Quantification and Analysis of Lung Involvement by Artificial Intelligence in Patients with Progressive Pulmonary Fibrosis Treated with Nintedanib
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis Using 3D Slicer
2.2. Lung Function Tests
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kondoh, Y.; Inoue, Y. Progressive Pulmonary Fibrosis: Current Status in Terminology and Future Directions. Adv. Ther. 2025, 42, 2988–3001. [Google Scholar] [CrossRef]
- Harari, S. Beyond idiopathic pulmonary fibrosis: The world of progressive-fibrosing interstitial lung disease. Eur. Respir. Rev. 2018, 27, 180110. [Google Scholar] [CrossRef]
- Rajan, S.K.; Cottin, V.; Dhar, R.; Danoff, S.; Flaherty, K.R.; Brown, K.K.; Mohan, A.; Renzoni, E.; Mohan, M.; Udwadia, Z.; et al. Progressive pulmonary fibrosis: An expert group consensus statement. Eur. Respir. J. 2023, 61, 2103187. [Google Scholar] [CrossRef]
- Naqvi, M.; Hannah, J.; Lawrence, A.; Myall, K.; West, A.; Chaudhuri, N. Antifibrotic therapy in progressive pulmonary fibrosis: A review of recent advances. Expert Rev. Respir. Med. 2024, 18, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.; Cottin, V. Epidemiology and real-life experience in progressive pulmonary fibrosis. Curr. Opin. Pulm. Med. 2022, 28, 407–413. [Google Scholar] [CrossRef]
- Olson, A.; Hartmann, N.; Patnaik, P.; Wallace, L.; Schlenker-Herceg, R.; Nasser, M.; Richeldi, L.; Hoffmann-Vold, A.-M.; Cottin, V. Estimation of the Prevalence of Progressive Fibrosing Interstitial Lung Diseases: Systematic Literature Review and Data from a Physician Survey. Adv. Ther. 2021, 38, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Hilberg, O.; Hoffmann-Vold, A.-M.; Smith, V.; Bouros, D.; Kilpeläinen, M.; Guiot, J.; Morais, A.; Clemente, S.; Daniil, Z.; Papakosta, D.; et al. Epidemiology of interstitial lung diseases and their progressive-fibrosing behaviour in six European countries. ERJ Open Res. 2022, 8, 00597–2021. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Hirani, N.A.; Hotchkin, D.L.; Nambiar, A.M.; Ogura, T.; Otaola, M.; Skowasch, D.; Park, J.S.; Poonyagariyagorn, H.K.; Wuyts, W.; et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018, 27, 180076. [Google Scholar] [CrossRef]
- Bernardinello, N.; Pezzuto, F.; D’sA, L.; Vedovelli, L.; Giraudo, C.; Chelu, A.; de Chellis, C.; Lunardi, F.; Fortarezza, F.; Boscaro, F.; et al. Predicting biomarkers of progressive pulmonary fibrosis: Morphological, cytokine profile, and clinical portrait. Front. Immunol. 2025, 16, 1514439. [Google Scholar] [CrossRef]
- Rosas, I.O.; Yao, J.; Avila, N.A.; Chow, C.K.; Gahl, W.A.; Gochuico, B.R. Automated quantification of high-resolution CT scan findings in individuals at risk for pulmonary fibrosis. Chest 2011, 140, 1590–1597. [Google Scholar] [CrossRef]
- Maldonado, F.; Moua, T.; Rajagopalan, S.; Karwoski, R.A.; Raghunath, S.; Decker, P.A.; Hartman, T.E.; Bartholmai, B.J.; Robb, R.A.; Ryu, J.H. Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 204–212. [Google Scholar] [CrossRef]
- Kim, H.J.; Brown, M.S.; Elashoff, R.; Li, G.; Gjertson, D.W.; Lynch, D.A.; Strollo, D.C.; Kleerup, E.; Chong, D.; Shah, S.K.; et al. Quantitative texture-based assessment of one-year changes in fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosphamide. Eur. Radiol. 2011, 21, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Min, X.; Nan, Y.; Feng, Z.; Li, B.; Cai, W.; Xi, X.; Wang, L. Assessment of the Severity of Coronavirus Disease: Quantitative Computed Tomography Parameters versus Semiquantitative Visual Score. Korean J. Radiol. 2020, 21, 998–1006. [Google Scholar] [CrossRef]
- Colombi, D.; Villani, G.D.; Maffi, G.; Risoli, C.; Bodini, F.C.; Petrini, M.; Morelli, N.; Anselmi, P.; Milanese, G.; Silva, M.; et al. Qualitative and quantitative chest CT parameters as predictors of specific mortality in COVID-19 patients. Emerg. Radiol. 2020, 27, 701–710. [Google Scholar] [CrossRef]
- Lanza, E.; Muglia, R.; Bolengo, I.; Santonocito, O.G.; Lisi, C.; Angelotti, G.; Morandini, P.; Savevski, V.; Politi, L.S.; Balzarini, L. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur. Radiol. 2020, 30, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Ghazipura, M.; Mammen, M.J.; Bissell, B.D.; Macrea, M.; Herman, D.D.; Hon, S.M.; Kheir, F.; Khor, Y.H.; Knight, S.L.; Raghu, G.; et al. Pirfenidone in Progressive Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2022, 19, 1030–1039. [Google Scholar] [CrossRef]
- Ghazipura, M.; Mammen, M.J.; Herman, D.D.; Hon, S.M.; Bissell, B.D.; Macrea, M.; Kheir, F.; Khor, Y.H.; Knight, S.L.; Raghu, G.; et al. Nintedanib in Progressive Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2022, 19, 1040–1049. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- 3D Slicer Image Computing Platform. Available online: https://www.slicer.org (accessed on 16 June 2024).
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- LungCTAnalyzer Extension. Available online: https://github.com/rbumm/SlicerLungCTAnalyzer (accessed on 16 June 2024).
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Cameli, P.; Alonzi, V.; D’alessandro, M.; Bergantini, L.; Pordon, E.; Guerrieri, M.; Refini, R.M.; Sestini, P.; Bargagli, E. The Effectiveness of Nintedanib in Patients with Idiopathic Pulmonary Fibrosis, Familial Pulmonary Fibrosis and Progressive Fibrosing Interstitial Lung Diseases: A Real-World Study. Biomedicines 2022, 10, 1973. [Google Scholar] [CrossRef]
- Wollin, L.; Distler, J.H.; Redente, E.F.; Riches, D.W.H.; Stowasser, S.; Schlenker-Herceg, R.; Maher, T.M.; Kolb, M. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur. Respir. J. 2019, 54, 1900161. [Google Scholar] [CrossRef]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef]
- Lamb, Y.N. Nintedanib: A Review in Fibrotic Interstitial Lung Diseases. Drugs 2021, 81, 575–586. [Google Scholar] [CrossRef]
- Yu, W.-K.; Chen, W.-C.; Su, V.Y.-F.; Shen, H.-C.; Wu, H.-H.; Chen, H.; Yang, K.-Y. Nintedanib Inhibits Endothelial Mesenchymal Transition in Bleomycin-Induced Pulmonary Fibrosis via Focal Adhesion Kinase Activity Reduction. Int. J. Mol. Sci. 2022, 23, 8193. [Google Scholar] [CrossRef]
- Grimminger, F.; Günther, A.; Vancheri, C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1426–1433. [Google Scholar] [CrossRef]
- Mondoni, M.; Varone, F.; Luppi, F.; Cameli, P.; Cerri, S.; Puci, M.V.; Cefalo, J.; Contino, S.; Martini, A.; Iovene, B.; et al. Effectiveness of Nintedanib in Progressive Pulmonary Fibrosis Assessed by Progression Criteria: An Italian, Observational, Multicenter Study. Lung 2025, 203, 77. [Google Scholar] [CrossRef] [PubMed]
- Narváez, J.; Aguilar-Coll, M.; Vicens-Zygmunt, V.; Alegre, J.J.; Bermudo, G.; Molina-Molina, M. Real-World Clinical Effectiveness and Safety of Antifibrotics in Progressive Pulmonary Fibrosis Associated with Rheumatoid Arthritis. J. Clin. Med. 2024, 13, 7074. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; Kokosi, M.; Nair, A.; Karwoski, R.; Raghunath, S.M.; Walsh, S.L.; Wells, A.U.; Hansell, D.M. Automated Quantitative Computed Tomography Versus Visual Computed Tomography Scoring in Idiopathic Pulmonary Fibrosis. J. Thorac. Imaging 2016, 31, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; van Moorsel, C.H.M.; van Es, H.W.; van Beek, F.T.; Struik, M.H.L.; Kokosi, M.; Egashira, R.; Brun, A.L.; et al. Predicting Outcomes in Idiopathic Pulmonary Fibrosis Using Automated Computed Tomographic Analysis. Am. J. Respir. Crit. Care Med. 2018, 198, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.M.; Mackintosh, J.A.; Jo, H.E.; Walsh, S.L.F.; Silva, M.; Calandriello, L.; Chapman, S.; Ellis, S.; Glaspole, I.; Goh, N.; et al. Quantitative computed tomography predicts outcomes in idiopathic pulmonary fibrosis. Respirology 2022, 27, 1045–1053. [Google Scholar] [CrossRef] [PubMed]

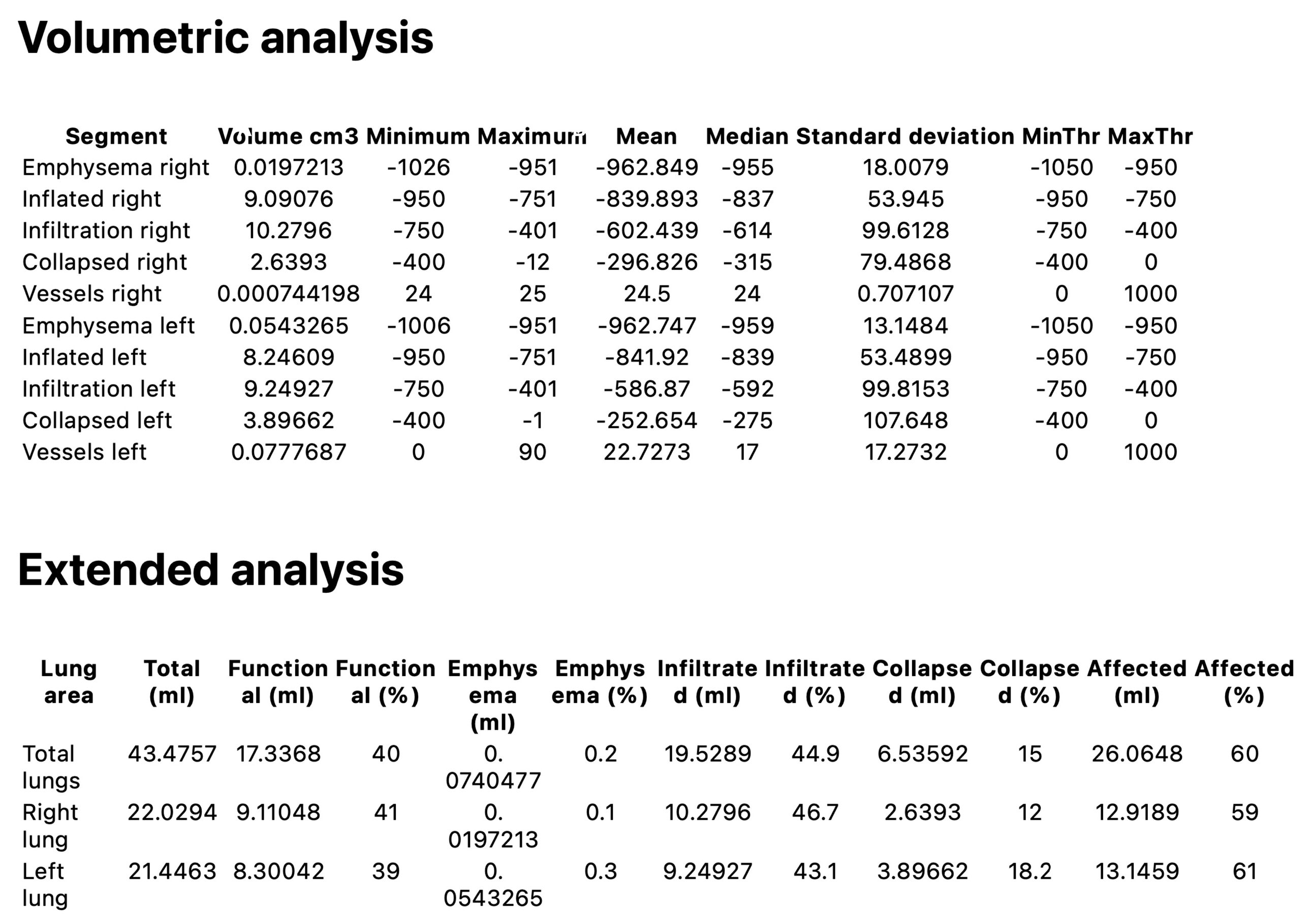

| Volumetric Analysis Parameter | Baseline | Follow-Up | p-Value |
|---|---|---|---|
| Infiltration right, mean value ± SD, cm3 | 5.56 ± 3.08 | 4.88 ± 2.77 | 0.041 |
| Collapsed right, mean value ± SD, cm3 | 1.79 ± 1.24 | 1.77 ± 1.61 | 0.919 |
| Vessels right, mean value ± SD, cm3 | 0.28 ± 0.27 | 0.33 ± 0.54 | 0.483 |
| Infiltration left, mean value ± SD, cm3 | 4.73 ± 2.35 | 5.03 ± 3.09 | 0.555 |
| Collapsed left, mean value ± SD, cm3 | 1.62 ± 1.50 | 1.77 ± 1.73 | 0.466 |
| Vessels left, mean value ± SD, cm3 | 0.21 ± 0.27 | 0.36 ± 0.70 | 0.174 |
| Extended Analysis Parameter | Baseline | Follow-Up | p-Value |
|---|---|---|---|
| Total, mean value ± SD, mL | 41.93 ± 1.21 | 41.85 ± 1.68 | 0.745 |
| Functional, mean value ± SD, mL | 22.62 ± 5.22 | 22.79 ± 5.87 | 0.800 |
| Functional, mean value ± SD, % | 53.90 ± 11.90 | 54.19 ± 13.30 | 0.855 |
| Infiltrated, mean value ± SD, mL | 10.30 ± 5.11 | 9.50 ± 4.73 | 0.199 |
| Infiltrated, mean value ± SD, % | 24.60 ± 12.26 | 22.81 ± 11.38 | 0.215 |
| Affected, mean value ± SD, mL | 13.60 ± 7.00 | 12.88 ± 6.80 | 0.400 |
| Affected, mean value ± SD, % | 32.51 ± 16.83 | 31.14 ± 17.03 | 0.510 |
| Total right, mean value ± SD, mL | 21.22 ± 0.69 | 21.01 ± 0.97 | 0.335 |
| Functional right, mean value ± SD, mL | 13.86 ± 4.14 | 5.98 ± 10.41 | 0.156 |
| Functional right, mean value ± SD, % | 65.49 ± 19.13 | 68.62 ± 18.41 | 0.128 |
| Infiltrated right, mean value ± SD, mL | 5.56 ± 3.08 | 4.88 ± 2.77 | 0.042 |
| Infiltrated right, mean value ± SD, % | 26.33 ± 14.55 | 23.31 ± 13.17 | 0.054 |
| Affected right, mean value ± SD, mL | 7.25 ± 4.04 | 6.53 ± 3.72 | 0.084 |
| Affected right, mean value ± SD, % | 34.51 ± 19.13 | 31.38 ± 18.41 | 0.128 |
| Total left, mean value ± SD, mL | 20.82 ± 0.99 | 20.84 ± 1.03 | 0.944 |
| Functional left, mean value ± SD, mL | 14.47 ± 3.78 | 14.48 ± 4.10 | 0.982 |
| Functional left, mean value ± SD, % | 69.35 ± 17.09 | 69.32 ± 18.57 | 0.100 |
| Infiltrated left, mean value ± SD, mL | 4.73 ± 2.35 | 4.63 ± 2.36 | 0.774 |
| Infiltrated left, mean value ± SD, % | 22.79 ± 11.40 | 22.30 ± 11.33 | 0.754 |
| Affected left, mean value ± SD, mL | 6.34 ± 3.51 | 6.35 ± 3.76 | 0.985 |
| Affected left, mean value ± SD, % | 30.63 ± 17.12 | 30.68 ± 18.60 | 0.128 |
| Lung Functional Parameter | Baseline | Follow-Up | p-Value |
|---|---|---|---|
| RV, mean value ± SD, L | 1.49 ± 0.56 | 1.29 ± 0.63 | 0.104 |
| RV, mean value ± SD, % | 60.96 ± 21.55 | 52.65 ± 24.61 | 0.093 |
| FVC, mean value ± SD, L | 2.58 ± 1.06 | 2.50 ± 0.82 | 0.469 |
| FVC, mean value ± SD, % | 72.68 ± 23.84 | 72.23 ± 16.53 | 0.885 |
| FEV1, mean value ± SD, L | 2.14 ± 0.83 | 2.06 ± 0.64 | 0.344 |
| FEV1, mean value ± SD, % | 78.82 ± 25.80 | 78.36 ± 18.66 | 0.894 |
| PEF, mean value ± SD, L/s | 7.35 ± 2.98 | 6.79 ± 1.82 | 0.300 |
| PEF, mean value ± SD, % | 101.80 ± 33.21 | 96.59 ± 20.92 | 0.447 |
| FEF25–75, mean value ± SD, L/s | 2.38 ± 1.18 | 2.37 ± 0.78 | 0.982 |
| FEF25–75, mean value ± SD, % | 115.00 ± 64.19 | 120.00 ± 45.09 | 0.563 |
| DLCO, mean value ± SD, mmol/min/kPa | 3.26 ± 1.21 | 2.76 ± 1.13 | 0.097 |
| DLCO, mean value ± SD, % | 43.06 ± 15.03 | 36.75 ± 12.68 | 0.096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battaglia, C.; Pelaia, C.; Lupia, C.; Mondelli, A.; Turco, F.; Zaffino, P.; Cosentino, C.; Manti, F.; Conti, G.; Montenegro, N.; et al. Quantification and Analysis of Lung Involvement by Artificial Intelligence in Patients with Progressive Pulmonary Fibrosis Treated with Nintedanib. Medicina 2025, 61, 1646. https://doi.org/10.3390/medicina61091646
Battaglia C, Pelaia C, Lupia C, Mondelli A, Turco F, Zaffino P, Cosentino C, Manti F, Conti G, Montenegro N, et al. Quantification and Analysis of Lung Involvement by Artificial Intelligence in Patients with Progressive Pulmonary Fibrosis Treated with Nintedanib. Medicina. 2025; 61(9):1646. https://doi.org/10.3390/medicina61091646
Chicago/Turabian StyleBattaglia, Caterina, Corrado Pelaia, Chiara Lupia, Alessia Mondelli, Francesco Turco, Paolo Zaffino, Carlo Cosentino, Francesco Manti, Giuliana Conti, Nicola Montenegro, and et al. 2025. "Quantification and Analysis of Lung Involvement by Artificial Intelligence in Patients with Progressive Pulmonary Fibrosis Treated with Nintedanib" Medicina 61, no. 9: 1646. https://doi.org/10.3390/medicina61091646
APA StyleBattaglia, C., Pelaia, C., Lupia, C., Mondelli, A., Turco, F., Zaffino, P., Cosentino, C., Manti, F., Conti, G., Montenegro, N., Maiorano, A., Pelaia, G., Romeo, P., & Laganà, D. (2025). Quantification and Analysis of Lung Involvement by Artificial Intelligence in Patients with Progressive Pulmonary Fibrosis Treated with Nintedanib. Medicina, 61(9), 1646. https://doi.org/10.3390/medicina61091646










