The Impact of Statin Use on Sepsis Mortality: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
- Articles not in the English language;
- Animal studies;
- Non-original articles (review, medical hypothesis, letter to the editor, etc.);
- Systematic reviews and meta-analyses;
- Duplicated papers;
- Studies that could not be obtained;
- Adolescents and children with sepsis;
- Absence of established data of sepsis;
- Absence of established data of statin use;
2.4. Data Extraction
2.5. Assessment of Bias
2.6. Quantitative Analysis
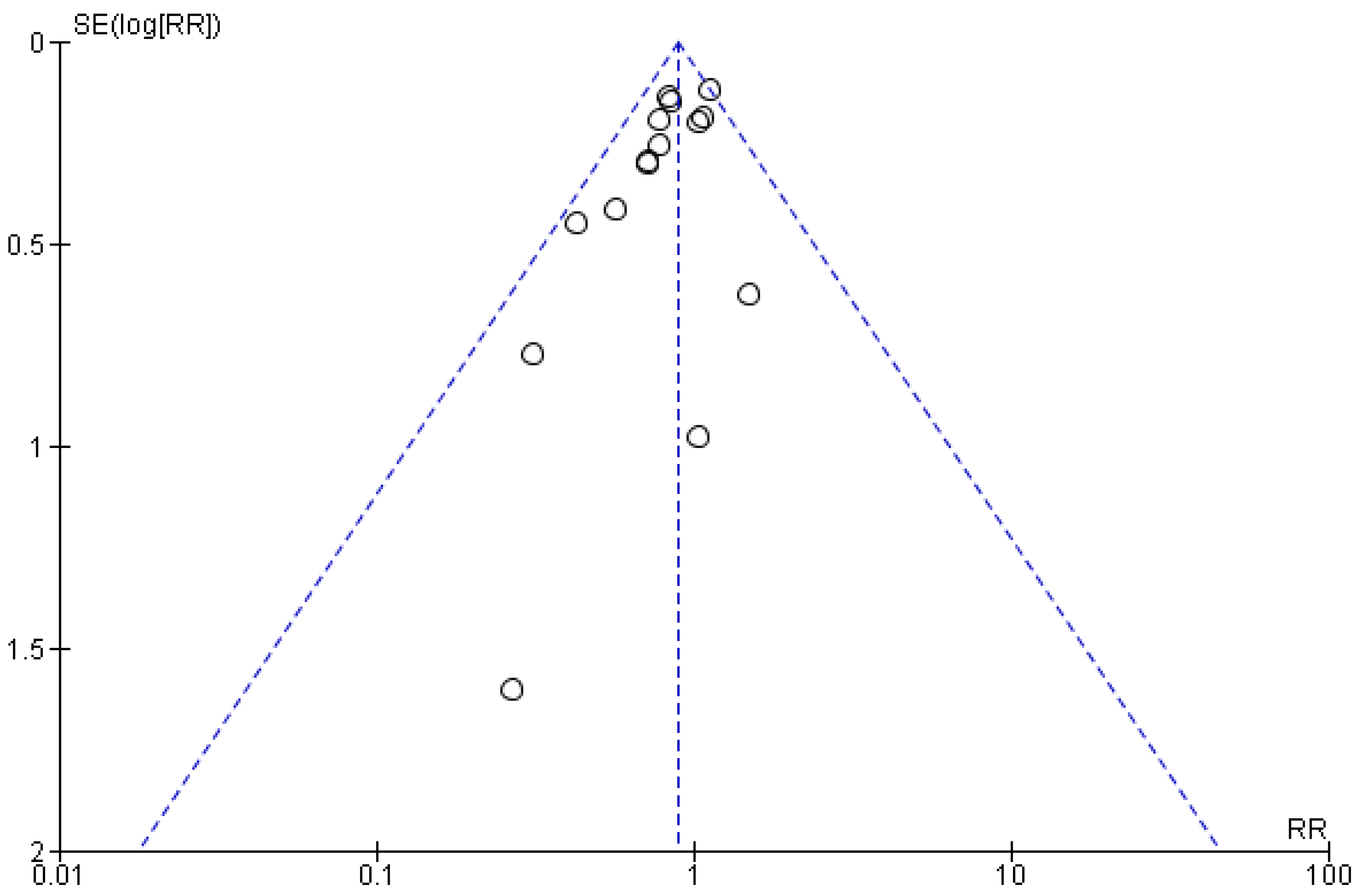
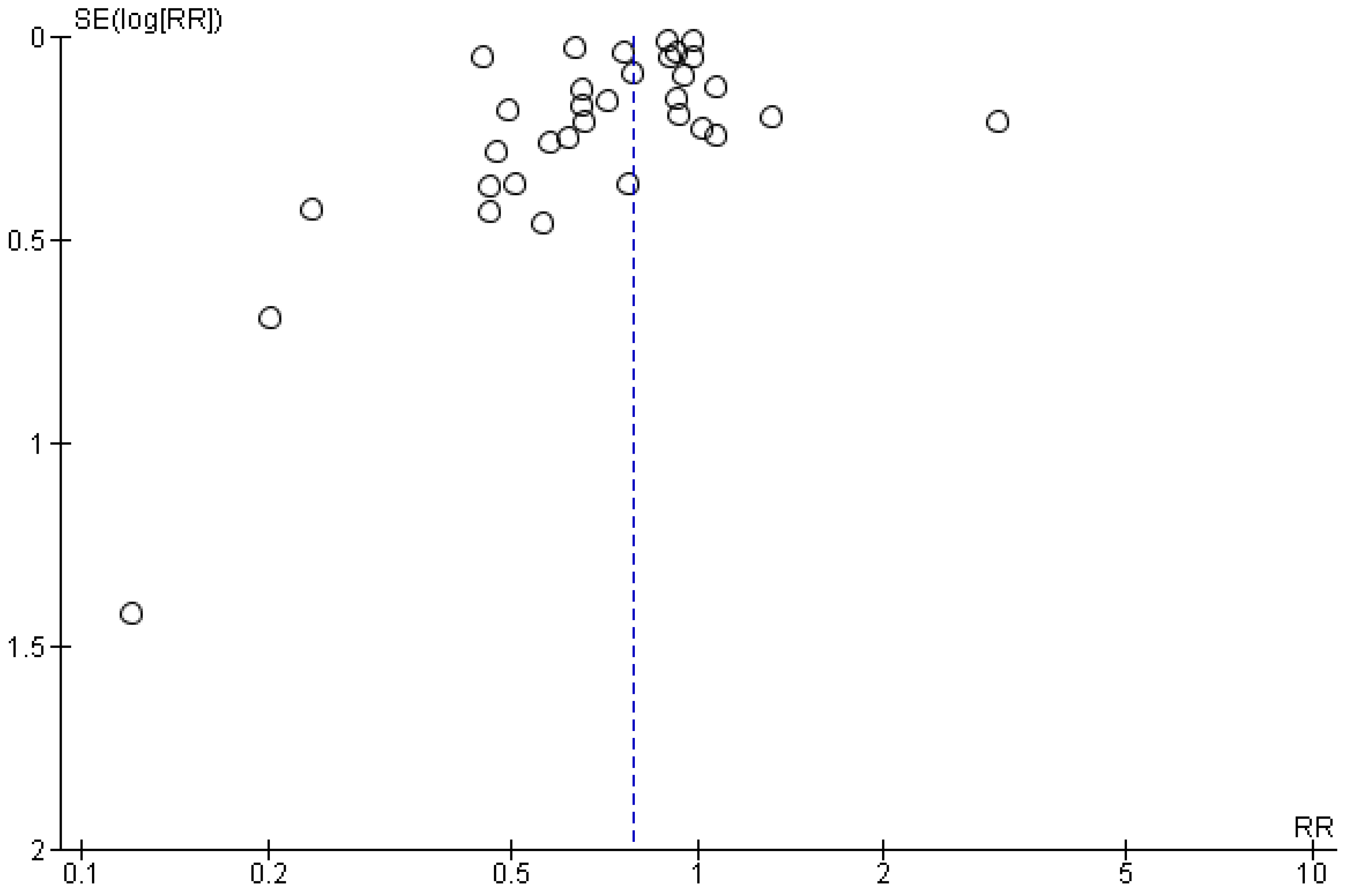
2.7. Assessment of Publication Bias
3. Results
3.1. Study Selection
3.2. Study and Population Characteristics
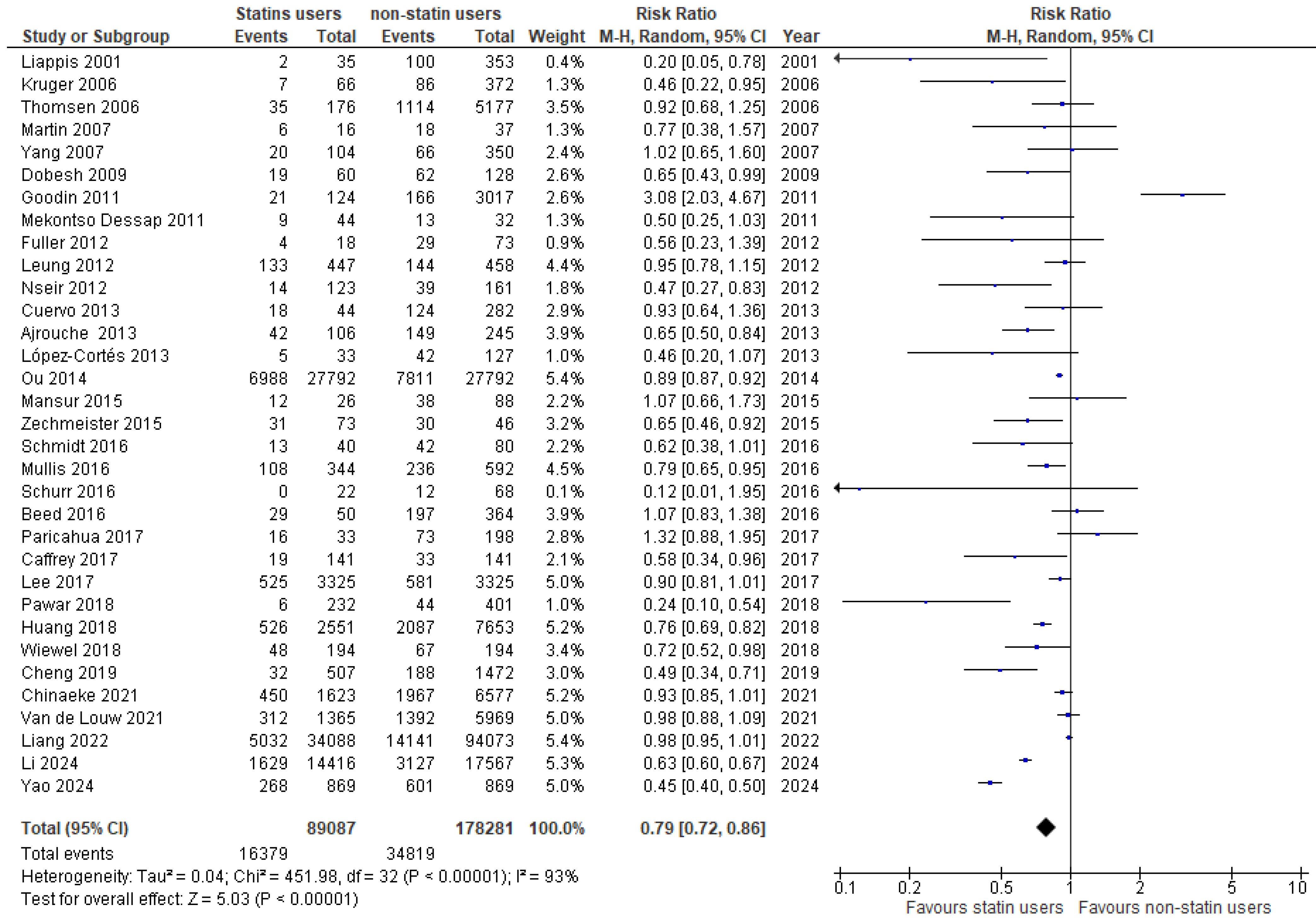
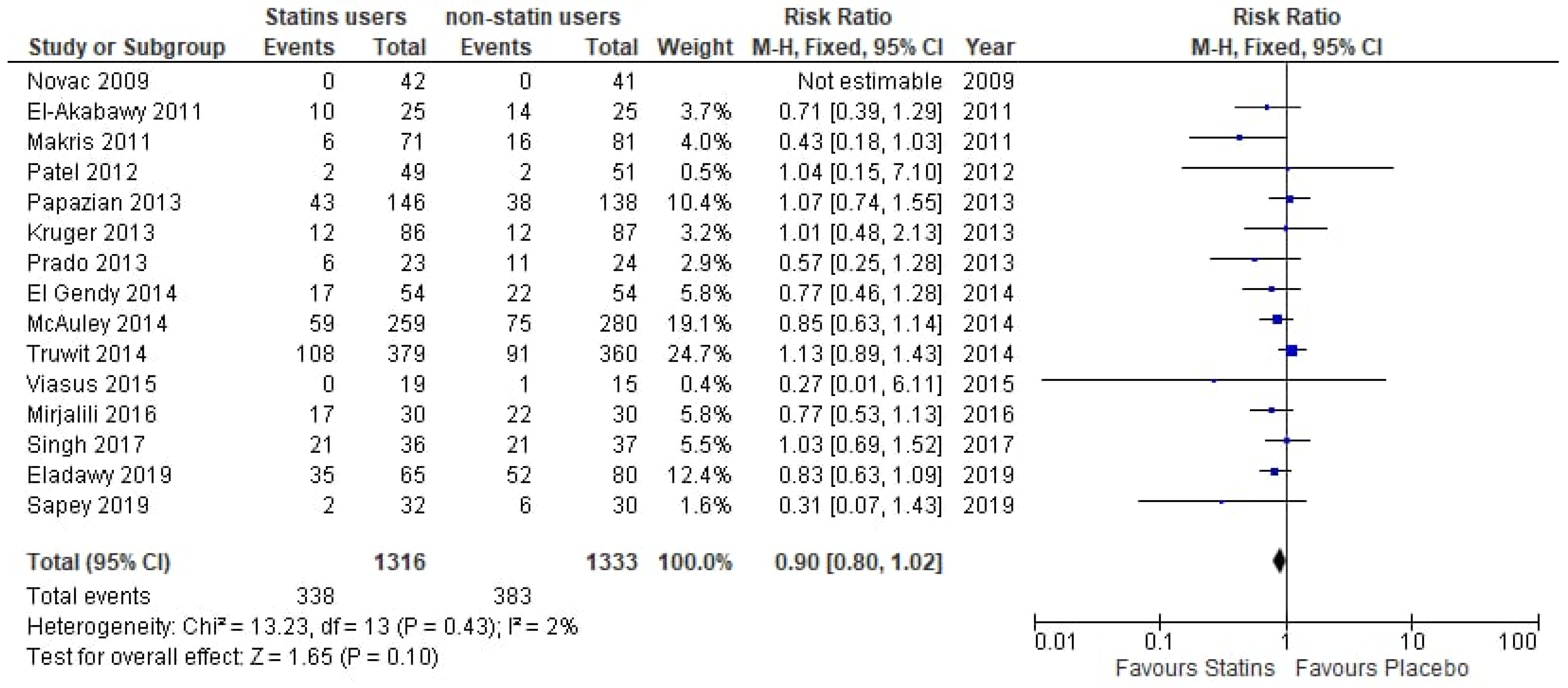
3.3. Analysis of Evidence
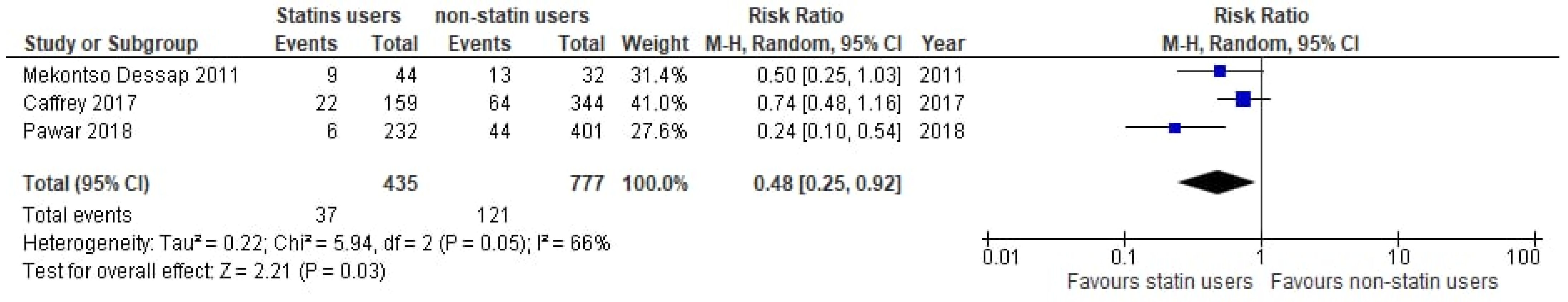
3.4. Subgroup Analyses
3.5. Sensitivity Analyses
3.6. Quality Appraisal
4. Discussion
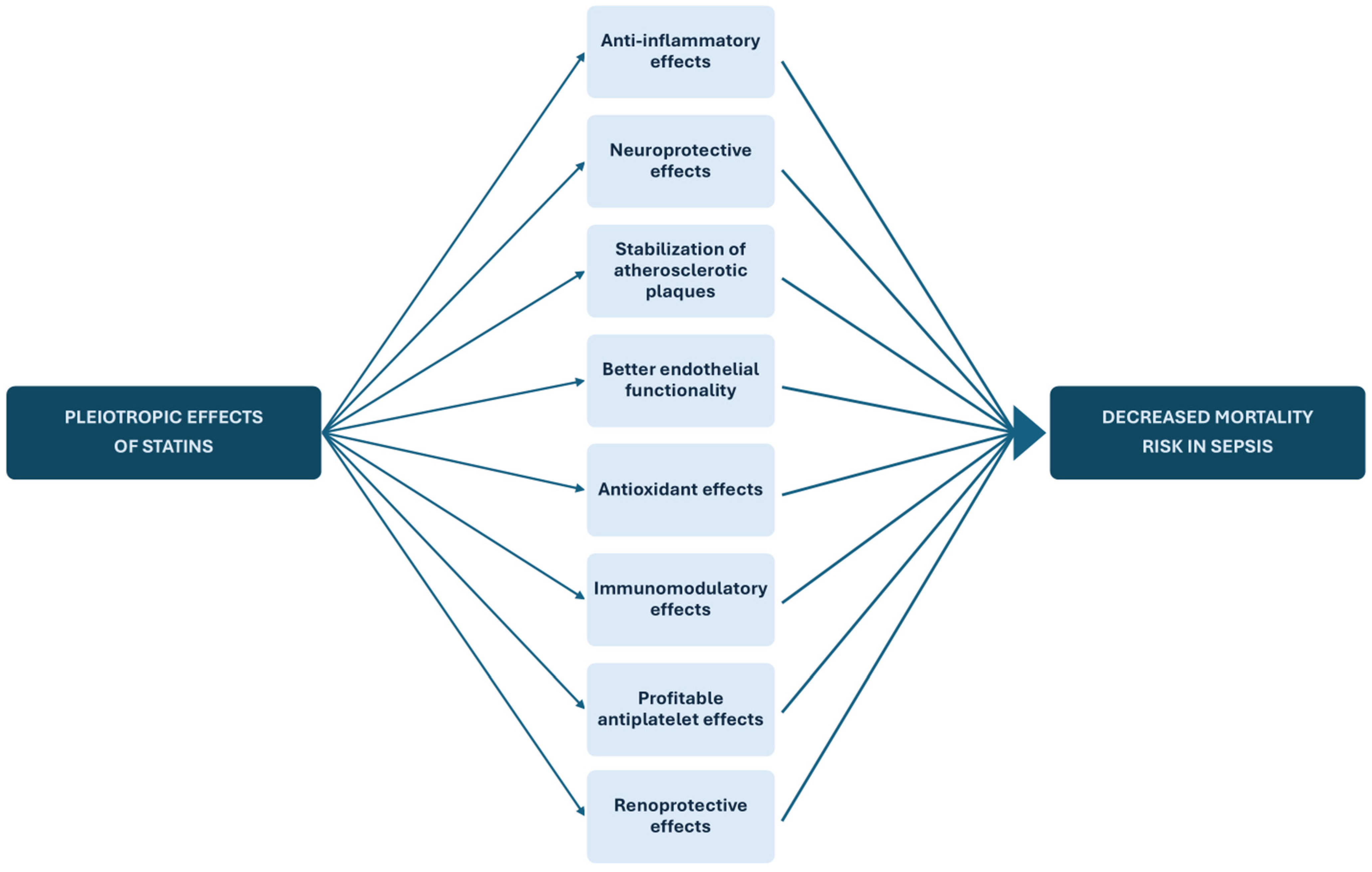
4.1. Pathophysiological Mechanisms of Statin Action on Sepsis
4.2. Strengths
4.3. Limitations
4.4. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- La Via, L.; Sangiorgio, G.; Stefani, S.; Marino, A.; Nunnari, G.; Cocuzza, S.; La Mantia, I.; Cacopardo, B.; Stracquadanio, S.; Spampinato, S.; et al. The Global Burden of Sepsis and Septic Shock. Epidemiologia 2024, 5, 456–478. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Elisaf, M.S.; Nair, D.R.; Mikhailidis, D.P. All for Statins and Statins for All; An Update. Curr. Pharm. Des. 2016, 22, 18–27. [Google Scholar] [CrossRef]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Navarese, E.P.; Kowalewski, M.; Andreotti, F.; van Wely, M.; Camaro, C.; Kolodziejczak, M.; Gorny, B.; Wirianta, J.; Kubica, J.; Kelm, M.; et al. Meta-analysis of time-related benefits of statin therapy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am. J. Cardiol. 2014, 113, 1753–1764. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B. Statins for the prevention of stroke: A meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e92388. [Google Scholar] [CrossRef]
- Kostapanos, M.S.; Milionis, H.J.; Elisaf, M.S. An overview of the extra-lipid effects of rosuvastatin. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Hraiech, S.; Loundou, A.; Truwit, J.; Kruger, P.; McAuley, D.F.; Papazian, L.; Roch, A. Statin therapy in critically-ill patients with severe sepsis: A review and meta-analysis of randomized clinical trials. Minerva Anestesiol. 2015, 81, 921–930. [Google Scholar]
- Janda, S.; Young, A.; Fitzgerald, J.M.; Etminan, M.; Swiston, J. The effect of statins on mortality from severe infections and sepsis: A systematic review and meta-analysis. J. Crit. Care 2010, 25, 656.e7–656.e22. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Makris, G.C.; Matthaiou, D.K.; Rafailidis, P.I. Statins for infection and sepsis: A systematic review of the clinical evidence. J. Antimicrob. Chemother. 2008, 61, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.D.; Sun, T.W.; Kan, Q.C.; Guan, F.X.; Zhang, S.G. Effect of statin therapy on mortality from infection and sepsis: A meta-analysis of randomized and observational studies. Crit. Care 2014, 18, R71. [Google Scholar] [CrossRef]
- Quinn, M.; Moody, C.; Tunnicliffe, B.; Khan, Z.; Manji, M.; Gudibande, S.; Murphy, N.; Whitehouse, T.; Snelson, C.; Veenith, T. Systematic review of statins in sepsis: There is no evidence of dose response. Indian J. Crit. Care Med. 2016, 20, 534–541. [Google Scholar] [CrossRef]
- Pertzov, B.; Eliakim-Raz, N.; Atamna, H.; Trestioreanu, A.Z.; Yahav, D.; Leibovici, L. Hydroxymethylglutaryl-CoA reductase inhibitors (statins) for the treatment of sepsis in adults—A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 280–289. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Novack, V.; Eisinger, M.; Frenkel, A.; Terblanche, M.; Adhikari, N.K.; Douvdevani, A.; Amichay, D.; Almog, Y. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: A randomized double-blind placebo controlled clinical trial. Intensive Care Med. 2009, 35, 1255–1260. [Google Scholar] [CrossRef]
- El-Akabawy, H.; Zaghla, H.; Samer, N.; Fahmy, A. Effect of statin therapy in early sepsis: Effect on endothelial function and prognostic implication. Med. J. Cairo Univ. 2010, 78, 113–122. [Google Scholar]
- Kruger, P.S.; Harward, M.L.; Jones, M.A.; Joyce, C.J.; Kostner, K.M.; Roberts, M.S.; Venkatesh, B. Continuation of statin therapy in patients with presumed infection: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2011, 183, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.; Manoulakas, E.; Komnos, A.; Papakrivou, E.; Tzovaras, N.; Hovas, A.; Zintzaras, E.; Zakynthinos, E. Effect of pravastatin on the frequency of ventilator-associated pneumonia and on intensive care unit mortality: Open-label, randomized study. Crit. Care Med. 2011, 39, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M.; Snaith, C.; Thickett, D.R.; Linhartova, L.; Melody, T.; Hawkey, P.; Barnett, A.H.; Jones, A.; Hong, T.; Cooke, M.W.; et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Crit. Care 2012, 16, R231. [Google Scholar] [CrossRef]
- Prado, K.; Ribeiro, C.; Furian, T.; Pinto Ribeiro, R.; Silvello, D.; Rohde, L.; Clausell, N.; Becker, L. A clinical randomized trial on the use of atorvastatin in patients with sepsis or septic shock: Effects on endothelial function. Crit. Care 2013, 17, P61. [Google Scholar] [CrossRef]
- Kruger, P.; Bailey, M.; Bellomo, R.; Cooper, D.J.; Harward, M.; Higgins, A.; Howe, B.; Jones, D.; Joyce, C.; Kostner, K.; et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am. J. Respir. Crit. Care Med. 2013, 187, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Roch, A.; Charles, P.E.; Penot-Ragon, C.; Perrin, G.; Roulier, P.; Goutorbe, P.; Lefrant, J.Y.; Wiramus, S.; Jung, B.; et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: A randomized clinical trial. JAMA 2013, 310, 1692–1700. [Google Scholar] [CrossRef]
- El Gendy, H.A.; Elsharnouby, N.M. Safety and vasopressor effect of rosuvastatin in septic patients. Egypt. J. Anaesth. 2014, 30, 311–317. [Google Scholar] [CrossRef][Green Version]
- Truwit, J.D.; Bernard, G.R.; Steingrub, J.; Matthay, M.A.; Liu, K.D.; Albertson, T.E.; Brower, R.G.; Shanholtz, C.; Rock, P.; Douglas, I.S.; et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N. Engl. J. Med. 2014, 370, 2191–2200. [Google Scholar] [CrossRef]
- McAuley, D.F.; Laffey, J.G.; O’Kane, C.M.; Perkins, G.D.; Mullan, B.; Trinder, T.J.; Johnston, P.; Hopkins, P.A.; Johnston, A.J.; McDowell, C.; et al. Simvastatin in the acute respiratory distress syndrome. N. Engl. J. Med. 2014, 371, 1695–1703. [Google Scholar] [CrossRef]
- Viasus, D.; Garcia-Vidal, C.; Simonetti, A.F.; Dorca, J.; Llopis, F.; Mestre, M.; Morandeira-Rego, F.; Carratalà, J. The effect of simvastatin on inflammatory cytokines in community-acquired pneumonia: A randomised, double-blind, placebo-controlled trial. BMJ Open 2015, 5, e006251. [Google Scholar] [CrossRef]
- Mirjalili, M.R.; Shiranian, F.; Rahimian, M.; Borjian, L.; Dehghani, A. The effect of simvastatinon the outcome of patients with sepsis: A clinical trial study. Acta Medica Mediterr. 2016, 32, 1203–1206. [Google Scholar]
- Singh, R.K.; Agarwal, V.; Baronia, A.K.; Kumar, S.; Poddar, B.; Azim, A. The Effects of Atorvastatin on Inflammatory Responses and Mortality in Septic Shock: A Single-center, Randomized Controlled Trial. Indian. J. Crit. Care Med. 2017, 21, 646–654. [Google Scholar] [CrossRef]
- Eladawy, S.; Bazan, N.S.; Elgendy, M.A.A.; Zaki, M.A. PSY7 Cost-Effective Analysis for Simvastatin Plus Standard Therapy Versus Standard Therapy Alone in Critically Ill Septic Patients. Value Health 2019, 22, S375. [Google Scholar] [CrossRef]
- Sapey, E.; Patel, J.M.; Greenwood, H.; Walton, G.M.; Grudzinska, F.; Parekh, D.; Mahida, R.Y.; Dancer, R.C.A.; Lugg, S.T.; Howells, P.A.; et al. Simvastatin Improves Neutrophil Function and Clinical Outcomes in Pneumonia. A Pilot Randomized Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Liappis, A.P.; Kan, V.L.; Rochester, C.G.; Simon, G.L. The effect of statins on mortality in patients with bacteremia. Clin. Infect. Dis. 2001, 33, 1352–1357. [Google Scholar] [CrossRef]
- Thomsen, R.W.; Hundborg, H.H.; Johnsen, S.P.; Pedersen, L.; Sørensen, H.T.; Schønheyder, H.C.; Lervang, H.H. Statin use and mortality within 180 days after bacteremia: A population-based cohort study. Crit. Care Med. 2006, 34, 1080–1086. [Google Scholar] [CrossRef]
- Schmidt, H.; Hennen, R.; Keller, A.; Russ, M.; Müller-Werdan, U.; Werdan, K.; Buerke, M. Association of statin therapy and increased survival in patients with multiple organ dysfunction syndrome. Intensive Care Med. 2006, 32, 1248–1251. [Google Scholar] [CrossRef]
- Kruger, P.; Fitzsimmons, K.; Cook, D.; Jones, M.; Nimmo, G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006, 32, 75–79. [Google Scholar] [CrossRef]
- Martin, C.P.; Talbert, R.L.; Burgess, D.S.; Peters, J.I. Effectiveness of statins in reducing the rate of severe sepsis: A retrospective evaluation. Pharmacotherapy 2007, 27, 20–26. [Google Scholar] [CrossRef]
- Yang, K.C.; Chien, J.Y.; Tseng, W.K.; Hsueh, P.R.; Yu, C.J.; Wu, C.C. Statins do not improve short-term survival in an oriental population with sepsis. Am. J. Emerg. Med. 2007, 25, 494–501. [Google Scholar] [CrossRef]
- Dobesh, P.P.; Klepser, D.G.; McGuire, T.R.; Morgan, C.W.; Olsen, K.M. Reduction in mortality associated with statin therapy in patients with severe sepsis. Pharmacotherapy 2009, 29, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Goodin, J.; Manrique, C.; Dulohery, M.; Sampson, J.; Saettele, M.; Dabbagh, O. Effect of statins on the clinical outcomes of patients with sepsis. Anaesth. Intensive Care 2011, 39, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Mekontso Dessap, A.; Ouanes, I.; Rana, N.; Borghi, B.; Bazin, C.; Katsahian, S.; Hulin, A.; Brun-Buisson, C. Effects of discontinuing or continuing ongoing statin therapy in severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2011, 15, R171. [Google Scholar] [CrossRef]
- Leung, S.; Pokharel, R.; Gong, M.N. Statins and outcomes in patients with bloodstream infection: A propensity-matched analysis. Crit. Care Med. 2012, 40, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.M.; Gajera, M.; Schorr, C.; Gerber, D.; Dellinger, R.P.; Zanotti, S. The association of prior statin use in septic shock treated with early goal directed therapy. Eur. J. Emerg. Med. 2012, 19, 226–230. [Google Scholar] [CrossRef]
- Nseir, W.; Mograbi, J.; Abu-Elheja, O.; Bishara, J.; Assy, N. The impact of prior long-term versus short-term statin use on the mortality of bacteraemic patients. Infection 2012, 40, 41–48. [Google Scholar] [CrossRef]
- Cuervo, G.; Garcia-Vidal, C.; Nucci, M.; Puchades, F.; Fernández-Ruiz, M.; Mykietiuk, A.; Manzur, A.; Gudiol, C.; Pemán, J.; Viasus, D.; et al. Effect of statin use on outcomes of adults with candidemia. PLoS ONE 2013, 8, e77317. [Google Scholar] [CrossRef]
- Ajrouche, R.; Al-Hajje, A.; El-Helou, N.; Awada, S.; Rachidi, S.; Zein, S.; Salameh, P. Statins decrease mortality in Lebanese patients with sepsis: A multicenter study. Pharm Pract. 2013, 11, 102–108. [Google Scholar] [CrossRef]
- López-Cortés, L.E.; Gálvez-Acebal, J.; Del Toro, M.D.; Velasco, C.; de Cueto, M.; Caballero, F.J.; Muniain, M.A.; Pascual, A.; Rodríguez-Baño, J. Effect of statin therapy in the outcome of bloodstream infections due to Staphylococcus aureus: A prospective cohort study. PLoS ONE 2013, 8, e82958. [Google Scholar] [CrossRef]
- Ou, S.Y.; Chu, H.; Chao, P.W.; Ou, S.M.; Lee, Y.J.; Kuo, S.C.; Li, S.Y.; Shih, C.J.; Chen, Y.T. Effect of the use of low and high potency statins and sepsis outcomes. Intensive Care Med. 2014, 40, 1509–1517. [Google Scholar] [CrossRef]
- Zechmeister, C.; Hurren, J.; McNorton, K. Continuation of Statin Therapy and Vasopressor Use in Septic Shock. Ann. Pharmacother. 2015, 49, 790–795. [Google Scholar] [CrossRef]
- Mansur, A.; Steinau, M.; Popov, A.F.; Ghadimi, M.; Beissbarth, T.; Bauer, M.; Hinz, J. Impact of statin therapy on mortality in patients with sepsis-associated acute respiratory distress syndrome (ARDS) depends on ARDS severity: A prospective observational cohort study. BMC Med. 2015, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Mullis, A.H.; Jogu, H.R.; Ahmad, M.I.; Feroz, A.; Mendapara, V.; Saleh, M.M.; Elfawal, R.S.; Onteddu, N.K.R.; Qureshi, W.T. Abstract 16215: Statin Use in Asymptomatic Patients With Sepsis and Elevated Troponin is Associated With Lower Risk of Mortality. Circulation 2016, 134, A16215. [Google Scholar]
- Beed, M.; Brindley, P.G.; Mahajan, R.; Juttner, I.; Campion-Smith, J.; Wilson, V.G. The association between prior statin use and long-term outcomes after critical care admission. J. Crit. Care 2016, 35, 63–68. [Google Scholar] [CrossRef][Green Version]
- Schurr, J.W.; Wu, W.; Smith-Hannah, A.; Smith, C.J.; Barrera, R. Incidence of Sepsis and Mortality With Prior Exposure of HMG-COA Reductase Inhibitors in a Surgical Intensive Care Population. Shock 2016, 45, 10–15. [Google Scholar] [CrossRef]
- Paricahua, L.I.; Goncalves, A.F.O.; Pacheco, S.; Pacheco, F.J. Sepsis Mortality in Critical Care and Prior Statin Therapy: A Retrospective Cohort Study in Central Argentina. J. Clin. Diagn. Res. 2017, 11, Oc17–Oc21. [Google Scholar] [CrossRef]
- Lee, M.G.; Lee, C.C.; Lai, C.C.; Hsu, T.C.; Porta, L.; Lee, M.; Chang, S.S.; Chien, K.L.; Chen, Y.M. Preadmission statin use improves the outcome of less severe sepsis patients—A population-based propensity score matched cohort study. Br. J. Anaesth. 2017, 119, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.R.; Timbrook, T.T.; Noh, E.; Sakoulas, G.; Opal, S.M.; Nizet, V.; LaPlante, K.L. Evidence To Support Continuation of Statin Therapy in Patients with Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2017, 61, e02228-16. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.M.; LaPlante, K.L.; Timbrook, T.T.; Caffrey, A.R. Improved survival with continuation of statins in bacteremic patients. SAGE Open Med. 2018, 6, 2050312118801707. [Google Scholar] [CrossRef]
- Wiewel, M.A.; Scicluna, B.P.; van Vught, L.A.; Hoogendijk, A.J.; Zwinderman, A.H.; Lutter, R.; Horn, J.; Cremer, O.L.; Bonten, M.J.; Schultz, M.J.; et al. The host response in critically ill sepsis patients on statin therapy: A prospective observational study. Ann. Intensive Care 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.M.; Wu, V.C.; Lin, Y.F.; Wang, J.J.; Shiao, C.C.; Chen, L.; Chueh, S.J.; Chueh, E.; Yang, S.Y.; Lai, T.S.; et al. Effects of Statin Use in Advanced Chronic Kidney Disease Patients. J. Clin. Med. 2018, 7, 285. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Kung, C.T.; Chen, F.C.; Cheng, H.H.; Tsai, T.C.; Hsiao, S.Y.; Su, C.M. Impact of prior statin use on mortality in patients with type 2 diabetes mellitus and bloodstream infection. J. Int. Med. Res. 2019, 47, 3636–3647. [Google Scholar] [CrossRef]
- Chinaeke, E.E.; Love, B.L.; Magagnoli, J.; Yunusa, I.; Reeder, G. The impact of statin use prior to intensive care unit admission on critically ill patients with sepsis. Pharmacotherapy 2021, 41, 162–171. [Google Scholar] [CrossRef]
- Van de Louw, A.; Cohrs, A.; Leslie, D. Effects of Statins on the Incidence and Mortality of Sepsis in Patients with New Cancer Diagnosis. J. Clin. Med. 2021, 10, 3427. [Google Scholar] [CrossRef]
- Liang, B.; Yang, S.T.; Wei, K.K.; Yu, A.S.; Kim, B.J.; Gould, M.K.; Sim, J.J. Statin Use and Mortality among Patients Hospitalized with Sepsis: A Retrospective Cohort Study within Southern California, 2008–2018. Crit Care Res Pract. 2022, 2022, 7127531. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, X.; Wang, M.; Zhou, F.; Li, C.; Le, X.; Zhang, S. Association between the use of statins and in-hospital mortality risk in patients with sepsis-induced coagulopathy during ICU stays: A study based on medical information mart for intensive care database. BMC Infect. Dis. 2024, 24, 738. [Google Scholar] [CrossRef]
- Li, M.; Noordam, R.; Trompet, S.; Winter, E.M.; Jukema, J.W.; Arbous, M.S.; Rensen, P.C.N.; Kooijman, S. The impact of statin use on sepsis mortality. J. Clin. Lipidol. 2024, 18, e915–e925. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Pasupuleti, V.; Rothberg, M.B. Statin therapy and mortality from sepsis: A meta-analysis of randomized trials. Am. J. Med. 2015, 128, 410–417.e411. [Google Scholar] [CrossRef]
- Tralhão, A.F.; Cés de Souza-Dantas, V.; Salluh, J.I.; Póvoa, P.M. Impact of statins in outcomes of septic patients: A systematic review. Postgrad. Med. 2014, 126, 45–58. [Google Scholar] [CrossRef] [PubMed]
- McGown, C.C.; Brookes, Z.L. Beneficial effects of statins on the microcirculation during sepsis: The role of nitric oxide. Br. J. Anaesth. 2007, 98, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Fraccarollo, D.; Vogt, C.; Flierl, U.; Hemberger, M.; Tas, P.; Ertl, G.; Bauersachs, J. Improved endothelial function and reduced platelet activation by chronic HMG-CoA-reductase inhibition with rosuvastatin in rats with streptozotocin-induced diabetes mellitus. Biochem. Pharmacol. 2007, 73, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Resch, U.; Tatzber, F.; Budinsky, A.; Sinzinger, H. Reduction of oxidative stress and modulation of autoantibodies against modified low-density lipoprotein after rosuvastatin therapy. Br. J. Clin. Pharmacol. 2006, 61, 262–274. [Google Scholar] [CrossRef]
- Kleemann, R.; Princen, H.M.; Emeis, J.J.; Jukema, J.W.; Fontijn, R.D.; Horrevoets, A.J.; Kooistra, T.; Havekes, L.M. Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE*3-Leiden transgenic mice: Evidence for antiinflammatory effects of rosuvastatin. Circulation 2003, 108, 1368–1374. [Google Scholar] [CrossRef]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Statins and blood coagulation. Arter. Thromb. Vasc. Biol. 2005, 25, 287–294. [Google Scholar] [CrossRef]
- Schäfer, A.; Fraccarollo, D.; Eigenthaler, M.; Tas, P.; Firnschild, A.; Frantz, S.; Ertl, G.; Bauersachs, J. Rosuvastatin reduces platelet activation in heart failure: Role of NO bioavailability. Arter. Thromb. Vasc. Biol. 2005, 25, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Kilic, U.; Bassetti, C.L.; Kilic, E.; Xing, H.; Wang, Z.; Hermann, D.M. Post-ischemic delivery of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor rosuvastatin protects against focal cerebral ischemia in mice via inhibition of extracellular-regulated kinase-1/-2. Neuroscience 2005, 134, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Shitara, Y.; Sugiyama, Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 2006, 112, 71–105. [Google Scholar] [CrossRef] [PubMed]
- Hylton Gravatt, L.A.; Flurie, R.W.; Lajthia, E.; Dixon, D.L. Clinical Guidance for Managing Statin and Antimicrobial Drug-Drug Interactions. Curr. Atheroscler. Rep. 2017, 19, 46. [Google Scholar] [CrossRef]




| Population | Patients with sepsis |
| Intervention | Statins |
| Comparator/controls | Placebo or no intervention |
| Outcomes | Mortality |
| Study design | Randomized control trials, case-control and cohort studies |
| First Author | Year | Country | Study Design | Exposure and Comparator | Mortality (n, Statin Patients) | Mortality (n, Controls) | Statin | Dose of Statin | Underlying Condition |
|---|---|---|---|---|---|---|---|---|---|
| Novack [16] | 2009 | Israel | RCT | De novo statin use vs. placebo | 0/42 | 0/41 | Simvastatin | 40 mg/day + 20 mg/day | Bacteremia |
| El-Akabawy [17] | 2011 | Egypt | RCT | De novo statin use vs. placebo | 10/25 | 14/25 | Atorvastatin | 80 mg/day | Sepsis |
| Kruger [18] | 2011 | Australia | RCT | Prior statin use and continuation vs. prior statin use and placebo | 6/75 | 4/75 | Atorvastatin | 20 mg/day | Bacteremia |
| Makris [19] | 2011 | Greece | RCT | De novo statin use vs. placebo | 6/71 | 16/81 | Pravastatin | 40 mg/day | VAP |
| Patel [20] | 2012 | UK | RCT | De novo statin use vs. placebo | 2/49 | 2/51 | Atorvastatin | 40 mg/day | Sepsis |
| Prado [21] | 2013 | Brazil | RCT | De novo statin use vs. placebo | 6/23 | 11/24 | Atorvastatin | 80 mg/day | Sepsis/Septic shock |
| Kruger [22] | 2013 | Australia + New Zeeland | RCT | De novo statin use vs. placebo | 12/86 | 12/87 | Atorvastatin | 20 mg/day | Sepsis |
| Papazian [23] | 2013 | France | RCT | De novo statin use vs. placebo | 43/146 | 38/138 | Simvastatin | 60 mg/day | VAP |
| El Gendy [24] | 2014 | Egypt | RCT | De novo statin use vs. placebo | 17/54 | 22/54 | Rosuvastatin | 20 mg/day | Sepsis |
| Truwit [25] | 2014 | USA | RCT | De novo statin use vs. placebo | 108/379 | 91/360 | Rosuvastatin | 20 mg/day | SA-ARDS |
| McAuley [26] | 2014 | UK + Ireland | RCT | De novo statin use vs. placebo | 59/259 | 75/280 | Simvastatin | 40 mg/day | ARDS |
| Viasus [27] | 2015 | Spain | RCT | De novo statin use vs. placebo | 0/19 | 1/15 | Simvastatin | 20 mg/day | CAP |
| Mirjalili [28] | 2016 | Iran | RCT | De novo statin use vs. placebo | 17/30 | 22/30 | Simvastatin | 40 mg/day | Sepsis |
| Singh [29] | 2017 | India | RCT | De novo statin use vs. placebo | 21/36 | 21/37 | Atorvastatin | 40 mg/day | Septic shock |
| Eladawy [30] | 2019 | Egypt | RCT | De novo statin use vs. placebo | 35/65 | 52/80 | Rosuvastatin | 20 mg/day | Sepsis |
| Sapey [31] | 2019 | UK | RCT | De novo statin use vs. placebo | 2/32 | 6/30 | Simvastatin | 40 mg/day | Pneumonia |
| First Author | Year | Country | Study Design | Exposure and Comparator | Mortality (n/Statin Patients) | Mortality (n/Controls) | Underlying Condition |
|---|---|---|---|---|---|---|---|
| Liappis [32] | 2001 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 2/35 | 100/353 | Bacteremia |
| Thompsen [33] | 2006 | Denmark | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 35/176 | 1114/5177 | Bacteremia |
| Schmidt [34] | 2006 | Germany | Retrospective Cohort study | De novo statin use vs. non-statin users | 13/40 | 42/80 | MODS |
| Kruger [35] | 2006 | UK | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 7/66 | 86/372 | Bacteremia |
| Martin [36] | 2007 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 6/16 | 18/37 | Sepsis |
| Yang [37] | 2007 | Taiwan | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 20/104 | 66/350 | Sepsis |
| Dobesh [38] | 2009 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 19/60 | 62/128 | Severe sepsis |
| Goodin [39] | 2011 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 21/124 | 87/444 | Sepsis |
| Mekontso Dessap [40] | 2011 | France | Retrospective Cohort study | Prior statin + continuation vs. prior statin + non-continuation | 9/44 | 13/32 | Severe Sepsis + Septic shock |
| Leung [41] | 2012 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 76/447 | 100/458 | Bacteremia |
| Fuller [42] | 2012 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 4/18 | 29/73 | Septic shock |
| Nseir [43] | 2012 | Israel | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 14/123 | 39/161 | Bacteremia |
| Cuervo [44] | 2013 | Argentina + Brazil + Spain | Retrospective Cohort study | Prior statin use + continuation vs. placebo | 18/44 | 124/282 | Candidemia |
| Ajrouche [45] | 2013 | Lebanon | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 42/106 | 149/245 | Sepsis |
| Lopez-Cortes [46] | 2013 | Spain | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 5/33 | 42/127 | Bacteremia |
| Ou [47] | 2014 | Taiwan | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 5253/27,792 | 6059/27,792 | Sepsis |
| Zechmeister [48] | 2015 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 31/73 | 30/46 | Septic shock |
| Mansur [49] | 2015 | Germany | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 12/26 | 38/88 | ARDS |
| Mullis [50] | 2016 | USA | Retrospective Cohort study | Statin vs. placebo | 108/344 | 236/592 | Sepsis |
| Beed [51] | 2016 | UK | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 13/88 | 53/584 | Sepsis |
| Schurr [52] | 2016 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 0/22 | 12/68 | Sepsis |
| Paricahua [53] | 2017 | Argentina | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 16/33 | 73/198 | Sepsis |
| Lee [54] | 2017 | Taiwan | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 525/3325 | 581/3325 | Severe Sepsis |
| Caffrey [55] | 2017 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 19/141 | 33/141 | Bacteremia |
| Pawar [56] | 2018 | USA | Retrospective Cohort study | Prior statin + continuation vs. prior statin + non-continuation | 6/232 | 44/401 | Bacteremia |
| Wiewel [57] | 2018 | Netherlands | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 48/194 | 67/194 | Sepsis |
| Huang [58] | 2018 | Taiwan | Retrospective Cohort study | De novo statin use vs. non-statin users | 526/2551 | 2087/7653 | CKD |
| Cheng [59] | 2019 | Taiwan | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 32/507 | 188/1472 | Bacteremia |
| Chinaeke [60] | 2021 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 450/1623 | 1967/6577 | Sepsis |
| Van de Louw [61] | 2021 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 312/1365 | 1390/5969 | Sepsis |
| Liang [62] | 2022 | USA | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 5032/34,088 | 14,141/94,073 | Sepsis |
| Yao [63] | 2024 | China | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 268/869 | 601/869 | Coagulopathy |
| Li [64] | 2024 | Netherlands | Retrospective Cohort study | Prior statin use + continuation vs. non-statin users | 1629/14,416 | 3127/17,567 | Sepsis |
| Study | Selection | Comparability | Outcome | Quality | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Cohort | Non-Exposed Cohort Selection | Exposure Ascertainment | Outcome of Interest | Assessment | Follow-Up Time | Follow-Up Adequacy | |||
| Liappis et al., 2001 [32] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Thompsen et al., 2006 [33] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | - | 8 |
| Schmidt et al., 2006 [34] | - | ★ | ★ | ★ | ★★ | ★ | ★ | - | 7 |
| Kruger et al., 2006 [35] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Martin et al., 2007 [36] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Yang et al., 2007 [37] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Dobesh et al., 2009 [38] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Goodin et al., 2011 [39] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Mekontso Dessap et al., 2011 [40] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Leung et al., 2012 [41] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Fuller et al., 2012 [42] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Nseir et al., 2012 [43] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Ajrouche et al., 2013 [45] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Lopez-Cortes et al., 2013 [46] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | - | 8 |
| Cuervo et al., 2013 [44] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Ou et al., 2014 [47] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Zechmeister et al., 2015 [48] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Mansur et al., 2015 [49] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Mullis et al., 2016 [50] | ★ | - | ★ | ★ | - | ★ | ★ | ★ | 6 |
| Beed et al., 2016 [51] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Schurr et al., 2016 [52] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Paricahua et al., 2017 [53] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Lee et al., 2017 [54] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Caffrey et al., 2017 [55] | - | - | - | ★ | ★★ | ★ | - | - | 4 |
| Pawar et al., 2018 [56] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Wiewel et al., 2018 [57] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Huang et al., 2018 [58] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | - | 8 |
| Cheng et al., 2019 [59] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Chinaeke et al., 2021 [60] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Van de Louw et al., 2021 [61] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Liang et al., 2022 [62] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Yao et al., 2024 [63] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Li et al., 2024 [64] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippou, C.; Tsioutis, C.; Tsiappari, M.; Spernovasilis, N.; Papadopoulos, D.; Agouridis, A.P. The Impact of Statin Use on Sepsis Mortality: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1563. https://doi.org/10.3390/medicina61091563
Philippou C, Tsioutis C, Tsiappari M, Spernovasilis N, Papadopoulos D, Agouridis AP. The Impact of Statin Use on Sepsis Mortality: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(9):1563. https://doi.org/10.3390/medicina61091563
Chicago/Turabian StylePhilippou, Constantinos, Constantinos Tsioutis, Maria Tsiappari, Nikolaos Spernovasilis, Dimitrios Papadopoulos, and Aris P. Agouridis. 2025. "The Impact of Statin Use on Sepsis Mortality: A Systematic Review and Meta-Analysis" Medicina 61, no. 9: 1563. https://doi.org/10.3390/medicina61091563
APA StylePhilippou, C., Tsioutis, C., Tsiappari, M., Spernovasilis, N., Papadopoulos, D., & Agouridis, A. P. (2025). The Impact of Statin Use on Sepsis Mortality: A Systematic Review and Meta-Analysis. Medicina, 61(9), 1563. https://doi.org/10.3390/medicina61091563










