Postoperative Pulmonary Complications After Laparoscopic Surgery: Risk Factors and Predictive Scores
Abstract
1. Introduction
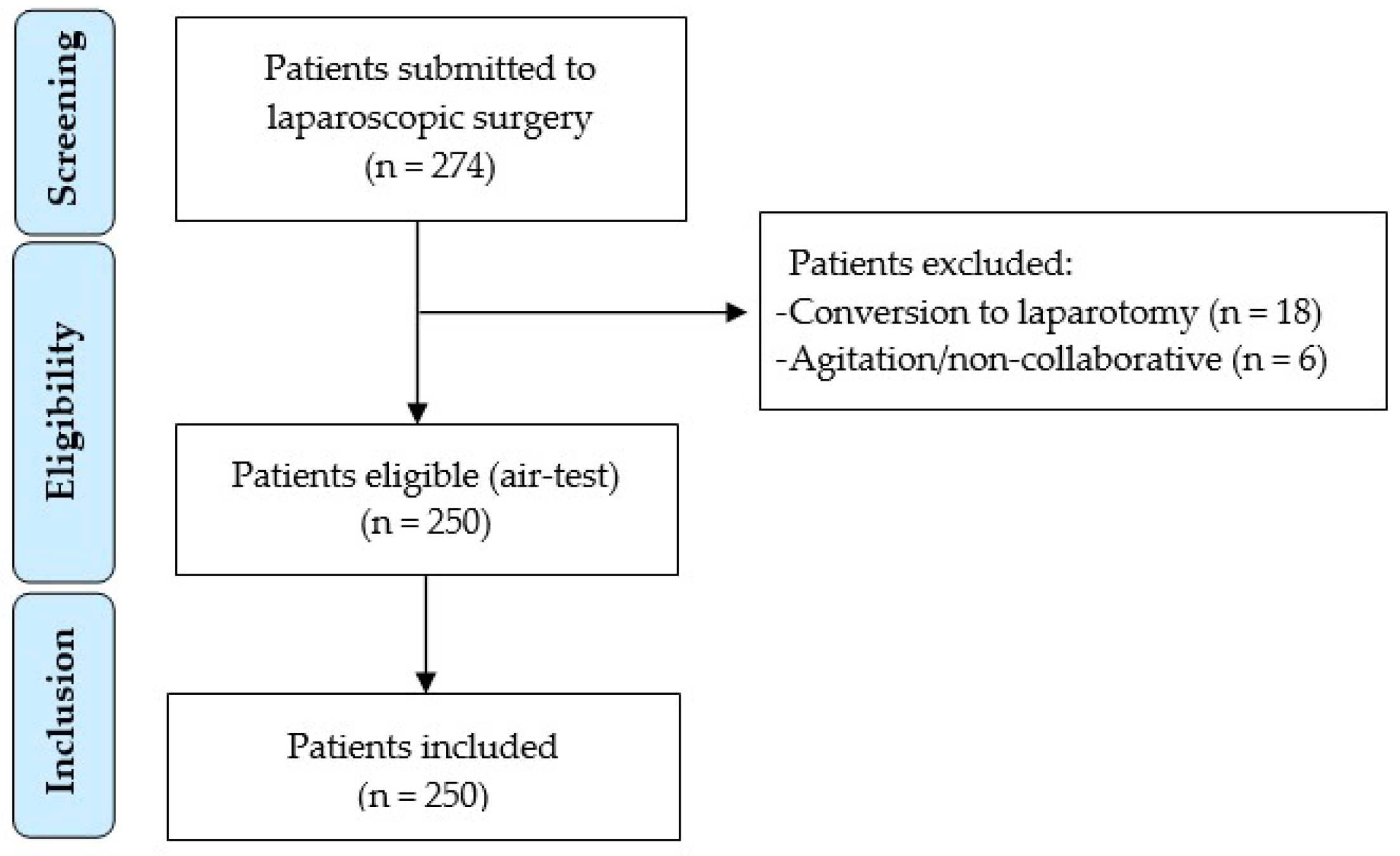
2. Materials and Methods
2.1. Routine Peri-Procedural Management
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPCs | Postoperative pulmonary complications |
| VAS | Visual analogue scale |
| PACU | Post-anesthesia care unit |
References
- Gerges, F.J.; Kanazi, G.E.; Jabbour-Khoury, S.I. Anesthesia for laparoscopy: A review. J. Clin. Anesth. 2006, 18, 67–78. [Google Scholar] [CrossRef]
- Riemma, G.; De Franciscis, P.; La Verde, M.; Ravo, M.; Fumiento, P.; Fasulo, D.D.; Della Corte, L.; Ronsini, C.; Torella, M.; Cobellis, L. Impact of the hemostatic approach after laparoscopic endometrioma excision on ovarian reserve: Systematic review and network meta-analysis of randomized controlled trials. Int. J. Gynaecol. Obstet. 2023, 162, 222–232. [Google Scholar] [CrossRef]
- Dokmak, S.; Scatton, O.; Fusco, G.; Belghiti, J.; Gayet, B.; Soubrane, O. Laparoscopy Decreases Pulmonary Complications in Patients 4Undergoing Major Liver Resection: A Propensity Score Analysis. Ann. Surg. 2016, 263, 353–361. [Google Scholar]
- Antoniou, S.A.; Antoniou, G.A.; Koch, O.O.; Köhler, G.; Pointner, R.; Granderath, F.A. Laparoscopic versus open obesity surgery: A meta-analysis of pulmonary complications. Dig. Surg. 2015, 32, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, F.; Ding, Y.; Sun, H.; Liu, S.; Li, A.; Li, F. Laparoscopic Major Gastrointestinal Surgery Is Safe for Properly Selected Patient with COPD: A Meta-Analysis. Biomed. Res. Int. 2019, 2019, 8280358. [Google Scholar] [CrossRef]
- Hasukić, Š.; Mešićć, D.; Dizdarević, E.; Keser, D.; Hadžiselimović, S.; Bazardžanović, M. Pulmonary function after laparoscopic and open cholecystectomy. Surg. Endosc. 2002, 16, 163–165. [Google Scholar] [CrossRef]
- Bablekos, G.D.; Michaelides, S.A.; Analitis, A.; Charalabopoulos, K.A. Effects of laparoscopic cholecystectomy on lung function: A systematic review. World J. Gastroenterol. 2014, 20, 17603–17617. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, C.; Soro, M.; Unzueta, C.; Suarez-Sipmann, F.; Canet, J.; Librero, J.; Pozo, N.; Peiró, S.; Llombart, A.; León, I.; et al. Individualized PeRioperative Open-lung VEntilation (iPROVE) Network. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): A randomised controlled trial. Lancet Respir. Med. 2018, 6, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Ortolá, C.; iPROVE Research Network Group for the PEALS study. Postoperative pulmonary complications in emergency abdominal surgery. A prospective international cohort study. Anaesth. Crit. Care Pain Med. 2025, 44, 101560. [Google Scholar] [CrossRef]
- Chandler, D.; Mosieri, C.; Kallurkar, A.; Pham, A.D.; Okada, L.K.; Kaye, R.J.; Cornett, E.M.; Fox, C.J.; Urman, R.D.; Kaye, A.D. Perioperative strategies for the reduction of postoperative pulmonary complications. Best. Pract. Res. Clin. Anaesthesiol. 2020, 34, 153–166. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bustamante, A.; Frendl, G.; Sprung, J.; Kor, D.J.; Subramaniam, B.; Martinez-Ruiz, R.; Lee, J.-W.; Henderson, W.G.; Moss, A.; Mehdiratta, N.; et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017, 152, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabate, S.; Mazo, V.; Briones, Z.; Sanchis, J.; et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, C.; Romero, C.; Tusman, G.; Suarez-Sipmann, F.; Canet, J.; Dosdá, R.; Valls, P.; Villena, A.; Serralta, F.; Jurado, A.; et al. The accuracy of postoperative, noninvasive Air-Test to diagnose atelectasis in healthy patients after surgery: A prospective, diagnostic pilot study. BMJ Open 2017, 7, e015560. [Google Scholar] [CrossRef]
- Jammer, I.B.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM); Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: A statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar]
- Ntutumu, R.; Liu, H.; Zhen, L.; Hu, Y.F.; Mou, T.Y.; Lin, T.; Yu, J.; Li, G.X. Risk factors for pulmonary complications following laparoscopic gastrectomy: A single-center study. Medicine 2016, 95, e4567. [Google Scholar] [CrossRef]
- Gong, J.; Xu, L.; Yu, H.; Qiu, F.; Zhang, Z.; Yin, Y.; Ma, H.; Cai, Z.; Zhong, J.; Ding, W.; et al. Increased postoperative complications after laparoscopic gastrectomy in patients with preserved ratio impaired spirometry. J. Gastrointest. Surg. 2024, 28, 889–895. [Google Scholar] [CrossRef]
- Yu, J.; Park, J.Y.; Kim, D.H.; Kim, S.; Hwang, J.H.; Seo, H.; Kim, Y.-K. Incidence and Risk Factors of Pulmonary Complications after Robot-Assisted Laparoscopic Prostatectomy: A Retrospective Observational Analysis of 2208 Patients at a Large Single Center. J. Clin. Med. 2019, 8, 1509. [Google Scholar] [CrossRef]
- Erice, F.; Fox, G.S.; Salib, Y.M.; Romano, E.; Meakins, J.L.; Magder, S.A. Diaphragmatic function before and after laparoscopic cholecystectomy. Anesthesiology 1993, 79, 966–975. [Google Scholar] [CrossRef]
- Ferreyra, G.; Long, Y.; Ranier, V.M. Respiratory complications after major surgery. Curr. Opin. Crit. Care 2009, 15, 342–348. [Google Scholar] [CrossRef]
- Brooks-Brunn, J.A. Predictors of postoperative pulmonary complications following abdominal surgery. Chest 1997, 111, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.W.; Tan, W.C.; Zhao, B.C.; Wen, S.H.; Shen, J.T.; Xu, M. Intraoperative ventilation strategies to prevent postoperative pulmonary complications: A network meta-analysis of randomised controlled trials. Br. J. Anaesth. 2020, 124, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef] [PubMed]
- Qadir, N.; Sahetya, S.; Munshi, L.; Summers, C.; Abrams, D.; Beitler, J.; Bellani, G.; Brower, R.G.; Burry, L.; Chen, J.-T.; et al. An Update on Management of Adult Patients with Acute Respiratory Distress Syndrome: An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2023, 209, 24–36. [Google Scholar] [CrossRef]
- Barbosa, F.T.; Castro, A.A.; de Sousa-Rodrigues, C.F. Positive end-expiratory pressure (PEEP) during anesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst Rev. 2014, 2014, CD007922. [Google Scholar]
- Karalapillai, D.; Weinberg, L.; Neto, A.S.; Peyton, P.J.; Ellard, L.; Hu, R.; Pearce, B.; Tan, C.O.; Story, D.; O’donnell, M.; et al. Low tidal volume ventilation for patients undergoing laparoscopic surgery: A secondary analysis of a randomised clinical trial. BMC Anesthesiol. 2023, 23, 71. [Google Scholar] [CrossRef]
- El-Khatib, M.; Zeeni, C.; Shebbo, F.M.; Karam, C.; Safi, B.; Toukhtarian, A.; Nafeh, N.A.; Mkhayel, S.; Shadid, C.A.; Chalhoub, S.; et al. Intraoperative mechanical power and postoperative pulmonary complications in low-risk surgical patients: A prospective observational cohort study. BMC Anesthesiol. 2024, 24, 82. [Google Scholar] [CrossRef]
- Wood, C.B.; Shinn, J.R.; Rees, A.B.; Patel, P.N.; Freundlich, R.E.; Smith, D.K.; McEvoy, M.D.; Rohde, S.L. Existing Predictive Models for Postoperative Pulmonary Complications Perform Poorly in a Head and Neck Surgery Population. J. Med. Syst. 2019, 43, 312. [Google Scholar] [CrossRef]
- Ferrando, C.; Suárez-Sipmann, F.; Librero, J.; Pozo, N.; Soro, M.; Unzueta, C.; Brunelli, A.; Peiró, S.; Llombart, A.; Balust, J.; et al. A noninvasive postoperative clinical score to identify patients at risk for postoperative pulmonary complications: The Air-Test Score. Minerva Anestesiol. 2020, 86, 404–415. [Google Scholar] [CrossRef]
- Ko, E.; Yoo, K.Y.; Lim, C.H.; Jun, S.; Lee, K.; Kim, Y.H. Is atelectasis related to the development of postoperative pneumonia? a retrospective single center study. BMC Anesthesiol. 2023, 23, 77. [Google Scholar] [CrossRef]
- Dhillon, G.; Buddhavarapu, V.S.; Grewal, H.; Munjal, R.; Verma, R.K.; Surani, S.; Kashyap, R. Evidence-based Practice Interventions for Reducing Postoperative Pulmonary Complications: A Narrative Review. Open Respir. Med. J. 2023, 17, e18743064271499. [Google Scholar] [CrossRef]
- Atilla, N.; Arpag, H.; Bozkus, F.; Kahraman, H.; Cengiz, E.; Bulbuloglu, E.; Atilla, S. Can We Predict the Perioperative Pulmonary Complications Before Laparoscopic Sleeve Gastrectomy: Original Research. Obes. Surg. 2017, 27, 1524–1528. [Google Scholar] [CrossRef]
- Neto, A.S.; da Costa, L.G.V.; Hemmes, S.N.T.; Canet, J.; Hedenstierna, G.; Jaber, S.; Hiesmayr, M.; Hollmann, M.; Mills, G.; Vidal Melo, M.; et al. The LAS VEGAS risk score for prediction of postoperative pulmonary complications: An observational study. Eur. J. Anaesthesiol. 2018, 35, 691–701. [Google Scholar] [CrossRef]
- Canet, J.; Sabaté, S.; Mazo, V.; Gallart, L.; de Abreu, M.G.; Belda, J.; Langeron, O.; Hoeft, A.; Pelosi, P. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: A prospective, observational study. Eur. J. Anaesthesiol. 2015, 32, 458–470. [Google Scholar] [CrossRef]
- Piccioni, F.; Spagnesi, L.; Pelosi, P.; Bignami, E.; Guarnieri, M.; Fumagalli, L.; Polati, E.; Schweiger, V.; Comi, D.; D’Andrea, R.; et al. Postoperative pulmonary complications and mortality after major abdominal surgery. An observational multicenter prospective study. Minerva Anestesiol. 2023, 89, 964–976. [Google Scholar] [CrossRef]
| Study Population | All Patients (n = 250) | PPC Group (n = 36) | Non PPC Group (n = 214) | p-Value | |
|---|---|---|---|---|---|
| Age, years | 61.5 ± 14.6 | 68.6 ± 8.8 | 60.4 ± 15 | 0.02 | |
| Male, n (%) | 137 (54.8) | 117 (54.7) | 20 (55.6) | 0.922 | |
| BMI, kg·m−1 | 27.8 ± 5.8 | 27.1 ± 4.6 | 28 ± 5.9 | 0.373 | |
| ASA physical status | 1, n (%) | 19 (7.6) | 0 | 19(8.9) | 0.185 |
| 2, n (%) | 141 (56.4) | 20 (55.6) | 121 (56.5) | ||
| 3, n (%) | 87 (34.8) | 16 (44.4) | 71 (32.3) | ||
| 4, n (%) | 3 (1.2) | 0 (0) | 3 (1.4) | ||
| ARISCAT score | 33.2 ± 12.6 | 33.5 ± 12.5 | 33.5 ± 12.7 | 0.914 | |
| FRAIL Score | 1.52 ± 0.64 | 1.58 ± 0.6 | 1.51 ± 0.63 | 0.549 | |
| Preoperative SpO2, % | 97.6 ± 1.5 | 97.3 ± 1.67 | 97.6 ± 1.5 | 0.209 | |
| Smoking status | Smoker, n (%) | 62 (24.8) | 10 (27.8) | 52 (24.3) | 0.678 |
| Nonsmoker, n (%) | 188 (75.2) | 26 (72.2) | 162 (75.7) | ||
| Comorbidities | COPD, n (%) | 12 (4.8) | 2 (5.6) | 10 (4.7) | 0.685 |
| Arterial hypertension, n (%) | 124 (49.6) | 19 (52.8) | 105 (49.1) | 0.721 | |
| Diabetes Mellitus, n (%) | 66 (26.4) | 11 (30.6) | 55 (25.7) | 0.544 | |
| Ischemic heart disease, n (%) | 8 (3.2) | 0 (0) | 8(3.7) | 0.607 | |
| Obesity, n (%) | 60 (24) | 6 (16.7) | 54 (25.2) | 0.3 | |
| Dyslipidaemia, n (%) | 119 (47.6) | 21 (58.3) | 98 (45.8) | 0.207 | |
| Indication for surgery | Colorectal cancer, n (%) | 93 (37.2) | 11(30.5) | 82 (38.3) | 0.563 |
| Cholelithiasis, n (%) | 83 (33.2) | 13 (36.1) | 70 (32.7) | ||
| Bariatric surgery, n (%) | 25 (10) | 4 (11.1) | 21(8.7) | ||
| Gastric cancer, n (%) | 45 (18) | 7 (19.4) | 38 (17.7) | ||
| Liver cancer, n (%) | 4 (1.6) | 2 (0.5) | 2 (0.93) | ||
| All Patients (n = 250) | PPC Group (n = 36) | Non PPC Group (n = 214) | p-Value | |
|---|---|---|---|---|
| Upper abdominal surgery, n (%) | 93 (37.2) | 18(50) | 75 (35) | 0.086 |
| Intraoperative bleeding, mL | 165.5 ± 156.5 | 202.5 ± 169 | 159.3 ± 153 | 0.126 |
| Length of surgery, min | 142.7 ± 108.1 | 173.4 ± 133 | 135 ± 86.3 | 0.025 |
| Tidal volume predicted body weight, mL·kg−1 | 7.8 ± 1.2 | 7.9 ± 1.1 | 7.7 ± 1.2 | 0.570 |
| PEEP, cmH2O | 6.5 ± 1.9 | 6.69 ± 2.2 | 6.49 ± 1.84 | 0.555 |
| Peak pressure, cmH2O | 23 ± 4.5 | 22.81 ± 5.5 | 23.20 ± 4.4 | 0.638 |
| FiO2, % | 57.4 ± 9.3 | 57.42 ± 7.5 | 57.42 ± 9.6 | 0.998 |
| Compliance, mL·cmH2O−1 | 34.7 ± 8.9 | 33.34 ± 8.5 | 34.95 ± 9 | 0.319 |
| Alveolar recruitment maneuver, n (%) | 64 (25.6) | 11 (30.6) | 53 (24.8) | 0.536 |
| Withdrawal ETT with positive pressure, n (%) | 98 (39.2) | 12 (33.3) | 89 (40.2) | 0.467 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Preoperative variables | ||||
| Male | 0.965 (0.474–1.96) | 0.922 | ||
| Age > 60 years | 2.34 (1.07–5.10) | 0.032 | 2.29 (1.03–5.08) | 0.041 |
| BMI >30, kg·m−2 | 2.34 (1.07–5.10) | 0.423 | ||
| COPD | 0.83 (0.17–3.9) | 0.8119 | ||
| Smoker | 0.83 (0.37–1.85) | 0.655 | ||
| ARISCAT score | 0.82 (0.46–1.46) | 0.519 | ||
| Preoperative SpO2 < 96% | 1.72 (0.32–1.22) | 0.172 | ||
| Arterial hypertension | 1.22 (0.61–1.82) | 0.765 | ||
| Diabetes mellitus | 0.83 (0.56–1.95) | 0.983 | ||
| Ischemic heart disease | 2.14 (1.12–4.10) | 0.125 | ||
| Dyslipidaemia | 0.53 (0.41–1.95) | 0.111 | ||
| Obesity | 2.11 (1.71–3.30) | 0.238 | ||
| Intraoperative variables | ||||
| Lower laparoscopic surgery | 1.85 (0.1–3.77) | 0.089 | ||
| Alveolar recruitment maneuver | 0.74 (0.34–1.62) | 0.463 | ||
| Withdrawal ETT with positive pressure | 1.34 (0.63–2.89) | 0.437 | ||
| Tidal volume predicted body weight, mL·kg−1 | 1.15 (0.53–2.47) | 0.567 | ||
| PEEP, cmH2O | 1.24 (0.73–2.69) | 0.456 | ||
| FiO2, % | 1.05 (0.23–1.47) | 0.234 | ||
| Peak pressure, cmH2O | 2.15 (0.53–2.47) | 0.952 | ||
| Compliance < 30, mL·cmH2O−1 | 1.15 (0.53–2.47) | 0.831 | ||
| Postoperative variables | ||||
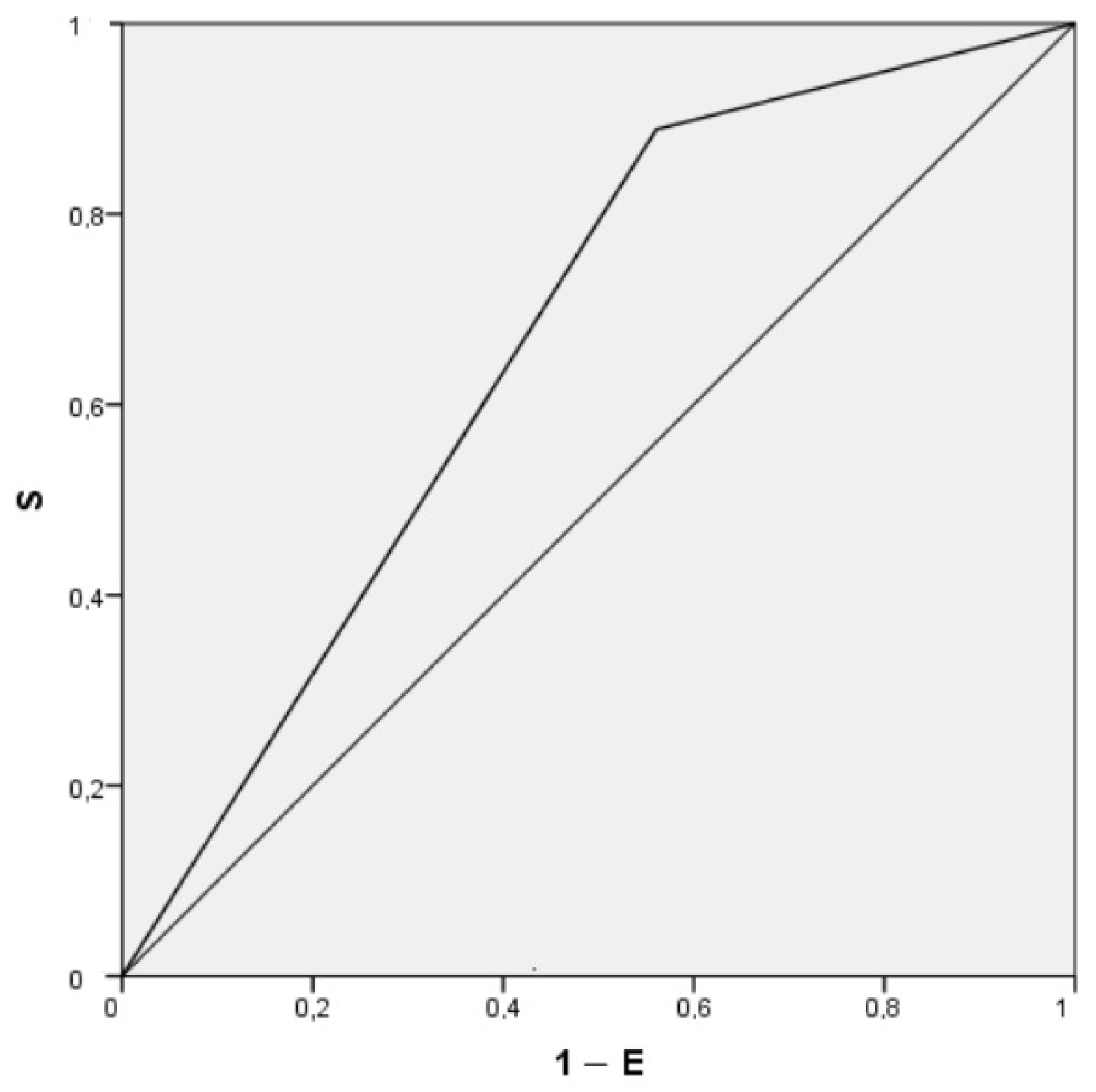
| Positive Air Test | 6.26 (2.14–18.34) | 0.001 | 6.22 (2.11–18.22) | 0.001 |
| All Patients (n = 250) | PPC Group (n = 36) | Non PPC Group (n = 214) | p-Value | |
|---|---|---|---|---|
| VAS | 3.46 ± 2 | 3.89 ± 2.2 | 3.39 ± 2 | 0.185 |
| Air test, % | 95.4 ± 2.98 | 93.97 ± 3 | 95.68 ± 2.9 | 0.001 |
| Positive Air test, n (%) | 152 (60.8) | 32 (88.9) | 120 (56.1) | 0.000 |
| Cardiovascular complications, n (%) | 12 (4.8) | 2 (5.6) | 10 (4.7) | 0.685 |
| Infectious complications, n (%) | 37 (14.8) | 11 (30) | 26 (12.1) | 0.009 |
| Other complications, n (%) | ||||
| Adynamic ileus | 13 (5.2) | 5 (13.8) | 8 (3.7) | 0.025 |
| Postoperative nausea and vomiting | 44 (17.6) | 10 (27.7) | 34 (15.8) | 0.098 |
| Delirium | 4 (1.6) | 2 (5.8) | 2 (0.9) | 0.1 |
| Acute kidney injury | 7 (2.8) | 2 (5.8) | 5 (2.3) | 0.266 |
| PACU stay, hours | 9 ± 15.4 | 15.9 ± 25.8 | 7.95 ± 12.7 | 0.005 |
| Hospital stay, days | 4.63 ± 5.7 | 8.2 ± 11.8 | 4 ± 3.6 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia, L.; Becerra-Bolaños, Á.; Rodríguez-Sánchez, R.; Ojeda, N.; Rodríguez-Pérez, A. Postoperative Pulmonary Complications After Laparoscopic Surgery: Risk Factors and Predictive Scores. Medicina 2025, 61, 1635. https://doi.org/10.3390/medicina61091635
Valencia L, Becerra-Bolaños Á, Rodríguez-Sánchez R, Ojeda N, Rodríguez-Pérez A. Postoperative Pulmonary Complications After Laparoscopic Surgery: Risk Factors and Predictive Scores. Medicina. 2025; 61(9):1635. https://doi.org/10.3390/medicina61091635
Chicago/Turabian StyleValencia, Lucía, Ángel Becerra-Bolaños, Rocío Rodríguez-Sánchez, Nazario Ojeda, and Aurelio Rodríguez-Pérez. 2025. "Postoperative Pulmonary Complications After Laparoscopic Surgery: Risk Factors and Predictive Scores" Medicina 61, no. 9: 1635. https://doi.org/10.3390/medicina61091635
APA StyleValencia, L., Becerra-Bolaños, Á., Rodríguez-Sánchez, R., Ojeda, N., & Rodríguez-Pérez, A. (2025). Postoperative Pulmonary Complications After Laparoscopic Surgery: Risk Factors and Predictive Scores. Medicina, 61(9), 1635. https://doi.org/10.3390/medicina61091635







