The Role of Biomarkers and Clinical Prediction Tools in the Diagnosis of Acute Aortic Syndromes: A Literature-Based Review
Abstract
1. Introduction
2. Methods
3. Classification and Clinical Presentation of Acute Aortic Syndromes
4. Diagnostic Imaging Techniques for Acute Aortic Syndromes
5. Artificial Intelligence and AAS
- Lactylation-related genes: PGK1, HMGA1 [24]
- Cellular senescence-related genes: CHEK1, FOXM1, BRCA1 [25]
- Disulfidptosis-related genes: INF2, CD2AP, CAPZB [26]
- Immune-related markers: CXCL1, ITGA5, PTX3, TIMP1 [27]
- Pyroptosis-related genes: CASP4, MLKL, PECAM1, HDAC6 [28]
- Ferroptosis-related genes: CA9, HMOX1, IL6, CDKN1A, HIF1A, MYC [29]
6. Diagnostic Scores in Acute Aortic Syndromes
6.1. Aortic Dissection Detection Risk Score (ADD-RS)
6.2. Aortic Dissection Detection Risk Score (ADD-RS) Combined with D-dimer
6.3. Simplified Acute Aortic Dissection (SAAD) Score
6.4. Aortic Occlusion Risk Tool for Acute Syndromes (AORTAs) Score
6.5. Comparative Analysis: ADD-RS vs. SAAD vs. AORTAs
6.6. Other Diagnostic Scores
6.7. An Integrative Approach: Coagulation, Laboratory Markers, and Scores
7. Biomarkers
7.1. D-Dimer
7.2. Inflammatory Markers
7.2.1. Soluble Suppression of Tumorigenesis-2 (sST2)
7.2.2. IL-6
7.2.3. IL-10
7.2.4. C-Reactive Protein (CRP)
7.3. Adiponectin
7.4. White Blood Cells (WBC), Platelet Count, Neutrophil/Lymphocytes Ratio (NLR)
7.5. Extracellular Matrix Markers (ECM)
7.5.1. Matrix Metalloproteinases (MMPs)
7.5.2. Soluble Elastin Fragments (sELAF)
7.5.3. Tenascin C (TNC)
7.6. Smooth Muscle Cell (SMC) Biomarkers
7.6.1. Smooth Muscle Myosin Heavy Chain (SmMHC)
7.6.2. Calponin
7.7. Cardiac Markers
7.7.1. N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP)
7.7.2. Troponin I and T
7.7.3. Copeptin
7.7.4. Ischemia-Modified Albumin (IMA)
7.8. Lipid Metabolism-Related Markers
7.8.1. Low-Density Lipoprotein (LDL)
7.8.2. Homocysteine (Hcy)
7.8.3. Lysophosphatidylcholine (LPC)
7.9. Non Coding Nucleic Acid (Micro and Circular RNA)
7.9.1. MicroRNA
7.9.2. Circular RNAs
7.10. Other Biomarkers
7.10.1. Polycystin-1
7.10.2. Lumican
7.10.3. Aggrecan
7.10.4. ADAMTS
7.10.5. Osteopontin
7.10.6. Semaphorin 7A
7.10.7. NOTCH Pathway
7.10.8. Other Emerging Biomarkers
7.10.9. Future Direction
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Erbel, R.; Alfonso, F.; Boileau, C.; Dirsch, O.; Eber, B.; Haverich, A.; Rakowski, H.; Struyven, J.; Radegran, K.; Sechtem, U.; et al. Diagnosis and management of aortic dissection. Eur. Heart J. 2001, 22, 1642–1681. [Google Scholar] [CrossRef] [PubMed]
- Von Kodolitsch, Y.; Schwartz, A.G.; Nienaber, C.A. Clinical prediction of acute aortic dissection. Arch. Intern. Med. 2000, 160, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Hagan, P.G.; Nienaber, C.A.; Isselbacher, E.M.; Bruckman, D.; Karavite, D.J.; Russman, P.L.; Evangelista, A.; Fattori, R.; Suzuki, T.; Oh, J.K.; et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. JAMA 2000, 283, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute aortic syndromes: Diagnosis and management, an update. Eur. Heart J. 2018, 39, 739–749d. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.A.; Eagle, K.A. Aortic dissection: New frontiers in diagnosis and management: Part I: From etiology to diagnostic strategies. Circulation 2003, 108, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Singh, A.A.; Safdar, N.Z.; Nandhra, S.; Vigneswaran, G.; CAASP Collaborators. Evaluating current acute aortic syndrome pathways: Collaborative Acute Aortic Syndrome Project (CAASP). BJS Open 2024, 8, zrae096. [Google Scholar] [CrossRef]
- DeBakey, M.E.; Henly Ebakey, M.E.; Henly, W.S.; Cooley, D.A.; Morris, G.C., Jr.; Crawford, E.S.; Beall, A.C., Jr. Surgical management of dissecting aneurysm of the aorta. J. Thorac. Cardiovasc. Surg. 1965, 49, 130–149. [Google Scholar] [CrossRef] [PubMed]
- Daily, P.O.; Trueblood, H.W.; Stinson, E.B.; Wuerflein, R.D.; Shumway, N.E. Management of acute aortic dissections. Ann. Thorac. Surg. 1970, 10, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Rylski, B.; Czerny, M.; Baier, A.L.M.; Kreibich, M.; Siepe, M.; Beyersdorf, F. Aortic dissection reconsidered: Type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 451–457. [Google Scholar] [CrossRef]
- Ramesh, P.; Al-Zubaidi, F.I.; Abdelghaffar, M.; Babiker, S.; Aspinall, A.; Butt, S.; Sabry, H.; Zeinah, M.; Harky, A. TEM Classification of Aortic Dissection-The Evolving Scoring System: A Literature Review. Heart Lung Circ. 2024, 33, 17–22. [Google Scholar] [CrossRef]
- Olin, J.W.; Fuster, V. Acute aortic dissection: The need for rapid, accurate, and readily available diagnostic strategies. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1721–1723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, I.A.; Nair, C.K. Clinical, diagnostic, and management perspectives of aortic dissection. Chest 2002, 122, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Van Arsdell, G.S.; David, T.E.; Butany, J. Autopsies in acute type A aortic dissection: Surgical implications. Circulation 1998, 98 (Suppl. S19), II299–II302. [Google Scholar] [PubMed]
- Davies, R.R.; Coe, M.P.; Mandapati, D.; Gallo, A.; Botta, D.M.; Elefteriades, J.A.; Coady, M.A. What is the optimal management of late-presenting survivors of acute type A aortic dissection? Ann. Thorac. Surg. 2007, 83, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Longhi, S.; Graziosi, M.; Biagini, E.; Terzi, F.; Cooke, R.M.; Quarta, C.; Sangiorgi, D.; Ciliberti, P.; Di Pasquale, G.; et al. Risk factors for diagnostic delay in acute aortic dissection. Am. J. Cardiol. 2008, 102, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Eagle, K.A. Acute aortic dissection. Lancet 2008, 372, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Eusanio, M.D.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018, 137, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e369, Erratum in Circulation 2010, 122, e410. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R. Aortenerkrankungen: Moderne diagnostische und therapeutische Empfehlungen [Aortic diseases: Modern diagnostic and therapeutic strategies]. Herz 2018, 43, 275–290. (In German) [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Kilinc, O.; Weiss, E.; Baraboo, J.; Mehta, C.; Hoel, A.; Malaisrie, S.C.; Markl, M.; Allen, B.D. Interval changes in four-dimensional flow-derived in vivo hemodynamics stratify aortic growth in type B aortic dissection patients. J. Cardiovasc. Magn. Reson. 2024, 26, 101078. [Google Scholar] [CrossRef]
- Tawakol, A.; Mezue, K.N. Molecular Imaging in Acute Aortic Syndrome: Lighting a Path to Better Risk Assessment. JACC Cardiovasc. Imaging 2022, 15, 1305–1307. [Google Scholar] [CrossRef]
- Syed, M.B.J.; Fletcher, A.J.; Debono, S.; Forsythe, R.O.; Williams, M.C.; Dweck, M.R.; Shah, A.S.V.; Macaskill, M.G.; Tavares, A.; Denvir, M.A.; et al. 18F-Sodium Fluoride Positron Emission Tomography and Computed Tomography in Acute Aortic Syndrome. JACC Cardiovasc. Imaging 2022, 15, 1291–1304. [Google Scholar] [CrossRef]
- Ye, J.; Fu, Y.; Ke, Y.; Liu, H.; Fang, S.; Lei, Y.; Mao, Y.; Yan, L.; Wang, Y. Lactylation associated biomarkers and immune infiltration in aortic dissection. Sci. Rep. 2025, 15, 21536. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tang, P. The role of senescence-related hub genes correlating with immune infiltration in type A aortic dissection: Novel insights based on bioinformatic analysis. PLoS ONE 2025, 20, e0326939. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Liu, H.; Zhang, Z.; Li, M.; Ge, X.; Bi, A.; Gao, C.; Tian, X.; Liu, K.; et al. Integrated bioinformatic analysis of immune infiltration and disulfidptosis related gene subgroups in type A aortic dissection. Sci. Rep. 2025, 15, 13719. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhao, Y.; Shi, Z.; Pan, Y.; Shi, K.; Zhao, C.; Liu, S.; Chen, Y.; Zhao, L.; Wu, J.; et al. Establishment of a nomogram model based on immune-related genes using machine learning for aortic dissection diagnosis and immunomodulation assessment. Int. J. Med. Sci. 2025, 22, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gao, L.; Ge, H.; Huang, W.; Zhao, R.; Gu, R.; Li, Z.; Wang, X. A neural network model was constructed by screening the potential biomarkers of aortic dissection based on genes associated with pyroptosis. Aging 2023, 15, 12388–12399. [Google Scholar] [CrossRef]
- Zou, H.X.; Qiu, B.Q.; Lai, S.Q.; Huang, H.; Zhou, X.L.; Gong, C.W.; Wang, L.J.; Yuan, M.M.; He, A.D.; Liu, J.C. Role of ferroptosis-related genes in Stanford type a aortic dissection and identification of key genes: New insights from bioinformatic analysis. Bioengineered 2021, 12, 9976–9990. [Google Scholar] [CrossRef]
- Asif, A.; Alsayyari, M.; Monekosso, D.; Remagnino, P.; Lakshminarayan, R. Role of Artificial Intelligence in Detecting and Classifying Aortic Dissection: Where Are We? A Systematic Review and Meta-Analysis. Radiol. Cardiothorac. Imaging 2025, 7, e240353. [Google Scholar] [CrossRef]
- Raj, A.; Allababidi, A.; Kayed, H.; Gerken, A.L.H.; Müller, J.; Schoenberg, S.O.; Zöllner, F.G.; Rink, J.S. Streamlining Acute Abdominal Aortic Dissection Management-An AI-based CT Imaging Workflow. J. Imaging Inform. Med. 2024, 37, 2729–2739. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Wu, C.; Shu, L.; Zhou, D. A deep-learning system integrating electrocardiograms and laboratory indicators for diagnosing acute aortic dissection and acute myocardial infarction. Int. J. Cardiol. 2025, 423, 133008. [Google Scholar] [CrossRef]
- Zhou, M.; Lei, L.; Chen, W.; Luo, Q.; Li, J.; Zhou, F.; Yang, X.; Pan, Y. Deep learning-based diagnosis of aortic dissection using an electrocardiogram: Development, validation, and clinical implications of the AADE score. Kardiol. Pol. 2024, 82, 63–71. [Google Scholar] [CrossRef]
- Duceau, B.; Alsac, J.M.; Bellenfant, F.; Mailloux, A.; Champigneulle, B.; Favé, G.; Neuschwander, A.; El Batti, S.; Cholley, B.; Achouh, P.; et al. Prehospital triage of acute aortic syndrome using a machine learning algorithm. Br. J. Surg. 2020, 107, 995–1003. [Google Scholar] [CrossRef]
- Rogers, A.M.; Hermann, L.K.; Booher, A.M.; Nienaber, C.A.; Williams, D.M.; Kazerooni, E.A.; Froehlich, J.B.; O’Gara, P.T.; Montgomery, D.G.; Cooper, J.V.; et al. IRAD investigators. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: Results from the international registry of acute aortic dissection. Circulation 2011, 123, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, P.; Mueller, C.; Soeiro, A.M.; Leidel, B.A.; Salvadeo, S.A.T.; Giachino, F.; Vanni, S.; Grimm, K.; Oliveira, M.T., Jr.; Pivetta, E.; et al. Diagnostic Accuracy of the Aortic Dissection Detection Risk Score Plus D-Dimer for Acute Aortic Syndromes: The ADvISED Prospective Multicenter Study. Circulation 2018, 137, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Gorla, R.; Erbel, R.; Kahlert, P.; Tsagakis, K.; Jakob, H.; Mahabadi, A.A.; Schlosser, T.; Eggebrecht, H.; Bossone, E.; Jánosi, R.A. Accuracy of a diagnostic strategy combining aortic dissection detection risk score and D-dimer levels in patients with suspected acute aortic syndrome. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 371–378. [Google Scholar] [CrossRef] [PubMed]
- McLatchie, R.; Reed, M.J.; Freeman, N.; Parker, R.A.; Wilson, S.; Goodacre, S.; Cowan, A.; Boyle, J.; Clarke, B.; Clarke, E.; et al. Diagnosis of Acute Aortic Syndrome in the Emergency Department (DAShED) study: An observational cohort study of people attending the emergency department with symptoms consistent with acute aortic syndrome. Emerg. Med. J. 2024, 41, 136–144. [Google Scholar] [CrossRef]
- Deng, L.; Xia, Q.; Diao, L.; Lin, F.; Cao, Y.; Han, H.; Song, M. Aortic Dissection Detection Risk Score and D-Dimer for Acute Aortic Syndromes in the Chinese Population: Exploration of Optimal Thresholds and Integrated Diagnostic Value. J. Cardiovasc. Transl. Res. 2023, 16, 886–895. [Google Scholar] [CrossRef]
- Kotani, Y.; Toyofuku, M.; Tamura, T.; Shimada, K.; Matsuura, Y.; Tawa, H.; Uchikawa, M.; Higashi, S.; Fujimoto, J.; Yagita, K.; et al. Validation of the diagnostic utility of D-dimer measurement in patients with acute aortic syndrome. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Essat, M.; Pandor, A.; Goodacre, S.; Ren, S.; Clowes, M.; Bima, P.; Toyofuku, M.; McLatchie, R.; Bossone, E. Diagnostic accuracy of the aortic dissection detection risk score alone or with D-dimer for acute aortic syndromes: Systematic review and meta-analysis. PLoS ONE 2024, 19, e0304401. [Google Scholar] [CrossRef] [PubMed]
- Ohle, R.; McIsaac, S.; Van Drusen, M.; Regis, A.; Montpellier, O.; Ludgate, M.; Bodunde, O.; Savage, D.W.; Yadav, K. Evaluation of the Canadian Clinical Practice Guidelines Risk Prediction Tool for Acute Aortic Syndrome: The RIPP Score. Emerg. Med. Int. 2023, 2023, 6636800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morello, F.; Bima, P.; Pivetta, E.; Santoro, M.; Catini, E.; Casanova, B.; Leidel, B.A.; de Matos Soeiro, A.; Nestelberger, T.; Mueller, C.; et al. Development and Validation of a Simplified Probability Assessment Score Integrated With Age-Adjusted d-Dimer for Diagnosis of Acute Aortic Syndromes. J. Am. Heart Assoc. 2021, 10, e018425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Bima, P.; Castelli, M.; Capretti, E.; de Matos Soeiro, A.; Cipriano, A.; Costantino, G.; Vanni, S.; Leidel, B.A.; Kaufmann, B.A.; et al. Diagnosis of acute aortic syndromes with ultrasound and d-dimer: The PROFUNDUS study. Eur. J. Intern. Med. 2024, 128, 94–103. [Google Scholar] [CrossRef]
- Bima, P.; Pivetta, E.; Nazerian, P.; Toyofuku, M.; Gorla, R.; Bossone, E.; Erbel, R.; Lupia, E.; Morello, F. Systematic Review of Aortic Dissection Detection Risk Score Plus D-dimer for Diagnostic Rule-out Of Suspected Acute Aortic Syndromes. Acad. Emerg. Med. 2020, 27, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Thokala, P.; Goodacre, S.; Cooper, G.; Hinchliffe, R.; Reed, M.J.; Thomas, S.; Wilson, S.; Fowler, C.; Lechene, V. Decision analytical modelling of strategies for investigating suspected acute aortic syndrome. Emerg. Med. J. 2024, 41, 728–735. [Google Scholar] [CrossRef]
- Song, D.H.; Choi, J.H.; Lee, J.Y. Predicting acute aortic syndrome using aortic dissection detection risk score, D-dimer, and X-ray. Heliyon 2023, 9, e20578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, J.; Duan, X.; Feng, R.; Zhao, Z.; Feng, X.; Lu, Q.; Jing, Q.; Zhou, J.; Bao, J.; Jing, Z. Diagnostic implication of fibrin degradation products and D-dimer in aortic dissection. Sci. Rep. 2017, 7, 43957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pepe, G.; Giusti, B.; Yacoub, M. An expanding role of biomarkers in acute aortic syndromes. Clin. Cardiol. 2006, 29, 432–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wren, J.; Goodacre, S.; Pandor, A.; Essat, M.; Clowes, M.; Cooper, G.; Hinchliffe, R.; Reed, M.J.; Thomas, S.; Wilson, S. Diagnostic accuracy of alternative biomarkers for acute aortic syndrome: A systematic review. Emerg. Med. J. 2024, 41, 678–685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Fu, W.; Wang, L. Biomarkers in aortic dissection: Diagnostic and prognostic value from clinical research. Chin. Med. J. 2024, 137, 257–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Li, Y.; Li, Z.; Shi, Y.; Zhu, H. Diagnostic biomarkers and aortic dissection: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asha, S.E.; Miers, J.W. A Systematic Review and Meta-analysis of D-dimer as a Rule-out Test for Suspected Acute Aortic Dissection. Ann. Emerg. Med. 2015, 66, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Lee, J.H. Low D-dimer in acute coronary syndrome and heart failure: Screening for large vessel diseases in patients with chest symptoms. Heliyon 2024, 10, e31210. [Google Scholar] [CrossRef]
- Suzuki, T.; Distante, A.; Zizza, A.; Trimarchi, S.; Villani, M.; Salerno Uriarte, J.A.; De Luca Tupputi Schinosa, L.; Renzulli, A.; Sabino, F.; Nowak, R.; et al. Diagnosis of acute aortic dissection by D-dimer: The International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009, 119, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Tan, X.; Gao, H.; Yuan, H.; Hu, R.; Jia, L.; Zhu, J.; Sun, L.; Zhang, H.; Huang, L.; et al. Magnitude of Soluble ST2 as a Novel Biomarker for Acute Aortic Dissection. Circulation 2018, 137, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Bartalucci, A.; Bironzo, M.; Santoro, M.; Pivetta, E.; Ianniello, A.; Rumbolo, F.; Mengozzi, G.; Lupia, E. Prospective diagnostic accuracy study of plasma soluble ST2 for diagnosis of acute aortic syndromes. Sci. Rep. 2020, 10, 3103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, M.H.; Gu, J.; Zhang, E.Y. Clinical significances of plasma interleukin-6, C-reaction protein and tumor necrosis factor-alpha in patients with aortic dissection. Sichuan Da Xue Xue Bao Yi Xue Ban 2015, 46, 234–237. (In Chinese) [Google Scholar] [PubMed]
- Wu, H.J.; Zhang, W.; Shu, Y.W.; Fan, H.; Li, H.; Zeng, Q.T. Comparison of plasma pro-inflammatory cytokine expressions in patients with different types of acute aortic. Chin. Crit. Care Med. 2014, 26, 740–742. [Google Scholar]
- Qu, H.J.; Zhu, J.J.; Li, X.M.; Yang, Y.N. Predictive value of interleukin-6 for mortality in patients with acute aortic dissection. Chin. J. Clin. (Electron. Ed.) 2015, 9, 3184–3187. [Google Scholar]
- Gorla, R.; Erbel, R.; Kahlert, P.; Tsagakis, K.; Jakob, H.; Mahabadi, A.A.; Schlosser, T.; Eagle, K.; Bossone, E.; Jánosi, R.A. Clinical features and prognostic value of stent-graft-induced post-implantation syndrome after thoracic endovascular aortic repair in patients with type B acute aortic syndromes. Eur. J. Cardiothorac. Surg. 2016, 49, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Forrer, A.; Schoenrath, F.; Torzewski, M.; Schmid, J.; Franke, U.F.W.; Göbel, N.; Aujesky, D.; Matter, C.M.; Lüscher, T.F.; Mach, F.; et al. Novel Blood Biomarkers for a Diagnostic Workup of Acute Aortic Dissection. Diagnostics 2021, 11, 615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, J.; Hu, J.; Zhang, H.W.; Xiao, Z.H.; Fang, Z.; Qian, H.; Zhong, M.H.; Guo, Y.Q.; Zhang, E.Y.; Shi, Y.K.; et al. Time-dependent changes of plasma inflammatory biomarkers in type A aortic dissection patients without optimal medical management. J. Cardiothorac. Surg. 2015, 10, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- del Porto, F.; Proietta, M.; Tritapepe, L.; Miraldi, F.; Koverech, A.; Cardelli, P.; Tabacco, F.; de Santis, V.; Vecchione, A.; Mitterhofer, A.P.; et al. Inflammation and immune response in acute aortic dissection. Ann. Med. 2010, 42, 622–629. [Google Scholar] [CrossRef]
- Peng, W.; Peng, Z.; Chai, X.; Zhu, Q.; Yang, G.; Zhao, Q.; Zhou, S. Potential biomarkers for early diagnosis of acute aortic dissection. Heart Lung 2015, 44, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Kubo, N.; Ako, J.; Wada, H.; Fujiwara, N.; Funayama, H.; Ikeda, N.; Nakamura, T.; Sugawara, Y.; Yasu, T.; et al. Peak C-reactive protein level predicts long-term outcomes in type B acute aortic dissection. Hypertension 2010, 55, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Henry, B.M.; Hsieh, C.C.; Maruna, P.; Omara, M.; Lindner, J. Prognostic Role of Admission C-Reactive Protein Level as a Predictor of In-Hospital Mortality in Type-A Acute Aortic Dissection: A Meta-Analysis. Vasc. Endovascular. Surg. 2019, 53, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wågsäter, D.; Vorkapic, E.; van Stijn, C.M.; Kim, J.; Lusis, A.J.; Eriksson, P.; Tangirala, R.K. Elevated Adiponectin Levels Suppress Perivascular and Aortic Inflammation and Prevent AngII-induced Advanced Abdominal Aortic Aneurysms. Sci. Rep. 2016, 6, 31414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Jiao, X.; Li, L.; Hu, C.; Zhang, X.; Pan, L.; Yu, H.; Li, J.; Chen, D.; Du, J.; et al. Increased Circulating Angiopoietin-Like Protein 8 Levels Are Associated with Thoracic Aortic Dissection and Higher Inflammatory Conditions. Cardiovasc. Drugs Ther. 2020, 34, 65–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morello, F.; Cavalot, G.; Giachino, F.; Tizzani, M.; Nazerian, P.; Carbone, F.; Pivetta, E.; Mengozzi, G.; Moiraghi, C.; Lupia, E. White blood cell and platelet count as adjuncts to standard clinical evaluation for risk assessment in patients at low probability of acute aortic syndrome. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sbarouni, E.; Georgiadou, P.; Analitis, A.; Voudris, V. High neutrophil to lymphocyte ratio in type A acute aortic dissection facilitates diagnosis and predicts worse outcome. Expert Rev. Mol. Diagn. 2015, 15, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yuan, N.; Guo, J.; Hou, M. Comparisons of potential values of D-dimer and the neutrophil- to-lymphocyte ratio in patients with suspected acute aortic syndrome. Am. J. Emerg. Med. 2023, 69, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Shimizu-Hirota, R.; Shimoda, M.; Adachi, T.; Shimizu, H.; Weiss, S.J.; Itoh, H.; Hori, S.; Aikawa, N.; Okada, Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation 2012, 126, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Giachino, F.; Loiacono, M.; Lucchiari, M.; Manzo, M.; Battista, S.; Saglio, E.; Lupia, E.; Moiraghi, C.; Hirsch, E.; Mengozzi, G.; et al. Rule out of acute aortic dissection with plasma matrix metalloproteinase 8 in the emergency department. Crit. Care 2013, 17, R33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katsuda, S.; Okada, Y.; Okada, Y.; Imai, K.; Nakanishi, I. Matrix metalloproteinase-9 (92-kd gelatinase/type IV collagenase equals gelatinase B) can degrade arterial elastin. Am. J. Pathol. 1994, 145, 1208–1218. [Google Scholar] [PubMed] [PubMed Central]
- Shinohara, T.; Suzuki, K.; Okada, M.; Shiigai, M.; Shimizu, M.; Maehara, T.; Ohsuzu, F. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K.; Yoshida, T.; Miyagawa-Tomita, S. Tenascin-C in development and disease of blood vessels. Anat. Rec. 2014, 297, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Nozato, T.; Sato, A.; Hirose, S.; Hikita, H.; Takahashi, A.; Endo, H.; Imanaka-Yoshida, K.; Yoshida, T.; Aonuma, K.; Hiroe, M. Preliminary study of serum tenascin-C levels as a diagnostic or prognostic biomarker of type B acute aortic dissection. Int. J. Cardiol. 2013, 168, 4267–4269. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhou, X.; Zhu, A.; Peng, W.; Zhong, Y.; Chai, X. The Role of Serum Tenascin-C in Predicting In-Hospital Death in Acute Aortic Dissection. Int. Heart J. 2019, 60, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Suzuki, T.; Hiroi, Y.; Ohtaki, E.; Suzuki, S.; Yazaki, Y.; Nagai, R. Diagnosis of aortic dissection by immunoassay for circulating smooth muscle myosin. Lancet 1995, 345, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Katoh, H.; Tsuchio, Y.; Hasegawa, A.; Kurabayashi, M.; Ohira, A.; Hiramori, K.; Sakomura, Y.; Kasanuki, H.; Hori, S.; et al. Diagnostic implications of elevated levels of smooth-muscle myosin heavy-chain protein in acute aortic dissection: The smooth muscle myosin heavy chain study. Ann. Intern. Med. 2000, 133, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Distante, A.; Zizza, A.; Trimarchi, S.; Villani, M.; Salerno Uriarte, J.A.; de Luca Tupputi Schinosa, L.; Renzulli, A.; Sabino, F.; Nowak, R.; et al. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur. Heart J. 2008, 29, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Lian, R.; Liu, J.; Zhang, G.; Hu, T.; Li, G.; Zhang, S.; Zhang, G. Routine use of a pocket-sized handheld echoscopic device plus a biomarker by emergency medicine residents with an early screening algorithm for suspected Type A acute aortic syndrome. J. Clin. Med. 2023, 12, 1346. [Google Scholar] [CrossRef] [PubMed]
- Sbarouni, E.; Georgiadou, P.; Marathias, A.; Geroulanos, S.; Kremastinos, D.T. D-dimer and BNP levels in acute aortic dissection. Int. J. Cardiol. 2007, 122, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Zhou, X.L.; Li, J.J.; Hui, R.T. Biomarkers in aortic dissection. Clin. Chim. Acta 2011, 412, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Sodeck, G.; Domanovits, H.; Schillinger, M.; Janata, K.; Thalmann, M.; Ehrlich, M.P.; Endler, G.; Laggner, A. Pre-operative N-terminal pro-brain natriuretic peptide predicts outcome in type A aortic dissection. J. Am. Coll. Cardiol. 2008, 51, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, E.; Godon, P.; Kirkorian, G.; Chabaud, S.; Touboul, P. Significance of serum troponin I elevation in patients with acute aortic dissection of the ascending aorta. Acta Cardiol. 2005, 60, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, Y.W.; Lee, D.; Kim, T.Y.; Lee, S.; Seo, J.S.; Lee, J.H. The D-Dimer to Troponin Ratio Is a Novel Marker for the Differential Diagnosis of Thoracic Acute Aortic Syndrome from Non-ST Elevation Myocardial Infarction. J. Clin. Med. 2023, 12, 3054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Folli, C.; Consonni, D.; Spessot, M.; Salvini, L.; Velati, M.; Ranzani, G.; Maiavacca, R.; Monzani, V. Diagnostic role of copeptin in patients presenting with chest pain in the emergency room. Eur. J. Intern. Med. 2013, 24, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Oddi, M.; Cavalot, G.; Ianniello, A.; Giachino, F.; Nazerian, P.; Battista, S.; Magnino, C.; Tizzani, M.; Settanni, F.; et al. Prospective diagnostic and prognostic study of copeptin in suspected acute aortic syndromes. Sci. Rep. 2018, 8, 16713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sbarouni, E.; Georgiadou, P.; Marathias, A.; Panagiotakos, D.; Geroulanos, S.; Voudris, V. Ischemia-modified albumin in acute aortic dissection. J. Clin. Lab. Anal. 2010, 24, 399–402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, G.; Zhou, Y.; He, H.; Pan, X.; Chai, X. Ischemia-Modified Albumin, a Novel Predictive Marker of In-Hospital Mortality in Acute Aortic Dissection Patients. Front. Physiol. 2019, 10, 1253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, N.; Hata, N.; Kume, N.; Yokoyama, S.; Takano, M.; Shinada, T.; Tomita, K.; Shirakabe, A.; Inami, T.; Seino, Y.; et al. Detection of acute aortic dissection by extremely high soluble lectin-like oxidized LDL receptor-1 (sLOX-1) and low troponin T levels in blood. Int. J. Cardiol. 2013, 165, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Veeranna, V.; Zalawadiya, S.K.; Niraj, A.; Pradhan, J.; Ference, B.; Burack, R.C.; Jacob, S.; Afonso, L. Homocysteine and reclassification of cardiovascular disease risk. J. Am. Coll. Cardiol. 2011, 58, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Giusti, B.; Porciani, M.C.; Brunelli, T.; Evangelisti, L.; Fedi, S.; Gensini, G.F.; Abbate, R.; Sani, G.; Yacoub, M.; Pepe, G. Phenotypic variability of cardiovascular manifestations in Marfan Syndrome. Possible role of hyperhomocysteinemia and C677T MTHFR gene polymorphism. Eur. Heart J. 2003, 24, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, R.; Zhang, T.; Liu, F.; Zhang, W.; Wang, G.; Gu, G.; Han, Q.; Xu, D.; Yao, C.; et al. Identification of Lysophosphatidylcholines and Sphingolipids as Potential Biomarkers for Acute Aortic Dissection via Serum Metabolomics. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Sbarouni, E.; Georgiadou, P. MicroRNAs in acute aortic dissection. J. Thorac. Dis. 2018, 10, 1256–1257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boon, R.A.; Seeger, T.; Heydt, S.; Fischer, A.; Hergenreider, E.; Horrevoets, A.J.; Vinciguerra, M.; Rosenthal, N.; Sciacca, S.; Pilato, M.; et al. MicroRNA-29 in aortic dilation: Implications for aneurysm formation. Circ. Res. 2011, 109, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Goliopoulou, A.; Oikonomou, E.; Antonopoulos, A.; Koumallos, N.; Gazouli, M.; Theofilis, P.; Mystakidi, V.C.; Pantelidis, P.; Vavuranakis, M.A.; Siasos, G.; et al. Expression of Tissue microRNAs in Ascending Aortic Aneurysms and Dissections. Angiology 2023, 74, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, S.; Della Corte, A.; Alajbegovic, A.; Krawczyk, K.K.; Bancone, C.; Galderisi, U.; Cipollaro, M.; De Feo, M.; Forte, A. Patients with bicuspid and tricuspid aortic valve exhibit distinct regional microrna signatures in mildly dilated ascending aorta. Heart Vessel. 2017, 32, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Song, H.F.; Li, S.H.; Guo, J.; Tsang, K.; Tumiati, L.; Butany, J.; Yau, T.M.; Ouzounian, M.; Fu, S.; et al. Progressive Aortic Dilation Is Regulated by miR-17-Associated miRNAs. J. Am. Coll. Cardiol. 2016, 67, 2965–2977. [Google Scholar] [CrossRef] [PubMed]
- Elia, L.; Quintavalle, M.; Zhang, J.; Contu, R.; Cossu, L.; Latronico, M.V.; Peterson, K.L.; Indolfi, C.; Catalucci, D.; Chen, J.; et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell Death Differ. 2009, 16, 1590–1598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, M.; Zou, S.; Weng, J.; Hou, L.; Yang, L.; Zhao, Z.; Bao, J.; Jing, Z. A microRNA profile comparison between thoracic aortic dissection and normal thoracic aorta indicates the potential role of microRNAs in contributing to thoracic aortic dissection pathogenesis. J. Vasc. Surg. 2011, 53, 1341–1349.e3. [Google Scholar] [CrossRef] [PubMed]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, J.; Bao, J.; Feng, R.; Zhao, Z.; Lu, Q.; Wang, G.; Li, H.; Su, D.; Zhou, J.; Jing, Q.; et al. Circulating microRNAs: A novel potential biomarker for diagnosing acute aortic dissection. Sci. Rep. 2017, 7, 12784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Z.; Wang, Q.; Pan, J.; Sheng, X.; Hou, D.; Chong, H.; Wei, Z.; Zheng, S.; Xue, Y.; Zhou, Q.; et al. Characterization of serum miRNAs as molecular biomarkers for acute Stanford type A aortic dissection diagnosis. Sci. Rep. 2017, 7, 13659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.J.; Huang, B.; Yang, Y.M.; Zhang, L.; Su, W.J.; Tian, L.; Lu, T.Y.; Zhang, S.; Fan, X.H.; Hui, R.T. Differential expression of microRNAs in aortic tissue and plasma in patients with acute aortic dissection. J. Geriatr. Cardiol. 2015, 12, 655–661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, C.; Tang, X.; Zhu, X.; Zhou, Q.; Guo, Y.; Zhao, R.; Wang, D.; Gong, B. Expression profiles of circRNAs and the potential diagnostic value of serum circMARK3 in human acute Stanford type A aortic dissection. PLoS ONE 2019, 14, e0219013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, J.; Ge, S.; Zhang, L.; Che, H.; Liang, C. Aortic dissection is associated with reduced polycystin-1 expression, an abnormality that leads to increased ERK phosphorylation in vascular smooth muscle cells. Eur. J. Histochem. 2016, 60, 2711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, J.; Hu, Y.; Peng, P.; Li, J.; Ge, S. PKD1 participates in the processing of aortic dissection via regulating vascular smooth muscle cell contraction and MAPK signaling. Cell. Mol. Biol. 2023, 69, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Wan, F.; Xue, Y.; Cheng, W.; Zheng, H.; Zhao, Y.; Fan, F.; Han, Y.; Tong, C.; Yao, C. Lumican as a novel potential clinical indicator for acute aortic dissection: A comparative study based on multi-slice computed tomography angiography. Exp. Ther. Med. 2016, 11, 923–928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.-W.; Chou, S.-H.; Tung, Y.-C.; Hsiao, F.-C.; Ho, C.-T.; Chan, Y.-H.; Wu, V.C.-C.; Chou, A.-H.; Hsu, M.-E.; Lin, P.-J.; et al. Expression and role of lumican in acute aortic dissection: A human and mouse study. PLoS ONE 2021, 16, e0255238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- König, K.C.; Lahm, H.; Dreßen, M.; Doppler, S.A.; Eichhorn, S.; Beck, N.; Kraehschuetz, K.; Doll, S.; Holdenrieder, S.; Kastrati, A.; et al. Aggrecan: A new biomarker for acute type A aortic dissection. Sci. Rep. 2021, 11, 10371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kemberi, M.; Salmasi, Y.; Santamaria, S. The Role of ADAMTS Proteoglycanases in Thoracic Aortic Disease. Int. J. Mol. Sci. 2023, 24, 12135. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Gan, J.; Liu, Y.; Shi, L.; Lu, Z.; Xua, Y.; Xiong, R.; Liu, L.; Yang, Z.; Lin, Y.; et al. ADAMTS-5 Decreases in Aortas and Plasma From Aortic Dissection Patients and Alleviates Angiotensin II-Induced Smooth Muscle-Cell Apoptosis. Front. Cardiovasc. Med. 2020, 7, 136. [Google Scholar] [CrossRef]
- Li, K.; Wang, Z.W.; Hu, Z.; Ren, Z.; Hu, X.; Li, L.; Wu, Z.; Wu, H.; Li, B.; Huang, J.; et al. Assessing Serum Levels of ADAMTS1 and ADAMTS4 as New Biomarkers for Patients with Type A Acute Aortic Dissection. Med. Sci. Monit. 2017, 23, 3913–3922. [Google Scholar] [CrossRef]
- Kaufmann, J.O.; Brangsch, J.; Kader, A.; Saatz, J.; Mangarova, D.B.; Zacharias, M.; Kempf, W.E.; Schwaar, T.; Ponader, M.; Adams, L.C.; et al. ADAMTS4-specific MR probe to assess aortic aneurysms in vivo using synthetic peptide libraries. Nat. Commun. 2022, 13, 2867. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, W.; Yu, C.; Zhong, F.; Li, G.; Kong, W.; Zheng, J. A disintegrin and metalloproteinase with thrombospondin motif 1 (ADAMTS1) expression increases in acute aortic dissection. Sci. China Life Sci. 2016, 59, 59–67. [Google Scholar] [CrossRef]
- Güneş, M.F.; Akpinar, M.B.; Cömertoğlu, I.; Akyol, S.; Demirçelik, B.; Gürel, Ö.M.; Aynekin, B.; Erdemli, H.K.; Ateş, M.; Eryonucu, B.; et al. The Investigation of a Disintegrin and Metalloproteinase with ThromboSpondin Motifs (ADAMTS) 1, 5 and 16 in Thoracic Aortic Aneurysms and Dissections. Clin. Lab. 2016, 62, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Taketani, T.; Imai, Y.; Morota, T.; Maemura, K.; Morita, H.; Hayashi, D.; Yamazaki, T.; Nagai, R.; Takamoto, S. Altered patterns of gene expression specific to thoracic aortic aneurysms: Microarray analysis of surgically resected specimens. Int. Heart J. 2005, 46, 265–277. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, L.; Xu, G.; Palmero, L.C.; Albini, P.T.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2013, 95, 570–577. [Google Scholar] [CrossRef]
- Kilic, T.; Okuno, K.; Eguchi, S.; Kassiri, Z. Disintegrin and Metalloproteinases (ADAMs [A Disintegrin and Metalloproteinase] and ADAMTSs [ADAMs With a Thrombospondin Motif]) in Aortic Aneurysm. Hypertension 2022, 79, 1327–1338. [Google Scholar] [CrossRef]
- Yuan, S.M.; Wang, J.; Huang, H.R.; Jing, H. Osteopontin expression and its possible functions in the aortic disorders and coronary artery disease. Rev. Bras. Cir. Cardiovasc. 2011, 26, 173–182, Erratum in Rev. Bras. Cir. Cardiovasc. 2012, 27, 173. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Liu, X.; Gong, H.; Liu, Y.; Zhou, Z.; Hu, M.; Zhang, X. Serum Sema7A is increased in patients with acute aortic dissection. Expert Rev. Mol. Diagn. 2023, 23, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Kostina, A.; Kostareva, A.; Irtyuga, O.; Gordeev, M.; Uspensky, V. Notch signaling in the pathogenesis of thoracic aortic aneurysms: A bridge between embryonic and adult states. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165631, Erratum in Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165732. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Ren, P.; Nguyen, M.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. Notch signaling in descending thoracic aortic aneurysm and dissection. PLoS ONE 2012, 7, e52833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, C.; Zhao, H.; Shi, F.; Li, M.; Liu, X.; Ji, C.; Han, Y. Serum Ceruloplasmin Is the Candidate Predictive Biomarker for Acute Aortic Dissection and Is Related to Thrombosed False Lumen: A Propensity Score-Matched Observational Case-Control Study. Biol. Trace Elem. Res. 2021, 199, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Yang, H.; Tang, Q.; Gong, Y.C.; Fu, Y.H.; Wan, F.; Yang, B.; Guo, R.; Zhong, Y.L.; Zhu, J.M.; et al. Identification of Vinculin as a Potential Diagnostic Biomarker for Acute Aortic Dissection Using Label-Free Proteomics. Biomed Res. Int. 2020, 2020, 7806409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, T.; Trimarchi, S.; Sawaki, D.; Grassi, V.; Costa, E.; Rampoldi, V.; Nagai, R.; Eagle, K. Circulating transforming growth factor-beta levels in acute aortic dissection. J. Am. Coll. Cardiol. 2011, 58, 775. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, P.; Ma, K.; Li, Y.; Yuan, H.; Zhu, J.; Duan, W.; Ou, J.; Huang, Y.; Wu, L.; et al. OPG/TRAIL ratio as a predictive biomarker of mortality in patients with type A acute aortic dissection. Nat. Commun. 2021, 12, 3401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Jiang, S.; He, J.; Li, N.; Fan, Y.; Zhao, X.; Hu, X. Uric acid in aortic dissection: A meta-analysis. Clin. Chim. Acta 2018, 484, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Mellnick, V.M.; Monteiro, S.; Patlas, M.N. Acute aortic syndrome: Yield of computed tomography angiography in patients with acute chest pain. Can. Assoc. Radiol. J. 2019, 70, 23–28. [Google Scholar] [CrossRef]
- Ma, M.; Chen, W.; Cao, H.L.; Pan, J.; Zhou, Q.; Tang, X.L.; Wang, D.J. The diagnostic value of tenascin-C in acute aortic syndrome. J. Geriatr. Cardiol. 2024, 21, 359–368. [Google Scholar] [CrossRef] [PubMed]
| The Bakey System | |
| Type 1 | The dissection begins in the ascending aorta and involves the aortic arch and descending aorta. |
| Type 2 | The dissection originates in and is confined to the ascending aorta. |
| Type 3 | The dissection starts in the descending aorta and extends distally. |
| 3a | Extends above the diaphragm. |
| 3b | Extends below the diaphragm. |
| Stanford classification | |
| Type A | Involves the ascending aorta, regardless of the location of the primary intimal tear. It is defined as a dissection occurring proximal to the brachiocephalic artery. |
| Type B | Originates distal to the left subclavian artery and is confined to the descending aorta. The entry tear is located beyond the origin of the innominate artery [6] |
| Authors | Cut-Off | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % | NPV % |
|---|---|---|---|---|---|
| Nazerian P. et al. [36] | ADD-RS ≥ 1 | 95 (91.5–97.4) | 26.4 (24.3–28.7) | 16.2 (14.3–18.3) | 97.3 (95.3–98.6) |
| Nazerian P. et al. [36] | D-dimer ˃ 0.5 g/L | 96.7% (93.6–98.6) | 64% (61.6–66.4) | 28.7% (25.6–32) | 99.2% (98.5–99.7) |
| Nazerian P. et al. [36] | ADD-RS ˃ 0 + D-dimer ˃ 0.5 g/L | 99.6 (97.7–100) | 18.2 (16.4–20.2) | 15.4 (13.7–17.3) | 99.7 (98.1–100) |
| Nazerian P. et al. [36] | ADD-RS ˃ 1 + D-dimer ˃ 0.5 g/L | 98.8 (96.4–99.7) | 57.3 (54.9–59.7) | 25.8 (23–28.7) | 99.7 (99.1–99.9) |
| Gorla R. et al. [37] | ADD-RS ˃ 0 | 98.8 | 64.6 | 44.9 | 99.5 |
| Gorla R. et al. [37] | D-dimer ˃ 0.5 g/L | 97.6 | 63.2 | 43.7 | 98.9 |
| Gorla R. et al. [37] | ADD-RS = 0 + D-dimer ˃ 0.5 g/L | 100 | 67.5 | 1.6 | 100 |
| Gorla R. et al. [37] | ADD-RS 1 + D-dimer ˃ 0.5 g/L | 93.5 | 63.2 | 21.5 | 98.9 |
| McLatchie R. et al. [38] | ADD-RS ≥ 1 | 69 (39–91) | 59 (58–60) | 40 (18–76) | 99.9 (99.7–100) |
| McLatchie R. et al. [38] | D-dimer ˃ 0.5 g/L | 57 (18–90) | 61 (57–65) | 1.9 (0.6–4.2) | 99.8 (99.6–99.9) |
| McLatchie R. et al. [38] | ADD-RS 1 + D-dimer ˃ 0.5 g/L | 100 (69–100) | 8 (7–9) | 42 (2–77) | 100 (98.2–100) |
| Deng L. et al. [39] | ADD-RS ˃ 1 + D-dimer ˃ 2.0 g/L | 93.1 | 70.2 | 34.6 | 98.4 |
| Kotani Y. et al. [40] | ADD-RS ˃ 1 + positive age adjusted d-dimer | 88.0 (68–97) | 45.0 (30–60) | - | - |
| Ren S. et al. [41] | ADD RS > 0 + D-dimer > 0.5 g/L | 99.8 (98.7–100) | 21.8 (12.1–32.6) | - | - |
| Ren S. et al. [41] | ADD RS > 1 + D-dimer > 0.5 g/L | 98.3 (94.9–99.5) | 51.4 (38.7–64.1) | - | - |
| Ohle R. [42] | RIPP ≥ 2 | 99.7 (98.5–99.9) | 53.0 (47.9–58.1) | 68.0 (64.0–71.7) | 99.5 (97.2–99.9) |
| Authors | Cut-Off | Sensitivity % (95% CI) | Specificity % (95% CI) | LR+ (95% CI) | LR− (95% CI) |
| Morello F. [43] | High prevalence population AORTAs ≤ 1/D-Dimer age adjusted | 98.3 (95.7–99.5) | 30 (26.9–33.4) | 1.41 (1.34–1.47) | 0.06 (0.02–0.15) |
| Morello F. [43] | High prevalence population AORTAs ≤ 1/D-Dimer 0.5 g/L | 99.1 (96.9–99.9) | 30.2 (27–33.5) | 1.42 (1.35–1.49) | 0.03 (0.01–0.11) |
| Morello F. [43] | Low prevalence population AORTAs ≤ 1/D-Dimer age adjusted | 100 (92.7–100) | 48.7 (43.8–53.7) | 1.95 (1.74–2.14) | 0 (0–0.12) |
| Morello F. [43] | Low prevalence population AORTAs ≤ 1/D-Dimer 0.5 g/L | 98 (89.3–99.6) | 52.8 (47.9–57.7) | 2.08 (1.86–2.33) | 0.04 (0–0.14) |
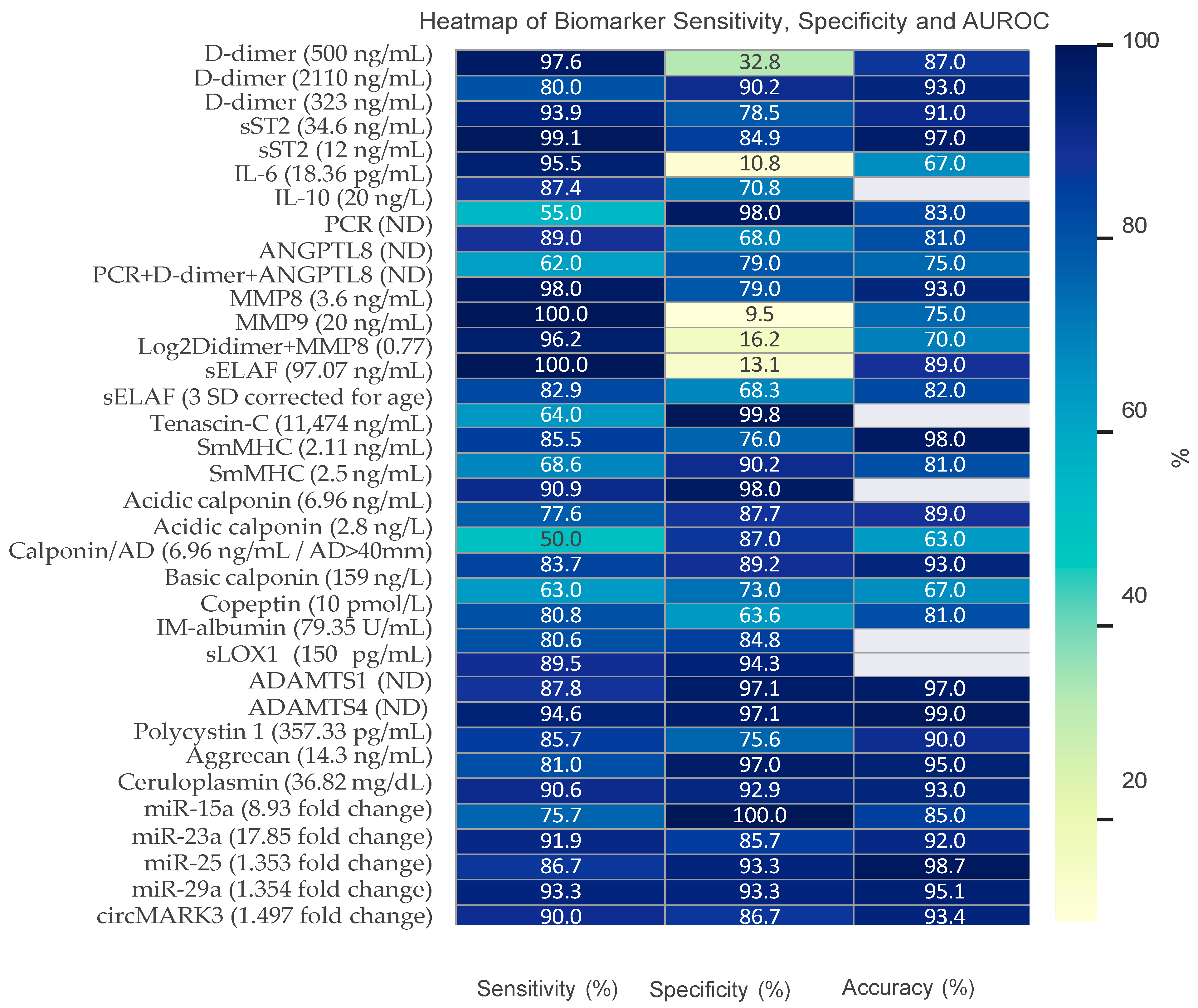
| Authors | Biomarker | Cut-Off | Sensitivity % (95% CI) | Specificity % (95% CI) | Accuracy (AUROC) |
|---|---|---|---|---|---|
| Giachino et al., 2013 [77] | D-dimer | 500 ng/mL | 97.6 (87.4–99.9) | 32.8 (21.3–46.0) | 0.87 (0.08–0.97) |
| Meng et al., 2019 [135] | D-dimer | 500 ng/mL | 100 | 51.3 | ND |
| Morello et al., 2018 [93] | D-dimer | 500 ng/mL | 95.2 (88.3–98.1) | 65.4 (58.5–71.8) | 0.92 (0.89–0.96) |
| Morello et al., 2020 [60] | D-dimer | 500 ng/mL | 95.8 (88.1–99.1) | 30.7 (19.6–43.7) | 0.84 (0.75–0.91) |
| Peng et al., 2015 [68] | D-dimer | 2.11 µg/mL | 80 | 90.2 | 0.93 (0.87–0.98) |
| Wang et al., 2017 [59] | D-dimer | 323 ng/mL | 93.9 | 78.5 | 0.91 (0.88–0.94) |
| Zhang et al., 2023 [75] | D-dimer | 500 ng/mL | 74 (69–79) | 76 (72–80) | 0.82 (0.79–0.85) |
| Suzuki I et al., 2009 [56] | D-dimer | 500 ng/mL | 96.6 (90.3–99.3) | 46.6 (37.9–55.5) | 0.84 (0.78–0.89) |
| Sodek G et al., 2008 [89] | D-dimer | 500 ng/mL | 97.0 (94–98) | 59 (53–64) | 0.94 |
| Wang et al., 2017 [59] | sST2 | 34.6 ng/mL | 99.1 (ND) | 84.9 | 0.97 (0.95–0.98) |
| Yang et al., 2020 [72] | D-dimer | ND | 67 | 98 | 0.88 (0.8–0.93) |
| Morello et al., 2020 [60] | sST2 | 12 ng/mL | 95.5 (88.8–98.7) | 10.8 (4.8–20.2) | 0.67 (0.61–0.74) |
| Qu et al., 2015 [63] | IL-6 | 18.36 pg/mL | 87.4 | 70.8 | ND |
| Forrer a et al., 2021 [65] | IL-10 | 20 ng/L | 55 | 98 | 0.83 (0.72–0.94) |
| Yang et al., 2020 [72] | PCR | ND | 89 | 68 | 0.81 (0.73–0.88) |
| Yang et al., 2020 [72] | ANGPTL8 | ND | 62 | 79 | 0.75 (0.66–0.83) |
| Yang et al., 2020 [72] | PCR + D-dimer + ANGPTL8 | ND | 98 | 79 | 0.93 (0.88–0.97) |
| Giachino et al., 2013 [77] | MMP8 | 3.6 ng/mL | 100 (93.2–100) | 9.5 (3.9–18.5) | 0.75 |
| Giachino et al., 2013 [77] | MMP9 | 20 ng/mL | 96.2 (86.8–99.5) | 16.2 (87.26.6) | 0.70 |
| Giachino et al., 2013 [77] | Log2Didimer + MMP8 | 0.77 | 100 (91.6–100) | 13.1 (5.8–24.2) | 0.89 (0.82–0.95) |
| Peng et al., 2015 [68] | sELAF | 97.07 ng/mL | 82.9 | 68.3 | 0.82 (0.73–0.91) |
| Shinohara et al., 2003 [79] | sELAF | 3 SD corrected for age | 64 | 99.8 | ND |
| Ma et al., 2024 [136] | Tenascin-C | 11474 ng/mL | 85.5 | 76.0 | 0.98 (0.96–0.99) |
| Peng et al., 2015 [68] | SmMHC | 2.11 ng/mL | 68.6 | 90.2 | 0.81 (0.71–0.91) |
| Suzuki et al., 2000 [84] | SmMHC | 2.5 ng/mL | 90.9 | 98 | ND |
| Suzuki et al., 2008 [85] | SmMHC | 2.5 ng/mL | 90 | 97 | ND |
| Lian et al., 2023 [86] | Acidic calponin | 6.96 ng/mL | 77.6 | 87.7 | 0.89 |
| Suzuki et al., 2008 [85] | Acidic calponin | 2.8 ng/L | 50 | 87 | 0.63 |
| Lian et al., 2023 [86] | Calponin and aortic diameter | 6.96 ng/mL AD > 40 mm | 83.7 | 89.2 | 0.93 |
| Suzuki et al., 2008 [85] | Basic calponin | 159 ng/L | 63 | 73 | 0.67 |
| Morello et al., 2018 [93] | Copeptin | 10 pmol/L | 80.8 (72.2–87.2) | 63.6 (56.9–69.9) | 0.81 (0.75–0.86) |
| Yang et al., 2019 [95] | IM-albumin | 79.35 U/mL | 80.6 | 84.8 | ND |
| Kobayashi et al., 2013 [96] | sLOX1 | 150 pg/mL | 89.5 | 94.3 | ND |
| Li et al., 2017 [119] | ADAMTS1 | ND | 87.8 | 97.1 | 0.97 (0.94–0.99) |
| Li et al., 2017 [119] | ADAMTS4 | ND | 94.6 | 97.1 | 0.99 (0.97–1.00) |
| Lyu et al., 2023 [127] | Sema7A | ND | ND | ND | 0.84 (0.78, 0.91) |
| Peng W et al., 2015 [68] | Polycystin 1 | 357.33 pg/mL | 85.7 | 75.6 | 0.90 (0.83–0.96) |
| Konig CK et al., 2021 [116] | Aggrecan | 14.3 ng/mL | 81 | 97 | 0.95 |
| Ma et al., 2021 [130] | Ceruloplasmin | 36.82 mg/dL | 90.6 | 92.9 | 0.93 (0.88–0.97) |
| Dong et al., 2017 [108] | miR-15a | 8.93 (fold change) | 75.7 | 100 | 0.85 (0.85–0.96) |
| Dong et al., 2017 [108] | miR-23a | 17.85 (fold change) | 91. | 85.7 | 0.92 (0.85–0.99) |
| Xu et al., 2017 [109] | miR-25 | 25 1.353 (fold change) | 86.7 | 93.3 | 0.987 (0.937–1.000) |
| Xu et al., 2017 [109] | miR-29a | 1.354 (fold change) | 93.3 | 93.3 | 0.951 (0.86–1.00) |
| Tian et al., 2019 [111] | circMARK3 | 1.497 (fold change) | 90.0 | 86.7 | 0.934 (0.87–1.0) |
| Biomarker | Clinical Rationale (Potential Utility) | Evidence, Size, Heterogeneity | Data Robustness | Clinical Adoption Potential | Limitation | Feasibility | Recommended Current Role |
|---|---|---|---|---|---|---|---|
| D-dimer | Fibrinolysis marker; helps rule-out AAS combined with low clinical probability | Large multicenter; mixed designs but consistent sensitivity within 24 h | Moderate/ High | High | Low specificity; false negatives possible) | Community ED, secondary and tertiary centers | Clinical use |
| sST2 | Myocardial stretch/inflammation | Small–moderate; heterogeneous cut-offs | Low | Low | Limited availability; non-specific | Tertiary | Research |
| IL-6 | Systemic inflammationmay track extent/complication | Small–moderate; variable timing assays | Low–Moderate | Low | Non-specific; slow TAT | Tertiary | Research |
| IL-10 | Anti-inflammatory response | Small; heterogeneous | Low | Low | Limited incremental value) | Tertiary | Research |
| CRP | Acute-phase, prognostic interest only | Large literature but non-specific | Low | Very low | Poor specificity | Community ED | Abandon |
| ANGPTL8 | Lipid/inflammation axis | Very small, exploratory | Very low | Low | Lack of esternal validation | Tertiary | Research |
| MMP-8 | ECM degradation | Small; assay heterogeneity | Low-moderate | Low | Limited rapid test | Tertiary | Research |
| MMP-9 | ECM remodeling | Small–moderate; conflicting thresholds | Low | Low | Non-specific; timing effects) | Tertiary | Research |
| Copeptin | Released in response to stess | Small, single centre study | Low | Low | Non-specific nor sensitive | Tertiary | Abandon |
| sELAF | Elastin degradation | Small; assay variability | Low | Low | Lack of standardization | Tertiary | Research |
| Tenascin-C | ECM stress protein | Small–moderate | Low-moderate | Low | Limited assays | Tertiary | Research |
| smMHC | Smooth-muscle injury; early release | Small–moderate; promising | Moderate | Low-moderate | Short diagnostic window | Tertiary | Research |
| Acid Calponin | SMC injury | Small; exploratory | Low | Low | Limited availability | Tertiary | Research |
| Basic Calponin | SMC injury | Small; exploratory | Low | Low | Limited availability | Tertiary | Research |
| IMA | Global ischemia marker | Small | Low | Low | Poor specificity | Tertiary | Abandon |
| sLOX-1 | Endothelial injury/oxidized LDL receptor | Small–moderate | Moderate | Low-moderate | Limited availibility | Tertiary | Research |
| ADAMTS-1 | Protease, ECM turnover | Small | Low | Low | Only preliminary data | Tertiary | Research |
| ADAMTS-4 | Protease, ECM turnover | Small | Low | Low | Only preliminary data | Tertiary | Research |
| Sema7A | Immune/semaphorin pathway | Very small | Very low | Very low | preliminary data | Tertiary | Research |
| Polycystin-1 | Structural protein | Minimal/indirect | Very low | Very low | preliminary data | Tertiary | Abandon |
| Aggrecan | ProteoglycanECM damage | Very small | Very low | Very low | Limited specificity | Tertiary | Research |
| Ceruloplasmin | Acute-phase/oxidative | Large | Very low | Very low | nonspecific | Tertiary | Abandon |
| miR-15a | Tissue injury signature | Small cohorts | Low | Low | Preliminary data, PCR-based; slow TAT | Tertiary | Research |
| miR-23a | Tissue injury signature | Small cohorts | Low | Low | Preliminary data, PCR-based; slow TAT | Tertiary | Research |
| miR-29a | ECM remodeling | Small cohorts | Low | Low | Preliminary data, PCR-based; slow TAT | Tertiary | Research |
| circMARK3 | Omics signal | Small cohorts, heterogeneous | Low | Low | Preliminary data | Tertiary | Research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignataro, G.; Scafetta, A.; De Luca, D.; Simeoli, L.; Piccioni, A.; Ojetti, V.; Franceschi, F.; Candelli, M. The Role of Biomarkers and Clinical Prediction Tools in the Diagnosis of Acute Aortic Syndromes: A Literature-Based Review. Medicina 2025, 61, 1551. https://doi.org/10.3390/medicina61091551
Pignataro G, Scafetta A, De Luca D, Simeoli L, Piccioni A, Ojetti V, Franceschi F, Candelli M. The Role of Biomarkers and Clinical Prediction Tools in the Diagnosis of Acute Aortic Syndromes: A Literature-Based Review. Medicina. 2025; 61(9):1551. https://doi.org/10.3390/medicina61091551
Chicago/Turabian StylePignataro, Giulia, Alice Scafetta, Donatella De Luca, Laura Simeoli, Andrea Piccioni, Veronica Ojetti, Francesco Franceschi, and Marcello Candelli. 2025. "The Role of Biomarkers and Clinical Prediction Tools in the Diagnosis of Acute Aortic Syndromes: A Literature-Based Review" Medicina 61, no. 9: 1551. https://doi.org/10.3390/medicina61091551
APA StylePignataro, G., Scafetta, A., De Luca, D., Simeoli, L., Piccioni, A., Ojetti, V., Franceschi, F., & Candelli, M. (2025). The Role of Biomarkers and Clinical Prediction Tools in the Diagnosis of Acute Aortic Syndromes: A Literature-Based Review. Medicina, 61(9), 1551. https://doi.org/10.3390/medicina61091551








