Retrospective Analysis of Therapeutic Modalities in Prosthetic Heart Valve Thrombosis: A 15-Year Single-Center Experience
Abstract
1. Introduction
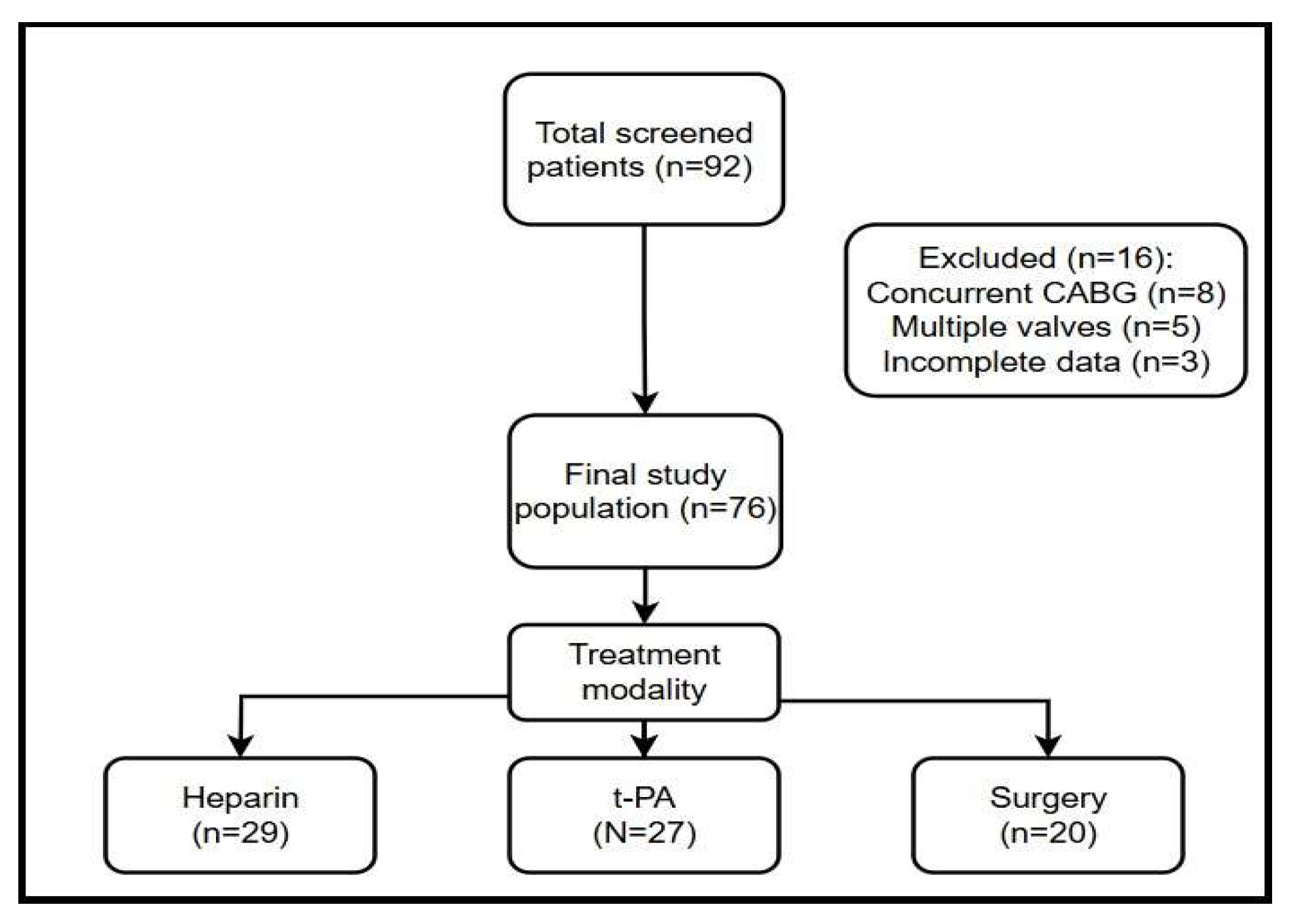
2. Materials and Methods
2.1. Treatment Strategies
2.2. Statistical Analysis
3. Results
3.1. Baseline Demographics and Clinical Characteristics
3.2. Treatment Modalities and Success Rates
3.3. Complication Analysis
4. Discussion
4.1. Treatment Efficacy and Comparative Outcomes
4.2. The NYHA Functional Class as a Prognostic Determinant
4.3. Thrombus Characteristics and Clinical Decision-Making
4.4. Anticoagulation Management and Prevention
4.5. Pregnancy and Special Populations
4.6. Implications for Clinical Practice
4.7. Future Directions
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EF | Ejection Fraction |
| CI | Confidence Interval |
| ESC | European Society of Cardiology |
| ICH | Intracranial Hemorrhage |
| INR | International Normalised Ratio |
| NYHA | New York Heart Association |
| PVT | Prosthetic Valve Thrombosis |
| TEE | Transesophageal Echocardiography |
| TTE | Transtoragic Echocardiography |
| t-PA | tissue Plasminogen Activator |
| UFH | Unfractionated Heparin |
References
- Renzulli, A.; Onorati, F.; De Feo, M.; Vitale, N.; Esposito, S.; Agozzino, L. Mechanical valve thrombosis: A tailored approach for a multiplex disease. J. Heart Valve Dis. 2004, 13 (Suppl 1), S37–S42. [Google Scholar] [PubMed]
- Núñez-Gil, I.J.; Alkhouli, M.; Centola, M.; Feltes, G.; Villablanca, P.; Ramakrishna, H. Analysis of Bioprosthetic Aortic Valve Thrombosis-Implications and Management Strategies. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, D.; Manzo, R.; Castiello, D.S.; Molaro, M.I.; Mariani, A.; lapicca, C.; Nappa, D.; Simonetti, F.; Leone, A.; Canonico, M.E.; et al. Echocardiographic Evaluation after Transcatheter Aortic Valve Implantation: A Comprehensive Review. Life 2023, 13, 1079. [Google Scholar] [CrossRef] [PubMed]
- Schaff, H.V. Progress in Management of Mechanical Valve Thrombosis. J. Am. Coll. Cardiol. 2022, 79, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.W.A.; Ruel, M.; Graeve, A.; Gerdisch, M.W.; Damiano, R.J.; Smith, R.L. Low-Dose vs Standard Warfarin After Mechanical Mitral Valve Replacement: A Randomized Trial. Ann. Thorac. Surg. 2023, 115, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Olie, R.H.; Winckers, K.; Rocca, B.; Ten Cate, H. Oral Anticoagulants Beyond Warfarin. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Özkan, M.; Gündüz, S.; Biteker, M.; Astarcioglu, M.A.; Çevik, C.; Kaynak, E. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: The TROIA trial. JACC Cardiovasc. Imaging 2013, 6, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.P.; Sun, J.C.; Fremes, S.E.; Rubens, F.D.; Teoh, K.H. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e576S–e600S. [Google Scholar] [CrossRef] [PubMed]
- Özkan, M.; Gündüz, S.; Güner, A.; Kalçık, M.; Gürsoy, M.O.; Uygur, B. Thrombolysis or Surgery in Patients With Obstructive Mechanical Valve Thrombosis: The Multicenter HATTUSHA Study. J. Am. Coll. Cardiol. 2022, 79, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sporn, Z.A.; Schaff, H.V.; Pellikka, P.A. ACC/AHA Versus ESC Guidelines on Prosthetic Heart Valve Management: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 73, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Lóriga, F.M.; Pérez-López, H.; Morlans-Hernández, K.; Facundo-Sánchez, H.; Santos-Gracia, J.; Valiente-Mustelier, J. Thrombolysis as first choice therapy in prosthetic heart valve thrombosis. A study of 68 patients. J. Thromb. Thrombolysis 2006, 21, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.Z.; Azzam, M.; Swailum, A.A.H.; Farouk, A. Early Outcomes of Emergency Surgery for Left-Sided Mechanical Valve Thrombosis. Heart Surg. Forum 2021, 24, E983–E987. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, K.H.; Kwan, C.K.; Chang, Y.K. Surgical Management of Mechanical Valve Thrombosis: Twenty-Six Years’ Experience. J. Korean Med. Sci. 2008, 23, 378. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.N.; Gehrig, T.; Bashore, T.M.; Kiefer, T.L. Treatment of mechanical aortic valve thrombosis with heparin and eptifibatide. J. Thromb. Thrombolysis 2014, 38, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Akins, C.W.; Miller, D.C.; Turina, M.I.; Kouchoukos, N.T.; Blackstone, E.H.; Grunkemeier, G.L. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann. Thorac. Surg. 2008, 85, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Castilho, F.M.; De Sousa, M.R.; Mendonça, A.L.P.; Ribeiro, A.L.P.; Cáceres-Lóriga, F.M. Thrombolytic therapy or surgery for valve prosthesis thrombosis: Systematic review and meta-analysis. J. Thromb. Haemost. 2014, 12, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, G.; Senguttuvan, N.B.; Joseph, J.; Devasenapathy, N.; Bahl, V.K.; Airan, B. Urgent surgery compared with fibrinolytic therapy for the treatment of left-sided prosthetic heart valve thrombosis: A systematic review and meta-analysis of observational studies. Eur. Heart J. 2013, 34, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Vándor, L. The role of thrombolysis in the management of left-sided prosthetic valve thrombosis: A study of 85 cases diagnosed by transesophageal echocardiography. J. Heart Valve Dis. 2001, 10, 636–649. [Google Scholar] [PubMed]
| Parameter | Overall (n = 76) | UFH (n = 29) | t-PA (n = 27) | Surgery (n = 20) | p-Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years (mean ± SD) | 53 ± 17 | 52 ± 18 | 50 ± 15 | 57 ± 17 | 0.47 |
| Female, n (%) | 48 (63.2) | 19 (65.5) | 18 (66.7) | 11 (55.0) | 0.72 |
| Pregnancy †, n (%) | 11/48 (22.9) | 5/19 (26.3) | 4/18 (22.2) | 2/11 (18.2) | 0.91 |
| Comorbidities, n (%) | |||||
| Atrial fibrillation | 30 (39.5) | 11 (37.9) | 10 (37.0) | 9 (45.0) | 0.88 |
| Hypertension | 28 (36.8) | 10 (34.5) | 9 (33.3) | 9 (45.0) | 0.77 |
| Diabetes mellitus | 15 (19.7) | 6 (20.7) | 5 (18.5) | 4 (20.0) | 0.98 |
| Laboratory | |||||
| INR at presentation, (median, range) | 1.60 (0.99–5.41) | 1.55 (1.0–3.5) | 1.60 (1.1–5.4) | 1.65 (1.2–4.5) | 0.62 |
| INR ≥ 2.5, n (%) | 16 (21.1) | 5 (17.2) | 6 (22.2) | 5 (25.0) | 0.81 |
| Valve type, n (%) | |||||
| Mechanical valve | 74 (97.4) | 28 (96.6) | 27 (100) | 19 (95.0) | 0.55 |
| Bioprosthetic valve | 2 (2.6) | 1 (3.4) | 0 (0) | 1 (5.0) | - |
| Valve position, n (%) | |||||
| Mitral position | 54 (71.1) | 20 (69.0) | 20 (74.1) | 14 (70.0) | 0.92 |
| Aortic position | 20 (26.3) | 8 (27.6) | 6 (22.2) | 6 (30.0) | 0.84 |
| Tricuspid position | 2 (2.6) | 1 (3.4) | 1 (3.7) | 0 (0) | 0.66 |
| Prosthesis age, years (median, range) | 7.5 (0.1–28) | 7.3 (0.2–25) | 7.6 (0.3–26) | 7.8 (0.1–28) | 0.89 |
| Clinical presentation, n (%) | |||||
| Acute heart failure | 37 (48.7) | 12 (41.4) | 13 (48.1) | 12 (60.0) | 0.56 |
| Cerebrovascular event | 17 (22.4) | 6 (20.7) | 6 (22.2) | 5 (25.0) | 0.94 |
| Peripheral embolism | 9 (11.8) | 3 (10.3) | 3 (11.1) | 3 (15.0) | 0.88 |
| Asymptomatic | 7 (9.2) | 3 (10.3) | 2 (7.4) | 2 (10.0) | 0.93 |
| NYHA I–II | 54 (71.1) | 20 (69.0) | 20 (74.1) | 14 (70.0) | 0.93 |
| NYHA III–IV | 22 (28.9) | 9 (31.0) | 7 (25.9) | 6 (30.0) | 0.93 |
| Parameter, mmHg (Median, Range) | Overall (n = 76) | UFH (n = 29) | t-PA (n = 27) | Surgery (n = 20) | p-Value |
|---|---|---|---|---|---|
| Mitral max gradient, | 20 (9–49) | 18 (9–40) | 20 (10–45) | 22 (12–49) | 0.41 |
| Mitral mean gradient, mmHg (median, range) | 10 (3.5–28) | 9 (3.5–20) | 10 (4–24) | 11 (4–28) | 0.39 |
| Aortic max gradient, mmHg (median, range) | 43 (14–120) | 40 (14–100) | 42 (15–110) | 45 (20–120) | 0.55 |
| Aortic mean gradient, mmHg (median, range) | 27 (8–80) | 25 (8–75) | 26 (10–78) | 30 (15–80) | 0.58 |
| Thrombus diameter, mm (median, range) | 10 (2–47) | 9 (2–25) | 10 (3–30) | 12 (5–47) | 0.44 |
| Thrombus surface area, mm2 (median, range) | 70 (3–1457) | 65 (5–850) | 70 (10–900) | 85 (15–1457) | 0.48 |
| Left ventricular EF, % (mean ± SD) | 51.7 ± 9.6 | 52.5 ± 9.3 | 51.2 ± 10.1 | 51.0 ± 9.5 | 0.83 |
| Outcome | UFH (n = 29) | t-PA (n = 27) | Surgery (n = 20) | p-Value |
|---|---|---|---|---|
| Treatment success, n (%) | 20 (69.0%) | 16 (59.3%) | 10 (50.0%) | 0.405 |
| Mortality, n (%) | 7 (24.1%) | 2 (7.4%) | 10 (50.0%) | 0.004 |
| Complication Type | UFH (n = 29) | t-PA (n = 27) | Surgery (n = 20) | Overall (n = 76) | p-Value |
|---|---|---|---|---|---|
| Embolic Events | |||||
| Major embolic events | 2 (6.9%) | 1 (3.7%) | 1 (5.0%) | 4 (5.3%) | 0.865 |
| Minor embolic events | 1 (3.4%) | 2 (7.4%) | 0 (0.0%) | 3 (3.9%) | 0.429 |
| Total embolic events | 3 (10.3%) | 3 (11.1%) | 1 (5.0%) | 8 (10.5%) | 0.727 |
| Bleeding Events | |||||
| Major bleeding | 2 (6.9%) | 0 (0.0%) | 3 (15.0%) | 5 (6.6%) | 0.122 |
| Minor bleeding | 3 (10.3%) | 2 (7.4%) | 2 (10.0%) | 8 (10.5%) | 0.921 |
| Intracranial hemorrhage | 1 (3.4%) | 2 (7.4%) | 0 (0.0%) | 3 (3.9%) | 0.372 |
| Other Complications | |||||
| Prosthesis dysfunction | 1 (3.4%) | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) | 0.440 |
| Infective endocarditis | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 1 (1.3%) | 0.242 |
| Wound infection | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 1 (1.3%) | 0.242 |
| Total patients with complications ‡ | 9 (31.0%) | 5 (18.5%) | 8 (40.0%) | 32 (42.1%) | 0.262 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| NYHA III–IV vs. I–II | 12.64 | 1.91–83.85 | 0.009 |
| Obstructive thrombus | 5.64 | 0.34–93.58 | 0.227 |
| t-PA treatment | 0.21 | 0.02–2.37 | 0.207 |
| Surgery | 3.10 | 0.43–22.46 | 0.263 |
| Age (per year) | 1.02 | 0.95–1.08 | 0.646 |
| INR at presentation | 1.36 | 0.13–14.28 | 0.796 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzunselvi, A.; Yüksel, S.; Uyanik, M. Retrospective Analysis of Therapeutic Modalities in Prosthetic Heart Valve Thrombosis: A 15-Year Single-Center Experience. Medicina 2025, 61, 1629. https://doi.org/10.3390/medicina61091629
Uzunselvi A, Yüksel S, Uyanik M. Retrospective Analysis of Therapeutic Modalities in Prosthetic Heart Valve Thrombosis: A 15-Year Single-Center Experience. Medicina. 2025; 61(9):1629. https://doi.org/10.3390/medicina61091629
Chicago/Turabian StyleUzunselvi, Alper, Serkan Yüksel, and Muhammet Uyanik. 2025. "Retrospective Analysis of Therapeutic Modalities in Prosthetic Heart Valve Thrombosis: A 15-Year Single-Center Experience" Medicina 61, no. 9: 1629. https://doi.org/10.3390/medicina61091629
APA StyleUzunselvi, A., Yüksel, S., & Uyanik, M. (2025). Retrospective Analysis of Therapeutic Modalities in Prosthetic Heart Valve Thrombosis: A 15-Year Single-Center Experience. Medicina, 61(9), 1629. https://doi.org/10.3390/medicina61091629







