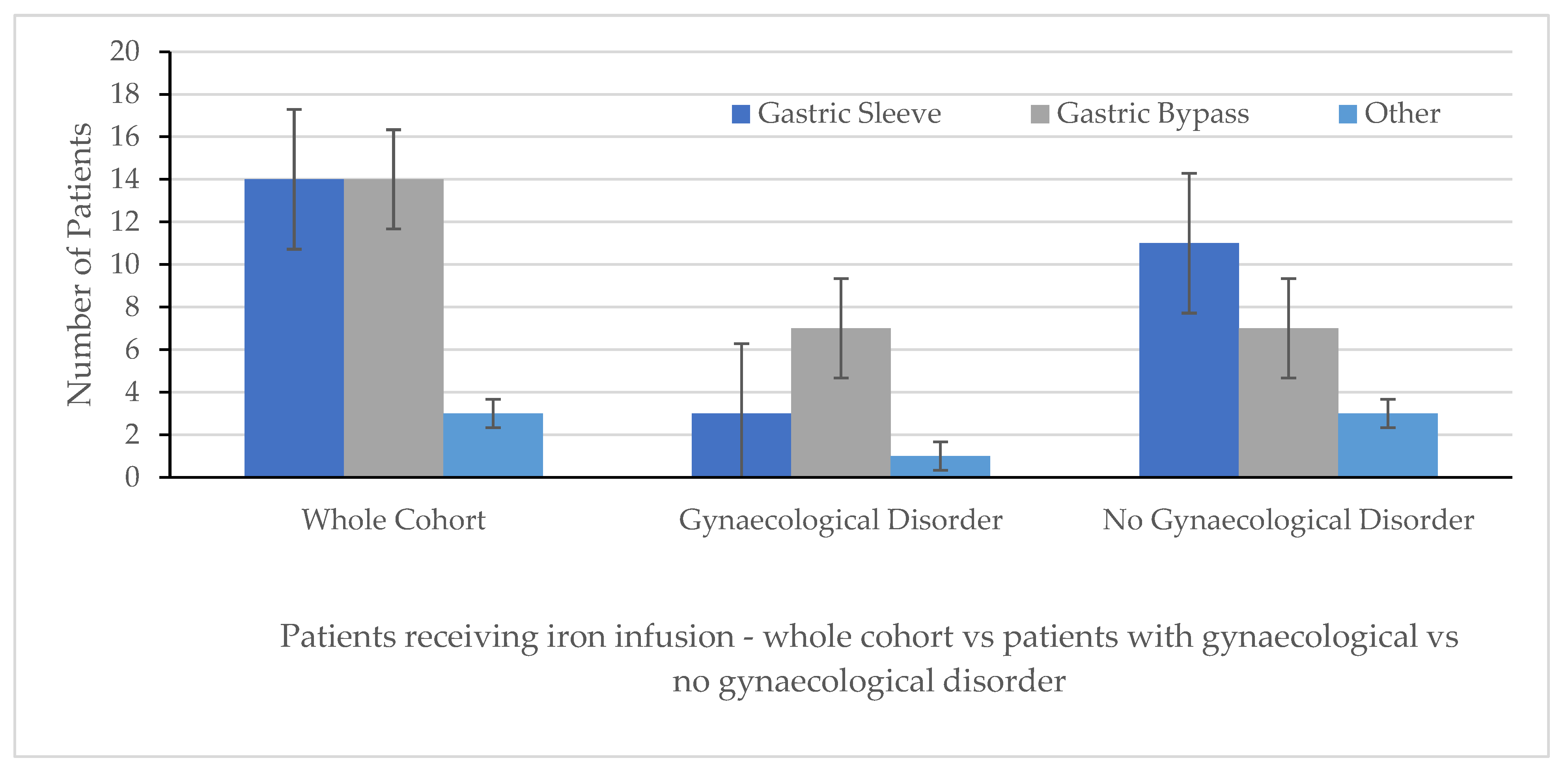
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMS | Bariatric and Metabolic Surgery. |
| IDA | Iron-deficient anaemia. |
| NICE | National Institute of Health and Care Excellence. |
| RIE | Royal Infirmary of Edinburgh. |
| qFIT | Quantitative Faecal Immunochemical Test. |
| USA | United States of America. |
| UK | United Kingdom. |
| PCOS | Polycystic ovarian syndrome. |
| NHS | National Health Service. |
Appendix A. Iron Infusion—Ferinject®—Dosage Calculation and Administration Regime
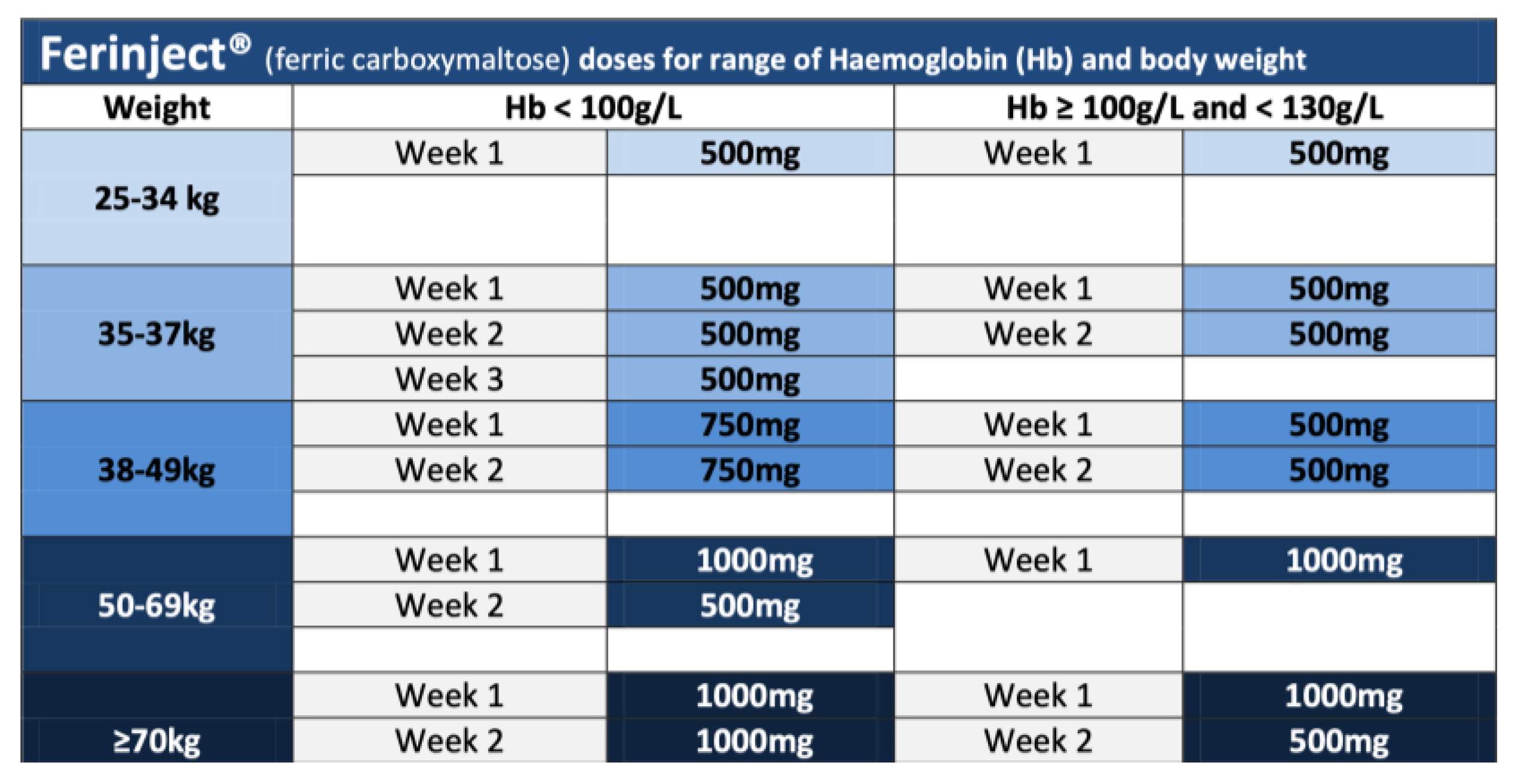
References
- Angrisani, L.; Santonicola, A.; Iovino, P.; Palma, R.; Know, L.; Prager, G.; Ramos, A.; Shikora, S.; Collaborative Study Group for the IFSO Worldwide Survey. IFSO Worldwide Survey 2020–2021: Current Trends for Bariatric and Metabolic Procedures. Obes. Surg. 2024, 34, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- von Drygalski, A.; Andris, D.A. Anemia after bariatric surgery: More than just iron deficiency. Nutr. Clin. Pract. 2009, 24, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; DeLoughery, T.G.; Tirnauer, J.S. Iron Deficiency in Adults: A Review. JAMA 2025, 333, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Mast, A.E.; Powers, J.M.; Kouides, P.A.; O’Brien, S.H.; Richards, T.; Lavin, M.; Levy, B.S. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am. J. Obstet. Gynecol. 2023, 229, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Jimenez, M.C.; Moreno, G.; Wright, I.; Shih, P.C.; Vaquero, M.P.; Remacha, A.F. Iron Deficiency in Menstruating Adult Women: Much More than Anemia. Women’s Health Rep. 2020, 1, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, K.M.; Legro, R.S.; Welt, C.K. A patient’s guide: Polycystic ovary syndrome (PCOS). J. Clin. Endocrinol. Metab. 2014, 99, 35A–36A. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.N.; Wiggins, T.; Robertson, F.P.; Huppler, L.; Doleman, B.; Harrison, E.M.; Hollyman, M.; Welbourn, R. Perioperative mortality in bariatric surgery: Meta-analysis. Br. J. Surg. 2021, 108, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Excellence NI for H and C. How Should I Diagnose Polycystic Ovary Syndrome? 2025. Available online: https://cks.nice.org.uk/topics/polycystic-ovary-syndrome/diagnosis/diagnosis/ (accessed on 20 July 2025).
- Excellence NI of C and H. Menorrhagia-Heavy Menstrual Bleeding. 2024. Available online: https://cks.nice.org.uk/topics/menorrhagia-heavy-menstrual-bleeding/ (accessed on 20 July 2025).
- Lucocq, J.; Homyer, K.; Geropoulos, G.; Thakur, V.; Stansfield, D.; Joyce, B.; Drummond, G.; Tulloh, B.; de Beaux, A.; Lamb, P.J.; et al. Long-Term Weight Loss and Comorbidity Resolution of Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-en-Y Gastric Bypass and the Impact of Preoperative Weight Loss on Overall Outcome. Surg. Laparosc. Endosc. Percutan. Tech. 2024, 34, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Lucocq, J.; Thakur, V.; Geropoulos, G.; Stansfield, D.; Irvine, L.; Duxbury, M.; de Beaux, A.C.; Tulloh, B.; Wallace, B.; Joyce, B.; et al. Intensive pre-operative information course (IPIC) and pre-operative weight loss results in long-term sustained weight loss following bariatric surgery: 11 year results from a Tertiary Referral Centre. Surg. Endosc. 2024, 38, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Medicine E and LL. Edinburgh and Lothian Laboratory Medicine Test Directory. 2025. Available online: https://edinburghlabmed.co.uk/TestDirectory/Pages/default (accessed on 20 July 2025).
- Vargas, C.M.; Gómez, D.; Madrigal, V.; Guilbert, L.; Sepúlveda, E.M.; Rodríguez, F.M.; Zerrweck, C. Women with Anemia Refractory to Oral Iron Treatment Following Bariatric Surgery: A Short-Term Analysis. Obes. Surg. 2023, 33, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Kermansaravi, M.; Shahsavan, M.; Hage, K.; Taskin, H.E.; ShahabiShahmiri, S.; Poghosyan, T.; Jazi, A.H.D.; Baratte, C.; Valizadeh, R.; Chevallier, J.M.; et al. Iron deficiency anemia after one anastomosis gastric bypass: A systematic review and meta-analysis. Surg. Endosc. 2025, 39, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Ottosson, J.; Clarkson, S.; Sjöberg, K. Anemia in patients ten years after bariatric surgery. Int. J. Obes. 2025, 49, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Group BBSC. Roux-en-Y gastric bypass, adjustable gastric banding, or sleeve gastrectomy for severe obesity (By-Band-Sleeve): A multicentre, open label, three-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2025, 13, 410–426. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.C.; Chen, H.J.; Leong, K.H.; Chang, K.L.; Wang, Y.T.T.; Wu, L.C.; Tung, P.-Y.; Kuo, C.-F.; Lin, C.-C.; Tsai, S.-Y. The risk of fibromyalgia in patients with iron deficiency anemia: A nationwide population-based cohort study. Sci. Rep. 2021, 11, 10496. [Google Scholar] [CrossRef]
- Medicines GG and C. Prescribing and Administration Information for Ferrinject. 2025. Available online: https://handbook.ggcmedicines.org.uk/media/1112/3-ferinject-ida-2020.pdf (accessed on 20 July 2025).


| N = 32 | |
|---|---|
| Female/Male | 32/0 (p value < 0.05) |
| Median Age, years (range) | 36.5 (23–55) |
| Pre-operative weight, median, range (kg) | 111 (88.5–155) |
| Pre-operative BMI, median, range (kg/m2) | 46.1 (36.1–61.9) |
| Location of Surgery: Local UK other Europe USA Asia | 18 (56.3%) 4 (12.5%) 8 (25.0%) 1 (3.1%) 1 (3.1%) |
| Co-morbidities (gynaecological) Uterine fibroid Menorrhagia Polycystic ovarian syndrome/Ovarian Cyst Nil | 1 (3.1%) 4 (12.5%) 6 (18.8%) 21 (65.6%) |
| Co-morbidities (non-gynaecological) Fibromyalgia T2DM Obstructive sleep apnoea Hypertension (HTN) Haematological disorder Nil | 3 (9.4%) 2 (6.3%) 2 (6.3%) 1 (3.1%) 1 (3.1%) 18 (56.3%) |
| Whole Cohort (n = 32) | Gynaecological Disorder (n = 11) | No Gynaecological Disorder (n = 21) | p Values | |
|---|---|---|---|---|
| Age median, (range) (years) | 36.5 (23–55) | 36 (26–48) | 33.5 (23–55) | p = ns |
| Pre-operative weight (kg) median, range Pre-operative BMI (kg/m2) median, range | 111 (88.5–155) 46.1 (36.1–61.9) | 110.8 (93–133) 41.6 (39–43.8) | 111 (88.5–155) 48.8 (36.1–61.9) | p = ns p = ns |
| Pre-initial iron Infusion Values: median (range) Hb (g/L) MCV (fL) Iron (µmol/L) Ferritin (µg/L) | 123 (87–142) 87 (73–99) 10 (4–25) 13 (4–265) | 119 (87–141) 84 (73–92) 13 (4–25) 11 (4–76) | 125 (104–142) 88 (82–99) 9.5 (5–24) 14.5 (5–265) | p = ns p = 0.0119 p = ns p = ns |
| Location of Primary Operation: Home Institution UK Other Abroad | 18 (54.3%) 4 (12.5%) 9 (28.1%) | 5 (45.5%)06 (54.5%) | 13 (61.9%) 4 (19.0%) 3 (14.3%) | |
| Number of iron infusions administered median, (range) | 2.0 (1–11) | 2.0 (1–11) | 2.0 (1–5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacVicar, E.; Saleh, N.; Tneoh, J.; Lucocq, J.; Geropoulos, G.; Wallace, B.; Ewing, A.; Lamb, P.J.; Drummond, G.; Joyce, B.; et al. Intravenous Iron Infusion in the Treatment of Iron Deficiency Anaemia Following Bariatric and Metabolic Surgery and Correlation with Gynaecological Disorders: Retrospective Review of Experience from a Tertiary Centre. Medicina 2025, 61, 1647. https://doi.org/10.3390/medicina61091647
MacVicar E, Saleh N, Tneoh J, Lucocq J, Geropoulos G, Wallace B, Ewing A, Lamb PJ, Drummond G, Joyce B, et al. Intravenous Iron Infusion in the Treatment of Iron Deficiency Anaemia Following Bariatric and Metabolic Surgery and Correlation with Gynaecological Disorders: Retrospective Review of Experience from a Tertiary Centre. Medicina. 2025; 61(9):1647. https://doi.org/10.3390/medicina61091647
Chicago/Turabian StyleMacVicar, Emma, Nivar Saleh, Joy Tneoh, James Lucocq, Georgios Geropoulos, Beverley Wallace, Anne Ewing, Peter J. Lamb, Gillian Drummond, Brian Joyce, and et al. 2025. "Intravenous Iron Infusion in the Treatment of Iron Deficiency Anaemia Following Bariatric and Metabolic Surgery and Correlation with Gynaecological Disorders: Retrospective Review of Experience from a Tertiary Centre" Medicina 61, no. 9: 1647. https://doi.org/10.3390/medicina61091647
APA StyleMacVicar, E., Saleh, N., Tneoh, J., Lucocq, J., Geropoulos, G., Wallace, B., Ewing, A., Lamb, P. J., Drummond, G., Joyce, B., & Robertson, A. G. (2025). Intravenous Iron Infusion in the Treatment of Iron Deficiency Anaemia Following Bariatric and Metabolic Surgery and Correlation with Gynaecological Disorders: Retrospective Review of Experience from a Tertiary Centre. Medicina, 61(9), 1647. https://doi.org/10.3390/medicina61091647





