Impact of Automated Insulin Delivery Systems in Children and Adolescents with Type 1 Diabetes Previously Treated with Multiple Daily Injections: A Single-Center Real-World Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Analysis of Glucose Outcomes and Insulin Data Across the Study
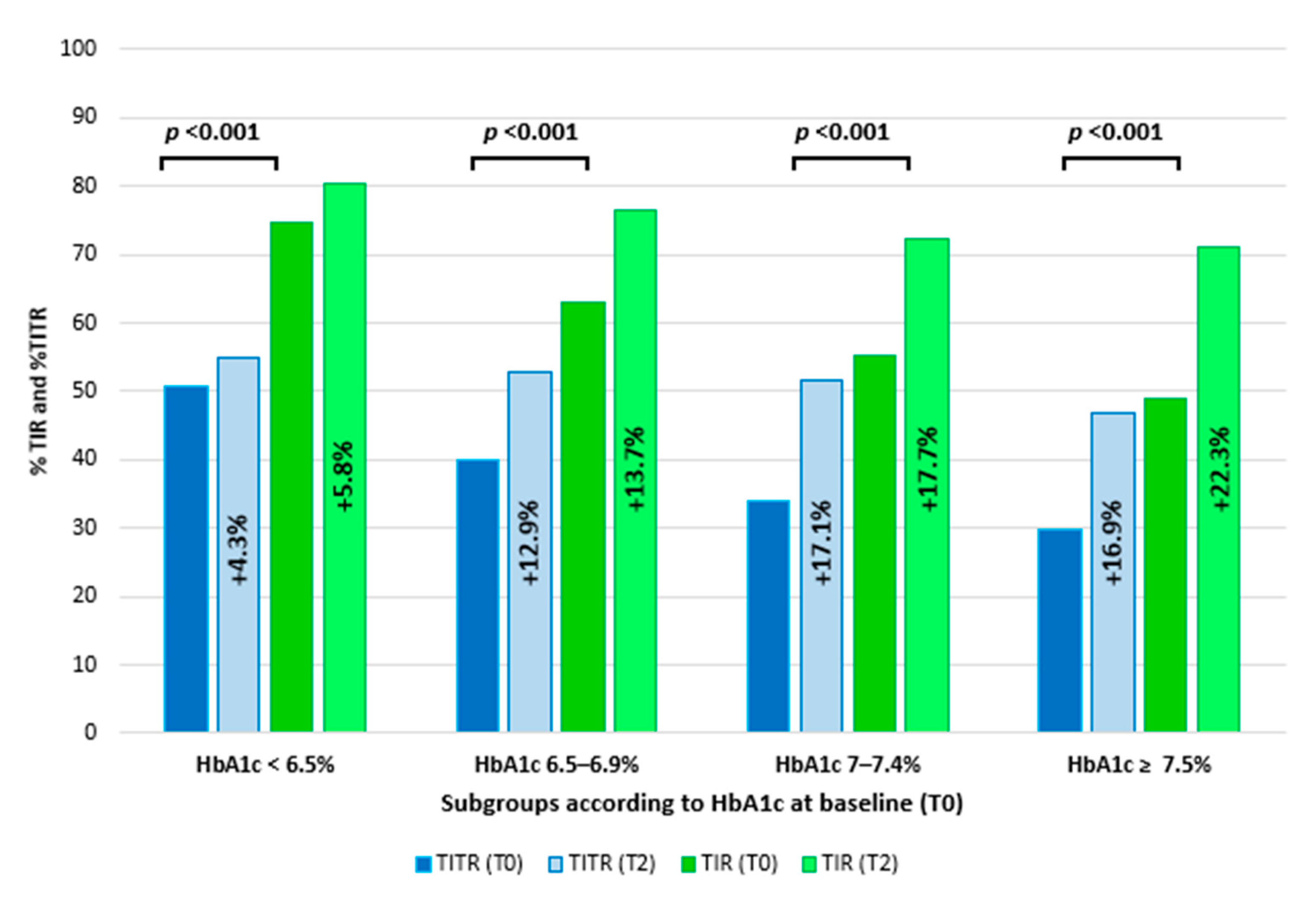
3.2. Comparison of Clinical Data and Glucose Metrics Between Different Subgroups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phillip, M.; Nimri, R.; Bergenstal, R.M.; Barnard-Kelly, K.; Danne, T.; Hovorka, R.; Kovatchev, B.P.; Messer, L.H.; Parkin, C.G.; Ambler-Osborn, L.; et al. Consensus Recommendations for the Use of Automated Insulin Delivery Technologies in Clinical Practice. Endocr. Rev. 2023, 44, 254–280. [Google Scholar] [CrossRef] [PubMed]
- Biester, T.; Berget, C.; Boughton, C.; Cudizio, L.; Ekhlaspour, L.; Hilliard, M.E.; Reddy, L.; Um, S.S.N.; Schoelwer, M.; Sherr, J.L.; et al. International Society for Pediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines 2024: Diabetes Technologies—Insulin Delivery. Horm. Res. Paediatr. 2024, 97, 636–662. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.; Lepore, G.; Battelino, T.; Arrieta, A.; Castañeda, J.; Grossman, B.; Shin, J.; Cohen, O. Real-World Performance of the MiniMedTM 780G System: First Report of Outcomes from 4120 Users. Diabetes Technol. Ther. 2022, 24, 113–119. [Google Scholar] [CrossRef]
- Arrieta, A.; Battelino, T.; Scaramuzza, A.E.; Da Silva, J.; Castañeda, J.; Cordero, T.L.; Shin, J.; Cohen, O. Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: Evidence from 12,870 real-world users. Diabetes Obes. Metab. 2022, 24, 1370–1379. [Google Scholar] [CrossRef]
- De Meulemeester, J.; Keymeulen, B.; De Block, C.; Van Huffel, L.; Taes, Y.; Ballaux, D.; Spincemaille, K.; Lapauw, B.; Vanhaverbeke, G.; Lowyck, I.; et al. One-year real-world benefits of Tandem Control-IQ technology on glucose management and person-reported outcomes in adults with type 1 diabetes: A prospective observational cohort study. Diabetologia 2025, 68, 948–960. [Google Scholar] [CrossRef]
- Graham, R.; Mueller, L.; Manning, M.; Habif, S.; Messer, L.H.; Pinsker, J.E.; Aronoff-Spencer, E. Real-World Use of Control-IQ Technology Is Associated with a Lower Rate of Severe Hypoglycemia and Diabetic Ketoacidosis Than Historical Data: Results of the Control-IQ Observational (CLIO) Prospective Study. Diabetes Technol. Ther. 2024, 26, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.E.; Chong, K.; Senior, P.A.; Lam, A.; Palumbo, P. A scoping review of Do-It-Yourself Automated Insulin Delivery system (DIY AID) use in people with type 1 diabetes. PLoS ONE 2022, 17, e0271096. [Google Scholar] [CrossRef]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Wang, J.; Rodbard, D.; Kohn, M.A.; Li, C.; Liepmann, D.; Kerr, D.; Ahn, D.; Peters, A.L.; Umpierrez, G.E.; et al. A glycemia risk index (GRI) of hypoglycemia and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J. Diabetes Sci. Technol. 2023, 17, 1226–1242. [Google Scholar] [CrossRef]
- Passanisi, S.; Lombardo, F.; Mameli, C.; Bombaci, B.; Macedoni, M.; Zuccotti, G.; Dovc, K.; Battelino, T.; Salzano, G.; Delvecchio, M. Safety, Metabolic and Psychological Outcomes of Medtronic MiniMed 780G™ in Children, Adolescents and Young Adults: A Systematic Review. Diabetes Ther. 2024, 15, 343–365. [Google Scholar] [CrossRef]
- Mameli, C.; Smylie, G.M.; Marigliano, M.; Zagaroli, L.; Mancioppi, V.; Maffeis, C.; Salpietro, V.; Zuccotti, G.; Delvecchio, M. Safety and Psychological Outcomes of Tandem t:Slim X2 Insulin Pump with Control-IQ Technology in Children, Adolescents, and Young Adults with Type 1 Diabetes: A Systematic Review. Diabetes Ther. 2024, 15, 2133–2149. [Google Scholar] [CrossRef]
- Michou, P.; Gkiourtzis, N.; Christoforidis, A.; Kotanidou, E.P.; Galli-Tsinopoulou, A. The efficacy of automated insulin delivery systems in children and adolescents with type 1 diabetes Mellitus: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2023, 199, 110678. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, V.; Faragalli, A.; Arnaldi, C.; Bassi, M.; Bonfanti, R.; Bracciolini, G.P.; Cardella, F.; Bo, S.D.; Delvecchio, M.; Di Candia, F.; et al. Glucometrics and device satisfaction in children and adolescents with type 1 diabetes using different treatment modalities: A multicenter real-world observational study. Diabetes Res. Clin. Pract. 2024, 210, 111621. [Google Scholar] [CrossRef]
- Passanisi, S.; Piona, C.; Salzano, G.; Marigliano, M.; Bombaci, B.; Morandi, A.; Alibrandi, A.; Maffeis, C.; Lombardo, F. Aiming for the Best Glycemic Control Beyond Time in Range: Time in Tight Range as a new CGM Metric in Children and Adolescents with Type 1 Diabetes using Different Treatment Modalities. Diabetes Technol. Ther. 2024, 26, 161–166. [Google Scholar] [CrossRef]
- Schiaffini, R.; Lumaca, A.; Martino, M.; Rapini, N.; Deodati, A.; Amodeo, M.E.; Ciampalini, P.; Matteoli, M.C.; Pampanini, V.; Cianfarani, S. Time In Tight Range in children and adolescents with type 1 diabetes: A cross-sectional observational single centre study evaluating efficacy of new advanced technologies. Diabetes Metab. Res. Rev. 2024, 40, e3826. [Google Scholar] [CrossRef]
- Dovc, K.; Lanzinger, S.; Cardona-Hernandez, R.; Tauschmann, M.; Marigliano, M.; Cherubini, V.; Preikša, R.; Schierloh, U.; Clapin, H.; AlJaser, F.; et al. Association of Achieving Time in Range Clinical Targets with Treatment Modality Among Youths with Type 1 Diabetes. JAMA Netw. Open 2023, 6, e230077. [Google Scholar] [CrossRef] [PubMed]
- Passanisi, S.; Salzano, G.; Bombaci, B.; Minuto, N.; Bassi, M.; Bonfanti, R.; Scialabba, F.; Mozzillo, E.; Di Candia, F.; Monti, S.; et al. Sustained Effectiveness of an Advanced Hybrid Closed-Loop System in a Cohort of Children and Adolescents with Type 1 Diabetes: A 1-Year Real-World Study. Diabetes Care 2024, 47, 1084–1091. [Google Scholar] [CrossRef]
- Seget, S.; Chobot, A.; Tarasiewicz, M.; Bielawska, A.; Rusak, E.; Ochab, A.; Polanska, J.; Jarosz-Chobot, P. Glycemic control in children with type 1 diabetes treated with the advanced hybrid closed loop system 2-year prospective, observational, two-center study. Front. Endocrinol. 2024, 15, 1332418. [Google Scholar] [CrossRef]
- Bismuth, É.; Tubiana-Rufi, N.; Rynders, C.A.; Dalla-Vale, F.; Bonnemaison, E.; Coutant, R.; Farret, A.; Poidvin, A.; Bouhours-Nouet, N.; Storey, C.; et al. Sustained 3-Year Improvement of Glucose Control with Hybrid Closed Loop in Children with Type 1 Diabetes While Going Through Puberty. Diabetes Care 2024, 47, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Breton, M.D.; Kovatchev, B.P. One Year Real-World Use of the Control-IQ Advanced Hybrid Closed-Loop Technology. Diabetes Technol. Ther. 2021, 23, 601–608. [Google Scholar] [CrossRef]
- Santova, A.; Plachy, L.; Neuman, V.; Pavlikova, M.; Petruzelkova, L.; Konecna, P.; Venhacova, P.; Skvor, J.; Pomahacova, R.; Neumann, D.; et al. Are all HCL systems the same? long term outcomes of three HCL systems in children with type 1 diabetes: Real-life registry-based study. Front. Endocrinol. 2023, 14, 1283181. [Google Scholar] [CrossRef] [PubMed]
- Beato-Víbora, P.I.; Chico, A.; Moreno-Fernandez, J.; Azriel-Mira, S.; Nattero-Chávez, L.; Mora, R.V.; Alonso-Carril, N.; Simó-Servat, O.; Aguilera-Hurtado, E.; Céspedes, L.M.R.; et al. Transitioning between automated insulin delivery systems: A focus on personalisation. Diabetes Res. Clin. Pract. 2025, 222, 112070. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.; Patti, L.; Silvestrini, I.; Strati, M.F.; Ponzano, M.; Minuto, N.; Maggi, D. One-year follow-up comparison of two hybrid closed-loop systems in Italian children and adults with type 1 diabetes. Front. Endocrinol. 2023, 14, 1099024. [Google Scholar] [CrossRef] [PubMed]
- Piona, C.; Passanisi, S.; Bombaci, B.; Marigliano, M.; Lombardo, F.; Mancioppi, V.; Morandi, A.; Maffeis, C.; Salzano, G.; ISPED Diabetes Study Group. Time in tight range in automated insulin delivery system users: Real-world data from children and adolescents with type 1 diabetes. Diabetes Obes. Metab. 2024, 26, 4767–4771. [Google Scholar] [CrossRef]
- Henry, Z.; Fimbel, S.V.; Bendelac, N.; Perge, K.; Thivolet, C. Beneficial effects of automated insulin delivery over one-year follow-up in real life for youths and adults with type 1 diabetes irrespective of patient characteristics. Diabetes Obes. Metab. 2024, 26, 557–566. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous glucose monitoring and metrics for clinical trials: An international consensus statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef]
- Pinsker, J.E.; Lee, J.B.; Dassau, E.; Seborg, D.E.; Bradley, P.K.; Gondhalekar, R.; Bevier, W.C.; Huyett, L.; Zisser, H.C.; Doyle, F.J. Randomized Crossover Comparison of Personalized MPC and PID Control Algorithms for the Artificial Pancreas. Diabetes Care 2016, 39, 1135–1142. [Google Scholar] [CrossRef]
- Cinar, A. Automated Insulin Delivery Algorithms. Diabetes Spectr. 2019, 32, 209–214. [Google Scholar] [CrossRef]
- Shannon, N.; Castañeda, J.; Choudhary, P.; Kolassa, R.; Keuthage, W.; Kroeger, J.; Thivolet, C.; Evans, M.; Ré, R.; Cellot, J.; et al. Twelve-month results of the ADAPT randomized controlled trial: Reproducibility and sustainability of advanced hybrid closed-loop therapy outcomes versus conventional therapy in adults with type 1 diabetes. Diabetes Obes. Metab. 2023, 25, 3212–3222. [Google Scholar]
- Choudhary, P.; Kolassa, R.; Keuthage, W.; Kroeger, J.; Thivolet, C.; Evans, M.; Ré, R.; de Portu, S.; Vorrink, L.; Shin, J.; et al. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): A randomised controlled study. Lancet Diabetes Endocrinol. 2022, 10, 720–731. [Google Scholar] [CrossRef]
- Castorani, V.; Rigamonti, A.; Frontino, G.; Morotti, E.; Sandullo, F.; Scialabba, F.; Arrigoni, F.; Dionisi, B.; Foglino, R.; Morosini, C.; et al. Turning the tides: Achieving rapid and safe glucose control in adolescents with suboptimally controlled type 1 diabetes using advanced hybrid closed loop systems. Front. Endocrinol. 2024, 15, 1243565. [Google Scholar] [CrossRef]
- Boucsein, A.; Watson, A.S.; Frewen, C.M.; Sanders, O.J.; Haszard, J.J.; Jones, S.D.; Milford-Hughes, P.J.; de Bock, M.I.; Wheeler, B.J. Impact of Advanced Hybrid Closed Loop on Youth with High-Risk Type 1 Diabetes Using Multiple Daily Injections. Diabetes Care 2023, 46, 628–632. [Google Scholar] [CrossRef]
- Criego, A.B.; Carlson, A.L.; Brown, S.A.; Forlenza, G.P.; Bode, B.W.; Levy, C.J.; Hansen, D.W.; Hirsch, I.B.; Bergenstal, R.M.; Sherr, J.L.; et al. Two Years with a Tubeless Automated Insulin Delivery System: A Single-Arm Multicenter Trial in Children, Adolescents, and Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2024, 26, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.; Mozzillo, E.; Di Candia, F.; Fanti, S.; Maines, E.; Guarnieri, C.; Maffeis, C.; Da Ros, A.; Piccoli, E.; Pertile, R.; et al. A Systematic Review and Metanalysis on the Impact of Automated Insulin Delivery Systems on Body Mass Index in Youths with Type 1 Diabetes and Potential Predictors. Diabetes Technol. Obes. Med. 2025, 1, 153–166. [Google Scholar] [CrossRef]
- Corbin, K.D.; Driscoll, K.A.; Pratley, R.E.; Smith, S.R.; Maahs, D.M.; Mayer-Davis, E.J. Advancing Care for Type 1 Diabetes and Obesity Network (ACT1ON). Obesity in Type 1 Diabetes: Pathophysiology, Clinical Impact, and Mechanisms. Endocr. Rev. 2018, 39, 629–663. [Google Scholar] [CrossRef] [PubMed]
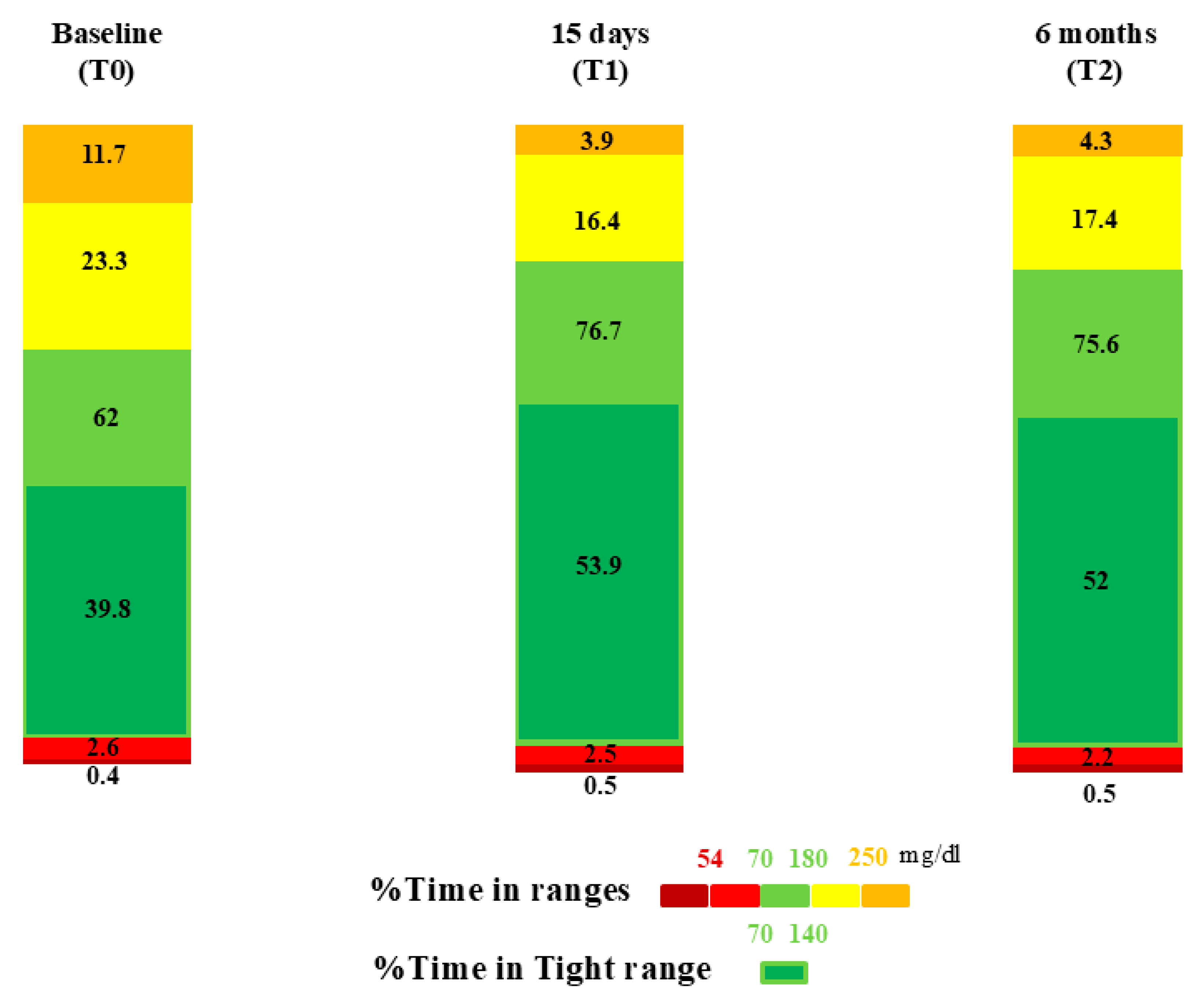
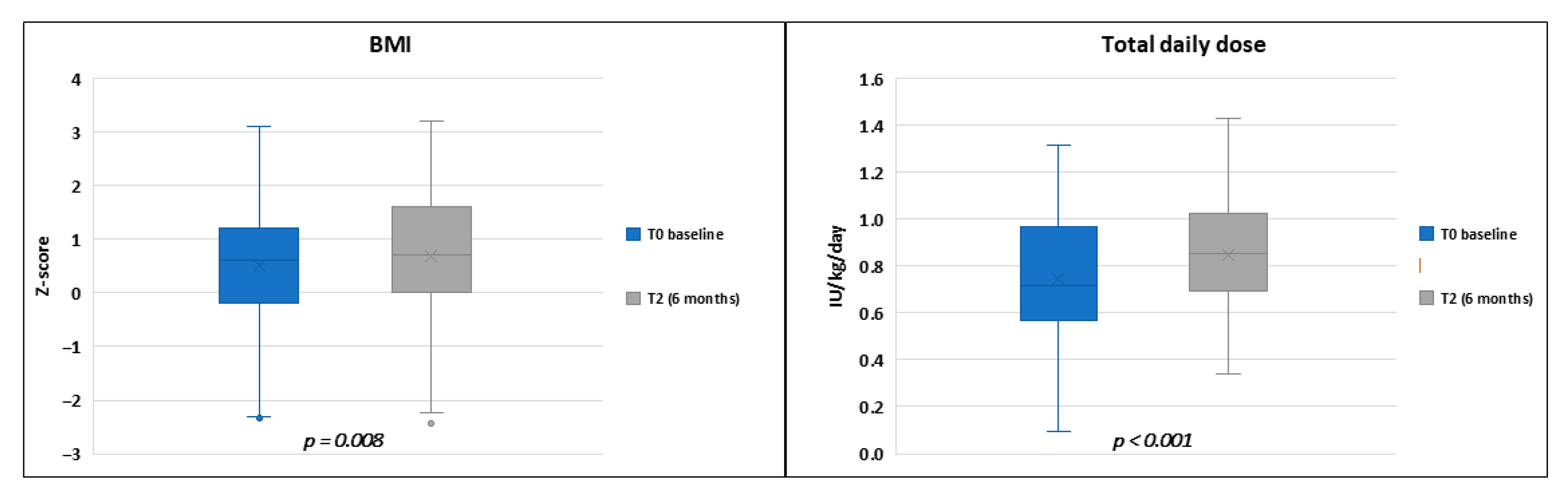
| Baseline | p a | 15 Days | p b | 6 Months | p c | |
|---|---|---|---|---|---|---|
| TIR (%) | 62 ± 15.3 | <0.001 * | 76.7 ± 8.4 | 0.253 | 75.6 ± 9.1 | <0.001 * |
| TITR 70–140 mg/dL (%) | 39.8 ± 13 | <0.001 * | 53.9 ± 10.4 | 0.130 | 52 ± 10.6 | <0.001 * |
| TAR > 180 mg/dL (%) | 34.8 ± 15.8 | <0.001 * | 20.3 ± 8.5 | 0.162 | 21.7 ± 9.7 | <0.001 * |
| TAR 180–250 mg/dL (%) | 23.3 ± 8 | <0.001 * | 16.4 ± 6.7 | 0.183 | 17.4 ± 7.5 | <0.001 * |
| TAR > 250 mg/dL (%) | 11.7 ± 9.8 | <0.001 * | 3.9 ± 3.5 | 0.270 | 4.3 ± 3.9 | <0.001 * |
| TBR < 70 mg/dL | 3 ± 3.6 | 0.515 | 3 ± 2.6 | 0.190 | 2.6 ± 2.5 | 0.180 |
| TBR 54–70 mg/dL (%) | 2.6 ± 3 | 0.389 | 2.5 ± 1.9 | 0.167 | 2.2 ± 1.9 | 0.088 |
| TBR < 54 mg/dL (%) | 0.4 ± 0.8 | 0.215 | 0.6 ± 0.9 | 0.381 | 0.5 ± 0.8 | 0.516 |
| GRI | 43 ± 21.6 | <0.001 * | 24 ± 8.7 | <0.001 * | 27.5 ± 10.3 | <0.001 * |
| GMI (%) | 7.2 ± 0.8 | <0.001 * | 6.7 ± 0.3 | 0.067 | 6.8 ± 0.4 | <0.001 * |
| Mean sensor glucose (mg/dL) | 163.4 ± 26.2 | <0.001 * | 141.3 ± 11.9 | 0.035 | 144.9 ± 14.9 | <0.001 * |
| CV (%) | 36.9 ± 6.4 | 0.005 | 35.1 ± 6.1 | 0.344 | 34.2 ± 6.1 | <0.001 * |
| 15-Day Use | 6-Month Use | |||||
|---|---|---|---|---|---|---|
| MM780G | CIQ | p-Value | MM780G | CIQ | p-Value | |
| TIR (%) | 77.6 (9.6) | 76.0 (7.4) | 0.422 | 75.9 (9.5) | 75.4 (8.7) | 0.816 |
| TITR (%) | 54.6 (11.1) | 53.4 (9.8) | 0.645 | 52.6 (8.5) | 51.5 (11.9) | 0.640 |
| TAR (%) | 20.1 (9.9) | 20.4 (7.3) | 0.896 | 22.0 (9.9) | 21.5 (9.5) | 0.808 |
| TAR1 (%) | 17.1 (8.4) | 15.8 (5.0) | 0.426 | 18.9 (8.5) | 16.3 (6.3) | 0.127 |
| TAR2 (%) | 3.0 (3.2) | 4.5 (3.5) | 0.068 | 3.1 (2.9) | 5.2 (4.3) | 0.019 * |
| TBR (%) | 2.2 (2.5) | 3.7 (2.5) | 0.015 * | 2.0 (2.0) | 3.1 (2.7) | 0.057 |
| TBR1 (%) | 1.9 (2.0) | 2.9 (1.7) | 0.034 * | 1.8 (1.6) | 2.4 (2.0) | 0.141 |
| TBR2 (%) | 0.3 (0.6) | 0.8 (1.0) | 0.031 * | 0.3 (0.5) | 0.7 (0.8) | 0.021 * |
| GRI | 21.8 (8.9) | 25.5 (8.2) | 0.129 | 25.2 (9.5) | 29.2 (10.6) | 0.475 |
| GMI (%) | 6.7 (0.3) | 6.7 (0.3) | 0.525 | 6.7 (0.3) | 6.8 (0.4) | 0.436 |
| Mean SG (mg/dL) | 140.1 (10.6) | 142.2 (12.7) | 0.468 | 143.4 (12.0) | 145.9 (16.7) | 0.467 |
| CV (%) | 33.3 (5.4) | 36.3 (6.3) | 0.035 * | 32.2 (4.8) | 35.6 (6.5) | 0.016 * |
| Insulin TDD (IU/kg) | 0.9 (0.3) | 0.9 (0.3) | 0.899 | 0.8 (0.2) | 0.8 (0.2) | 0.541 |
| Basal delivery (%) | 46.1 (8.9) | 54.7 (8.6) | <0.001 * | 40.7 (7.6) | 55.1 (9.6) | <0.001 * |
| Autocorrection boluses (%) | 33.1 (11.2) | 17.2 (10.3) | <0.001 * | 34.3 (10.7) | 17.8 (12.1) | <0.001 * |
| AutoMode use (%) | 98.4 (5.0) | 92.6 (5.9) | <0.001 * | 97.3 (4.7) | 94.1 (5.5) | 0.011 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bombaci, B.; Calderone, M.; Di Pisa, A.; La Rocca, M.; Torre, A.; Lombardo, F.; Salzano, G.; Passanisi, S. Impact of Automated Insulin Delivery Systems in Children and Adolescents with Type 1 Diabetes Previously Treated with Multiple Daily Injections: A Single-Center Real-World Study. Medicina 2025, 61, 1602. https://doi.org/10.3390/medicina61091602
Bombaci B, Calderone M, Di Pisa A, La Rocca M, Torre A, Lombardo F, Salzano G, Passanisi S. Impact of Automated Insulin Delivery Systems in Children and Adolescents with Type 1 Diabetes Previously Treated with Multiple Daily Injections: A Single-Center Real-World Study. Medicina. 2025; 61(9):1602. https://doi.org/10.3390/medicina61091602
Chicago/Turabian StyleBombaci, Bruno, Marco Calderone, Alessandra Di Pisa, Mariarosaria La Rocca, Arianna Torre, Fortunato Lombardo, Giuseppina Salzano, and Stefano Passanisi. 2025. "Impact of Automated Insulin Delivery Systems in Children and Adolescents with Type 1 Diabetes Previously Treated with Multiple Daily Injections: A Single-Center Real-World Study" Medicina 61, no. 9: 1602. https://doi.org/10.3390/medicina61091602
APA StyleBombaci, B., Calderone, M., Di Pisa, A., La Rocca, M., Torre, A., Lombardo, F., Salzano, G., & Passanisi, S. (2025). Impact of Automated Insulin Delivery Systems in Children and Adolescents with Type 1 Diabetes Previously Treated with Multiple Daily Injections: A Single-Center Real-World Study. Medicina, 61(9), 1602. https://doi.org/10.3390/medicina61091602










