The Impact of the COVID-19 Pandemic on New Lung Cancer Diagnosis in Mureș County, Romania: A 5-Year Retrospective, Comprehensive Study
Abstract
1. Introduction
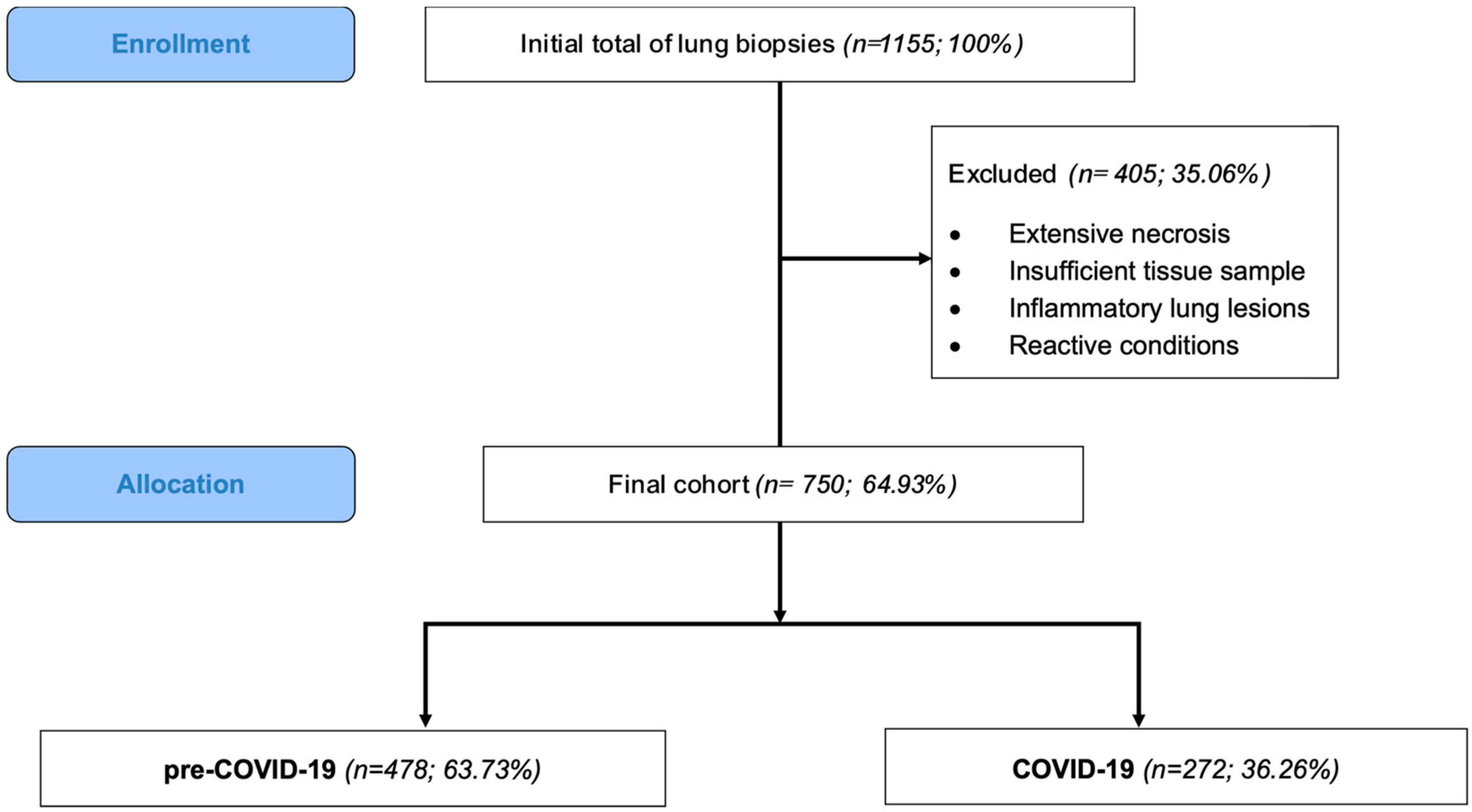
2. Materials and Methods
2.1. Study Design and Data Collection
- Pre-COVID 19 cohort (1 January 2018–15 March 2020);
- COVID-19 cohort (16 March 2020–31 December 2022).
2.2. Pathological Data
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Pathological Data
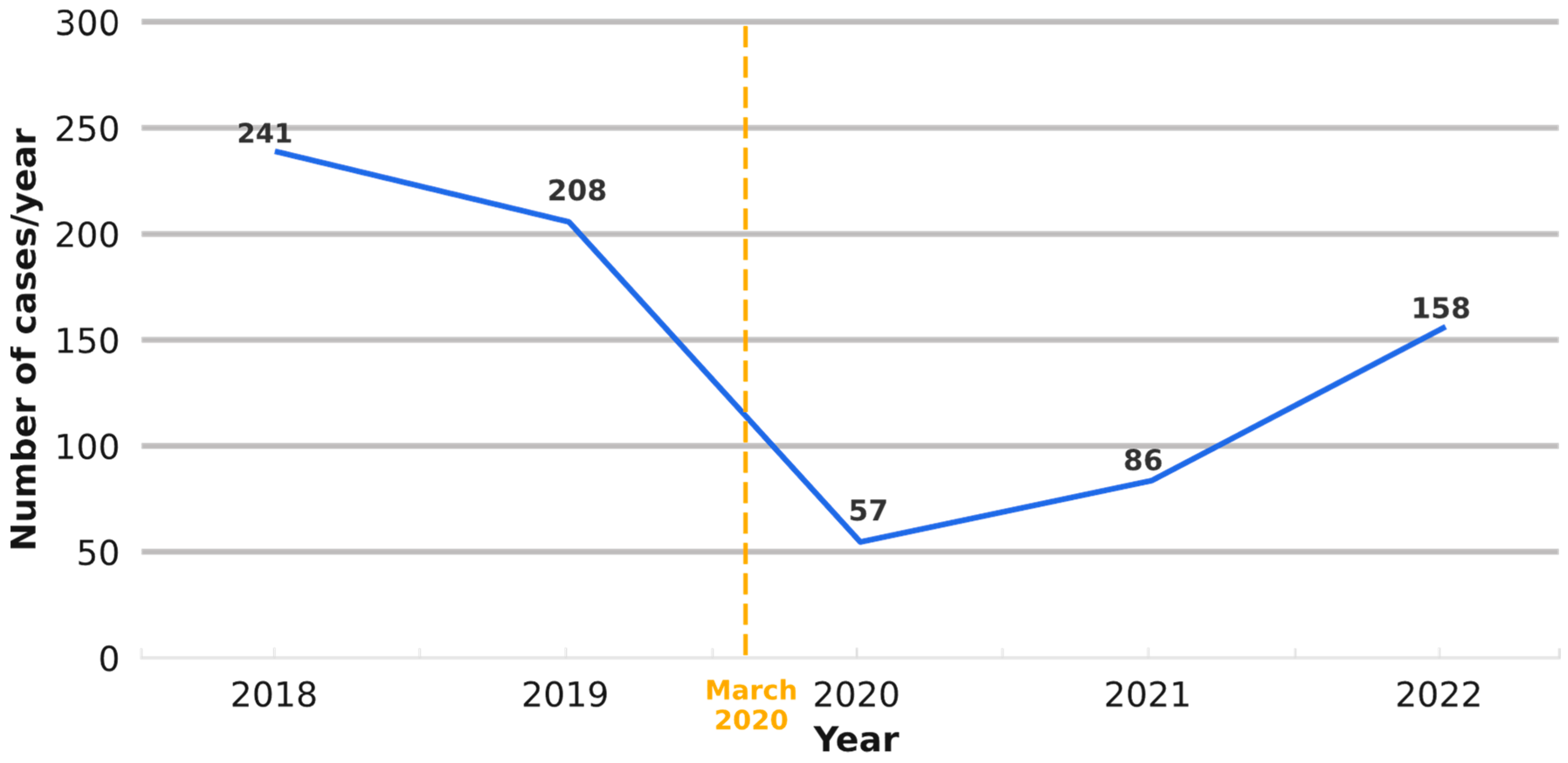
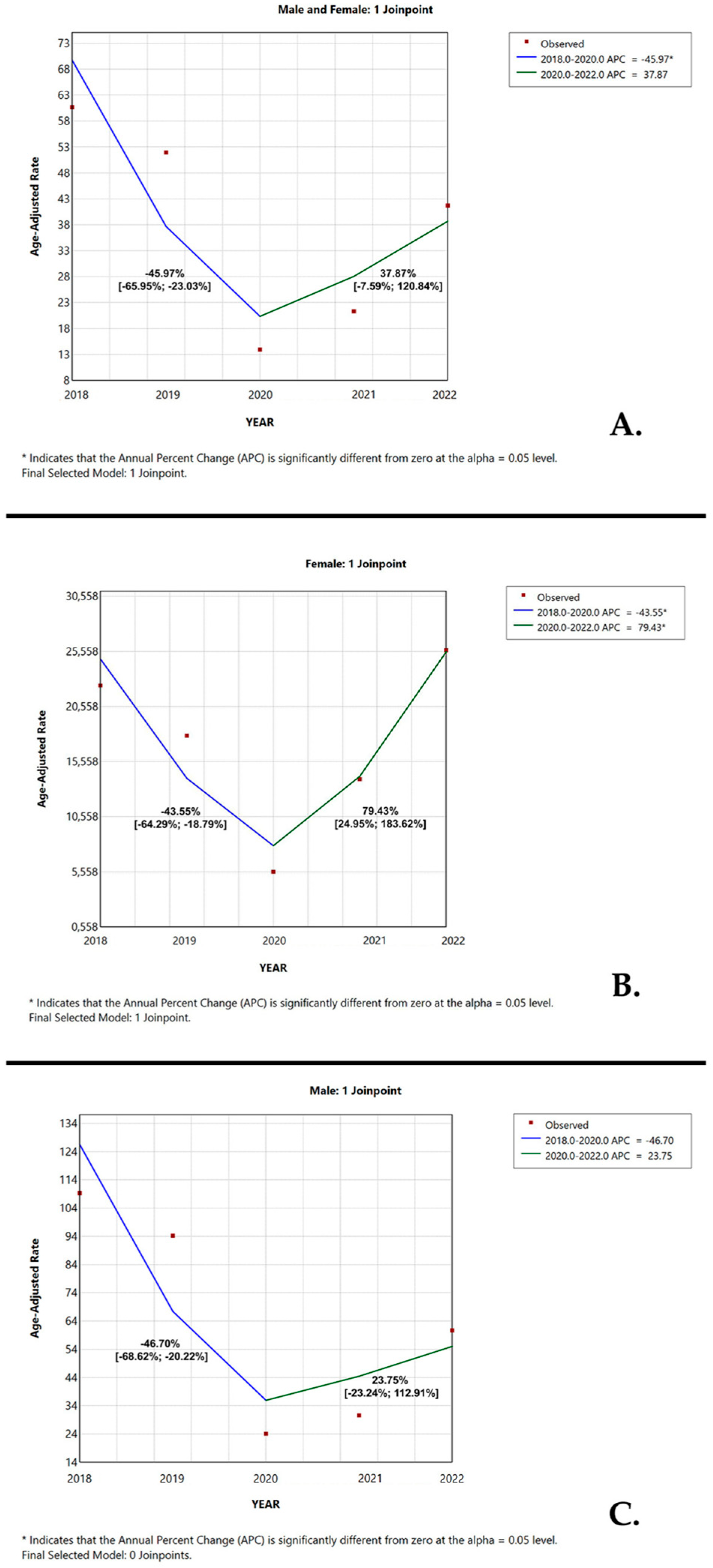
3.3. Temporal Trend
3.4. Associations Between Demographic and Morphologic Characteristics of the Study Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADK | Lung adenocarcinoma |
| APC | Annual percentage change |
| BRAF | V-Raf Murine Sarcoma Viral Oncogene Homolog B |
| CD 56 | Cluster of Differentiation 56 |
| COVID-19 | Coronavirus disease 2019 |
| CYP1A1 | Cytochrome P450 Family 1 Subfamily A Member 1 |
| CK AE1/AE3 | Cytokeratin AE1/AE3 |
| CK 7 | Cytokeratin 7 |
| DNA | Deoxyribonucleic acid |
| EGFR | Epidermal growth factor receptor |
| GATS | Global Adult Tobacco Survey |
| GRPR | Gastrin-releasing peptide receptor |
| KRAS | Kirsten rat sarcoma virus |
| LC | Lung cancer |
| LT | Lung tumor |
| MET | Mesenchymal–epithelial transition factor |
| M/F | Male-to-female ratio |
| NSCLC NOS | Non-small-cell lung cancer not otherwise specified |
| NSCLC | Non-small-cell lung cancer |
| NTRK | Neurotrophic tyrosine receptor kinase |
| p40 | Nuclear marker with expression in squamous-cell differentiation (p63 isoform) |
| p53 | Tumor suppressor gene at 17p13 |
| RR | Relative risk |
| SCLC | Small-cell lung cancer |
| SD | Standard deviation |
| SQC | Squamous-cell carcinoma |
| WHO | World Health Organization |
| TTF1 | Thyroid transcription factor 1; nuclear marker |
References
- Global Cancer Observatory. Available online: https://gco.iarc.who.int/ (accessed on 8 January 2025).
- Smolarz, B.; Łukasiewicz, H.; Samulak, D.; Piekarska, E.; Kołaciński, R.; Romanowicz, H. Lung Cancer-Epidemiology, Pathogenesis, Treatment and tiMolecular Aspect (Review of Literature). Int. J. Mol. Sci. 2025, 26, 2049. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global epidemiology of lung cancer. Ann. Global Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, S. The Successes and Failures of the Initial COVID-19 Pandemic Response in Romania. Front. Public Health 2020, 8, 344. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Huber, R.M.; Cavic, M.; Kerpel-Fronius, A.; Viola, L.; Field, J.; Jiang, L.; Kazerooni, E.A.; Koegelenberg, C.F.N.; Mohan, A.; dos Santos, R.S.; et al. Lung Cancer Screening Considerations During Respiratory Infection Outbreaks, Epidemics or Pandemics: An International Association for the Study of Lung Cancer Early Detection and Screening Committee Report. J. Thorac. Oncol. 2022, 17, 228–238. [Google Scholar] [CrossRef]
- Mitjà, P.S.; Àvila, M.; García-Olivé, I. Impact of the COVID-19 pandemic on lung cancer diagnosis and treatment. Med. Clínica 2022, 158, 138–139. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Bradley, S.H.; Kennedy, M.P.; Neal, R.D. Recognising Lung Cancer in Primary Care. Adv. Ther. 2020, 37, 1701. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhang, Y.; Etxeberria, J.; Arnold, M.; Cai, X.; Hao, Y.; Zou, H. Projections of Lung Cancer Incidence by 2035 in 40 Countries Worldwide: Population-Based Study. JMIR Public Health Surveill. 2023, 9, e43651. [Google Scholar] [CrossRef]
- Aden, D.; Zaheer, S.; Raj, S. Challenges faced in the cancer diagnosis and management—COVID-19 pandemic and beyond—Lessons for future. Heliyon 2022, 8, e12091. [Google Scholar] [CrossRef]
- Yang, L.; Chai, P.; Yu, J.; Fan, X. Effects of cancer on patients with COVID-19: A systematic review and meta-analysis of 63,019 participants. Cancer Biol. Med. 2021, 18, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; De Vincentiis, L.; Ambrosini-Spaltro, A.; Barbareschi, M.; Bertolini, V.; Contato, E.; Crivelli, F.; Feyles, E.; Mariani, M.P.; Morelli, L.; et al. Cancer diagnostic delay in northern and central italy during the 2020 lockdown due to the coronavirus disease 2019 pandemic: Assessment of the magnitude of the problem and proposals for corrective actions. Am. J. Clin. Pathol. 2021, 155, 64–68. [Google Scholar] [CrossRef]
- Kasymjanova, G.; Anwar, A.; Cohen, V.; Sultanem, K.; Pepe, C.; Sakr, L.; Friedmann, J.; Agulnik, J.S. The impact of COVID-19 on the diagnosis and treatment of lung cancer at a canadian academic center: A retrospective chart review. Curr. Oncol. 2021, 28, 4247–4255. [Google Scholar] [CrossRef]
- Patt, D.; Gordan, L.; Diaz, M.; Okon, T.; Grady, L.; Harmison, M.; Markward, N.; Sulivan, M.; Peng, J.; Zhou, A. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin. Cancer Inform. 2020, 4, 1059–1071. [Google Scholar] [CrossRef]
- Morais, S.; Antunes, L.; Rodrigues, J.; Fontes, F.; Bento, M.J.; Lunet, N. The impact of the coronavirus disease 2019 pandemic on the diagnosis and treatment of cancer in Northern Portugal. Eur. J. Cancer Prev. 2022, 31, 204–214. [Google Scholar] [CrossRef]
- Coma, E.; Guiriguet, C.; Mora, N.; Marzo-Castillejo, M.; Benitez, M.; Mendez-Boo, L.; Fina, F.; Fabregas, M.; Mercade, A.; Medina, M. Impact of the COVID-19 pandemic and related control measures on cancer diagnosis in Catalonia: A time-series analysis of primary care electronic health records covering about five million people. BMJ Open 2021, 11, e047567. [Google Scholar] [CrossRef]
- Greene, G.; Griffiths, R.; Han, J.; Akbari, A.; Jones, M.; Lyons, J.; Lyons, R.A.; Rolles, M.; Torabi, F.; Warlow, J.; et al. Impact of the SARS-CoV-2 pandemic on female breast, colorectal and non-small cell lung cancer incidence, stage and healthcare pathway to diagnosis during 2020 in Wales, UK, using a national cancer clinical record system. Br. J. Cancer 2022, 127, 558–568. [Google Scholar] [CrossRef]
- Bennett, D.; Murray, I.; Mitchell, H.; Gavin, A.; Donnelly, D. Impact of COVID-19 on cancer incidence, presentation, diagnosis, treatment and survival in Northern Ireland. Int. J. Cancer 2024, 154, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Burau, V.; Mejsner, S.B.; Falkenbach, M.; Fehsenfeld, M.; Kotherova, Z.; Neri, S.; Wallenburg, I.; Kuhnlmann, E. Post-COVID health policy responses to healthcare workforce capacities: A comparative analysis of health system resilience in six European countries. Health Policy 2024, 139, 104962. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Craina, M.; Pantea, S.; Pirvu, C.; Chiriac, V.D.; Marincu, I.; Bratosin, F.; Bogdan, I.; Hosin, S.; Citu, C.; et al. COVID-19 Pandemic Effects on Cervical Cancer Diagnosis and Management: A Population-Based Study in Romania. Diagnostics 2022, 12, 907. [Google Scholar] [CrossRef]
- Ungureanu, L.; Apostu, A.P.; Vesa, Ș.C.; Cășeriu, A.E.; Frățilă, S.; Iancu, G.; Bejinariu, N.; Munteanu, M.; Șenilă, S.C.; Vasilovici, A. Impact of the COVID-19 Pandemic on Melanoma Diagnosis in Romania—Data from Two University Centers. Int. J. Environ. Res. Public Health 2022, 19, 15129. [Google Scholar] [CrossRef]
- Burciu, C.; Miutescu, B.; Bende, R.; Burciu, D.; Moga, T.V.; Popescu, A.; Popa, A.; Bende, F.; Gadour, E.; Burdan, A.; et al. Effects of the COVID-19 Pandemic and Post-Pandemic Changes on the Diagnosis, Treatment, and Mortality of Hepatocellular Carcinoma in a Tertiary Center in Western Romania. Cancers 2025, 17, 1660. [Google Scholar] [CrossRef]
- Mayne, N.R.; Bajaj, S.S.; Powell, J.; Elser, H.C.; Civiello, B.S.; Fintelmann, F.J.; Li, X.; Yang, C.-F.J. Extended Delay to Treatment for Stage III-IV Non–Small-Cell Lung Cancer and Survival: Balancing Risks During the COVID-19 Pandemic. Clin. Lung Cancer 2022, 23, e362–e376. [Google Scholar] [CrossRef] [PubMed]
- Kasymjanova, G.; Rizzolo, A.; Pepe, C.; Friedmann, J.E.; Small, D.; Spicer, J.; Lecavalier-Barsoum, M.; Sultanem, K.; Wang, H.; Spatz, A.; et al. The Impact of COVID-19 on the Diagnosis and Treatment of Lung Cancer over a 2-Year Period at a Canadian Academic Center. Curr. Oncol. 2022, 29, 8677–8685. [Google Scholar] [CrossRef]
- Maxwell, S.S.; Weller, D. Lung cancer and COVID-19: Lessons learnt from the pandemic and where do we go from here? NPJ Prim. Care Respir. Med. 2022, 32, 19. [Google Scholar] [CrossRef]
- Mangone, L.; Marinelli, F.; Bisceglia, I.; Filice, A.; De Leonibus, L.; Rapicetta, C.; Paci, M. The Influence of COVID-19 on New Lung Cancer Diagnoses, by Stage and Treatment, in Northern Italy. Biology 2023, 12, 390. [Google Scholar] [CrossRef]
- Paraschiv, B.; Diaconu, C.C.; Cucu, A.; Bogdan, M.A.; Toma, C.L. The trend of epidemiological data in patients with lung cancer addressed to a Romanian tertiary pneumology service. Arch. Balk. Med. Union 2019, 54, 281–287. [Google Scholar] [CrossRef]
- Beatrice, S.; Valentin, B.M.; Elena, M.; Oana, I.; Sergiu, C. About Lung Cancer in Romania and Constanta County. ARS Medica Tomitana 2020, 26, 145–149. [Google Scholar] [CrossRef]
- Guarga, L.; Ameijide, A.; Marcos-Gragera, R.; Carulla, M.; Delgadillo, J.; Borràs, J.M.; Galceran, J. Trends in lung cancer incidence by age, sex and histology from 2012 to 2025 in Catalonia (Spain). Sci. Rep. 2021, 11, 23274. [Google Scholar] [CrossRef] [PubMed]
- Centre for Diseases Control. Global Adult Tobacco Survey 2018 Romania; CDC: Atlanta, GA, USA, 2021. [Google Scholar]
- Global Adult Tobacco Survey (GATS). Romania Country Report; Ministry of Health Romania: Bucharest, Romania, 2011. [Google Scholar]
- Mederos, N.; Friedlaender, A.; Peters, S.; Addeo, A. Gender-specific aspects of epidemiology, molecular genetics and outcome: Lung cancer. ESMO Open 2020, 5 (Suppl. 4), e000796. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.; Huang, J.Y.; Chen, H.C.; Lin, C.H.; Lin, S.H.; Hung, W.H.; Cheng, Y.F. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J. Cancer Res. Clin. Oncol. 2020, 146, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Cruz, C.S.D. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Olteanu, G.-E.; Vigdorovits, A.; Barna, R.A.; Mazilu, L.; Manolache, V.; Preteasa, V.; Curcean, S.; Roman, A.; Motas, N.; Dediu, M.; et al. Lung Cancer in Romania. J. Thorac. Oncol. 2024, 19, 1492–1503. [Google Scholar] [CrossRef]
- Chen, H.; Stoltzfus, K.C.; Lehrer, E.J.; Horn, S.R.; Siva, S.; Trifiletti, D.M.; Meng, M.-B.; Verma, V.; Louie, A.V.; Zaorsky, N.G. The Epidemiology of Lung Metastases. Front. Med. 2021, 8, 723396. [Google Scholar] [CrossRef]
- Gui, P.; Bivona, T.G. Evolution of metastasis: New tools and insights. Trends Cancer 2022, 8, 98–109. [Google Scholar] [CrossRef]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. Cancer 2020, 122, 943–952. [Google Scholar] [CrossRef]
- Pîslaru, A.I.; Albișteanu, S.-M.; Ilie, A.C.; Ștefaniu, R.; Mârza, A.; Moscaliuc, Ș.; Nicoară, M.; Turcu, A.-M.; Grigoraș, G.; Alexa, I.D. Lung Cancer: New Directions in Senior Patients Assessment. Geriatrics 2024, 9, 101. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | pre-COVID-19 (n = 478) | COVID-19 (n = 272) | p * |
|---|---|---|---|
| Sex n (%) | |||
| Females | 95 (19.87%) | 87 (31.99%) | - |
| Males | 383 (80.13%) | 185 (68.01%) | - |
| M/F ratio | 4/1 | 2/1 | 0.0002 ** |
| Age | |||
| Mean | 65.51 ± 9.42 | 65.59 ± 9.41 | |
| ≤40 | 8 (1.67%) | 3 (1.10%) | 0.17 |
| 41–50 | 20 (4.18%) | 13 (4.78%) | 0.23 |
| 51–60 | 99 (20.71%) | 57 (20.96%) | 0.0012 |
| 61–70 | 201 (42.05%) | 116 (42.65%) | p < 0.001 |
| >70 | 150 (31.38%) | 83 (30.51%) | p < 0.001 |
| Histology n (%) | |||
| Squamous carcinoma | 169 (35.36%) | 88 (32.35%) | p < 0.001 |
| Adenocarcinoma | 141 (29.50%) | 78 (28.68%) | p < 0.001 |
| Non-small-cell carcinoma NOS | 14 (2.93%) | 28 (10.29%) | 0.0406 |
| Adenosquamous carcinoma | 2 (0.42%) | 0 (0%) | - |
| Small-cell carcinoma | 62 (12.97%) | 44 (16.18%) | 0.0845 |
| Carcinoids | 5 (1.05%) | 2 (0.74%) | 0.2965 |
| Metastases | 29 (6.07%) | 5 (1.84%) | 0.0008 |
| Others: benign | 6 (1.26%) | 4 (1.47%) | 0.5351 |
| Others: malignant | 16 (3.35%) | 16 (5.88%) | 1 |
| Squamous dysplasia | 34 (7.11%) | 7 (2.57%) | 0.0004 |
| OR | 95% CI | Tjur’s R Squared | p * | |
|---|---|---|---|---|
| SQC | ||||
| Gender | 2.477 | 1.679–3.733 | 0.02763 | <0.001 |
| Age > 60 years | 1.016 | 0.7242–1.435 | 0.00001148 | 0.926 |
| ADK | ||||
| Gender | 0.7152 | 0.5018–1.025 | 0.004545 | 0.0678 |
| Age > 60 years | 1.241 | 0.8662–1.798 | 0.001801 | 0.2416 |
| SCLC | ||||
| Gender | 0.6659 | 0,4276–1,055 | 0.004221 | 0.0822 |
| Age > 60 years | 1.015 | 0.6441–1.640 | 0.000005327 | 0.9496 |
| Metastases | ||||
| Gender | 0.2995 | 0.1487–0.6030 | 0.01711 | <0.001 |
| Age > 60 years | 0.8669 | 0.4184–1.930 | 0.000183 | 0.7138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, G.-N.; Chinezu, L.; Cătană, R.T.; Carabașa, P.; Nechifor-Boilă, A. The Impact of the COVID-19 Pandemic on New Lung Cancer Diagnosis in Mureș County, Romania: A 5-Year Retrospective, Comprehensive Study. Medicina 2025, 61, 1548. https://doi.org/10.3390/medicina61091548
Radu G-N, Chinezu L, Cătană RT, Carabașa P, Nechifor-Boilă A. The Impact of the COVID-19 Pandemic on New Lung Cancer Diagnosis in Mureș County, Romania: A 5-Year Retrospective, Comprehensive Study. Medicina. 2025; 61(9):1548. https://doi.org/10.3390/medicina61091548
Chicago/Turabian StyleRadu, Georgian-Nicolae, Laura Chinezu, Ramona Teodora Cătană, Petre Carabașa, and Adela Nechifor-Boilă. 2025. "The Impact of the COVID-19 Pandemic on New Lung Cancer Diagnosis in Mureș County, Romania: A 5-Year Retrospective, Comprehensive Study" Medicina 61, no. 9: 1548. https://doi.org/10.3390/medicina61091548
APA StyleRadu, G.-N., Chinezu, L., Cătană, R. T., Carabașa, P., & Nechifor-Boilă, A. (2025). The Impact of the COVID-19 Pandemic on New Lung Cancer Diagnosis in Mureș County, Romania: A 5-Year Retrospective, Comprehensive Study. Medicina, 61(9), 1548. https://doi.org/10.3390/medicina61091548







