Using Computerised Gait Analysis to Assess Changes After Rehabilitation in Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Gait Speed Improvement
Abstract
1. Introduction
1.1. The State of Knowledge
1.2. Rationale of This Review
1.3. Objective
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
- -
- Articles written in English;
- -
- Articles published between 2015 and 2025;
- -
- Randomised controlled trials;
- -
- Studies including patients who were diagnosed with primary knee osteoarthritis and over 40 years of age (according to ACR diagnosis criteria) [26];
- -
- Studies evaluating gait changes in patients with KOA before and after a specific rehabilitation treatment;
- -
- Studies assessing walking using computerised devices for gait parameter analysis.
- -
- Studies available only as abstracts, conference posters or without full data access;
- -
- Studies conducted on non-human subjects;
- -
- Studies conducted on patients with secondary KOA;
- -
- Studies involving patients who have undergone orthopaedic surgical interventions on the knee, hip, ankle, or foot;
- -
- Studies involving patients with neurological diseases (central or peripheral);
- -
- Studies with extremely small sample sizes (<10 participants per group);
- -
- Studies with a follow-up period shorter than 2 weeks.
2.3. Data Extraction
2.4. Quality Assessment
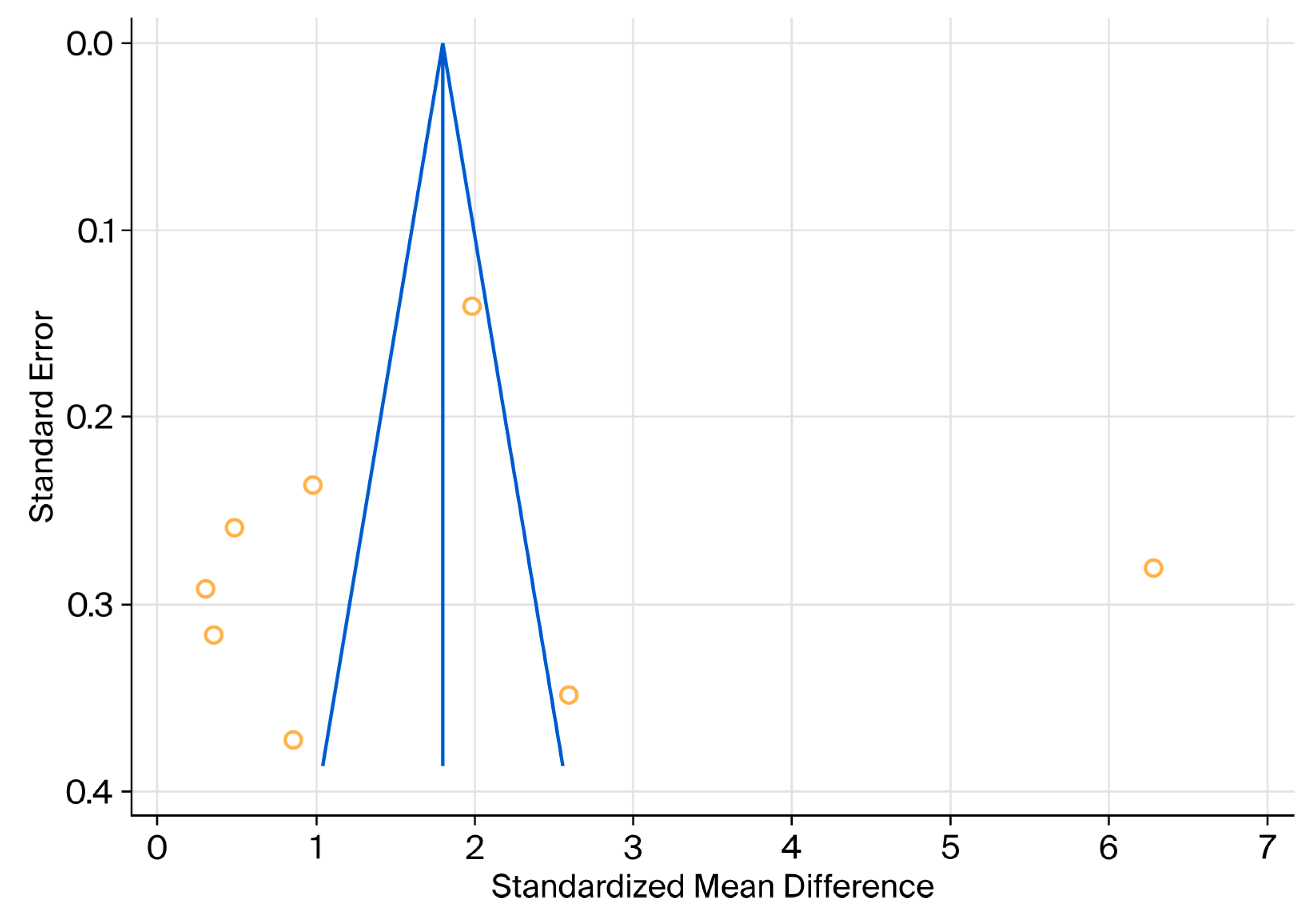
2.5. Statistical Analysis
3. Results
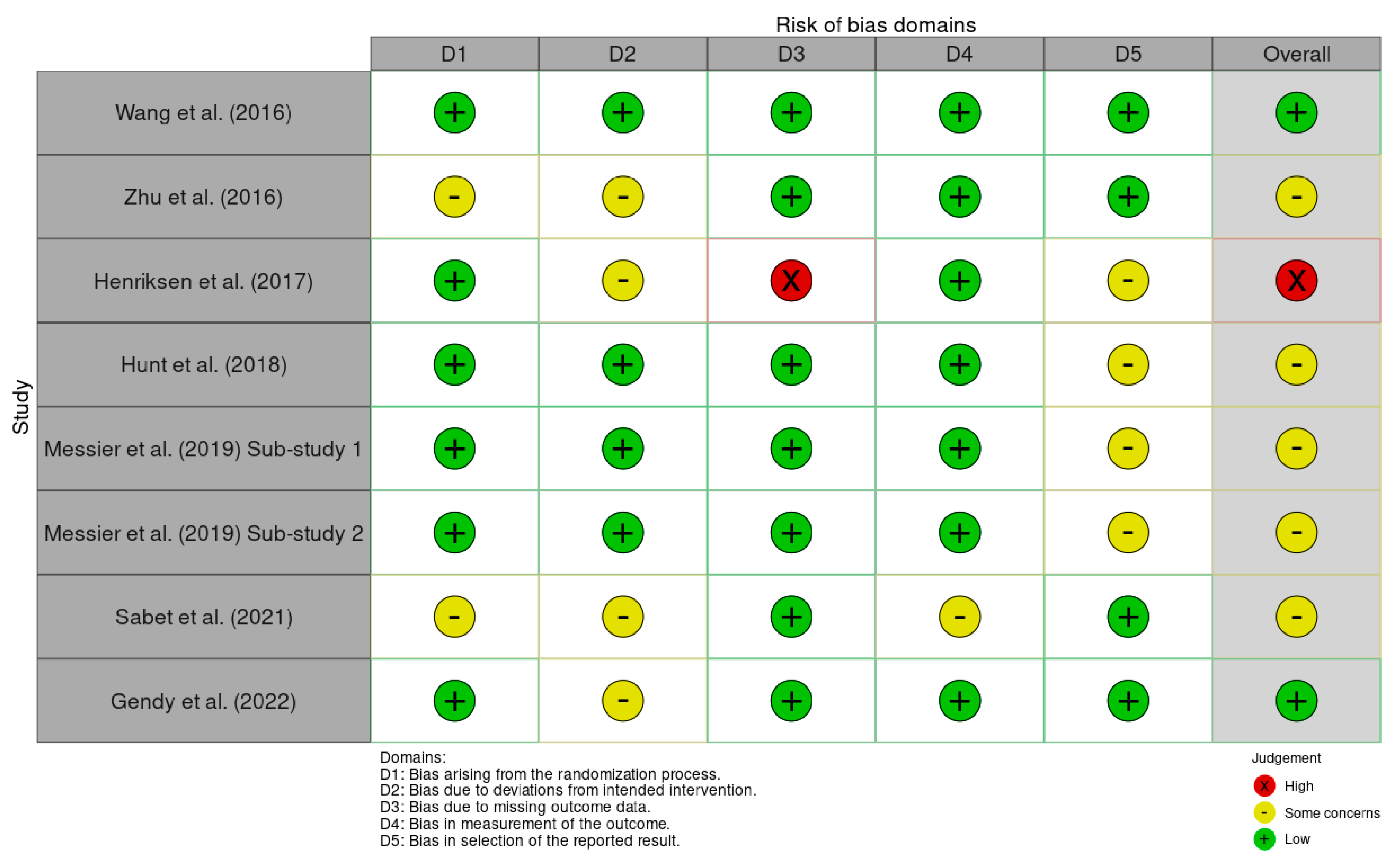
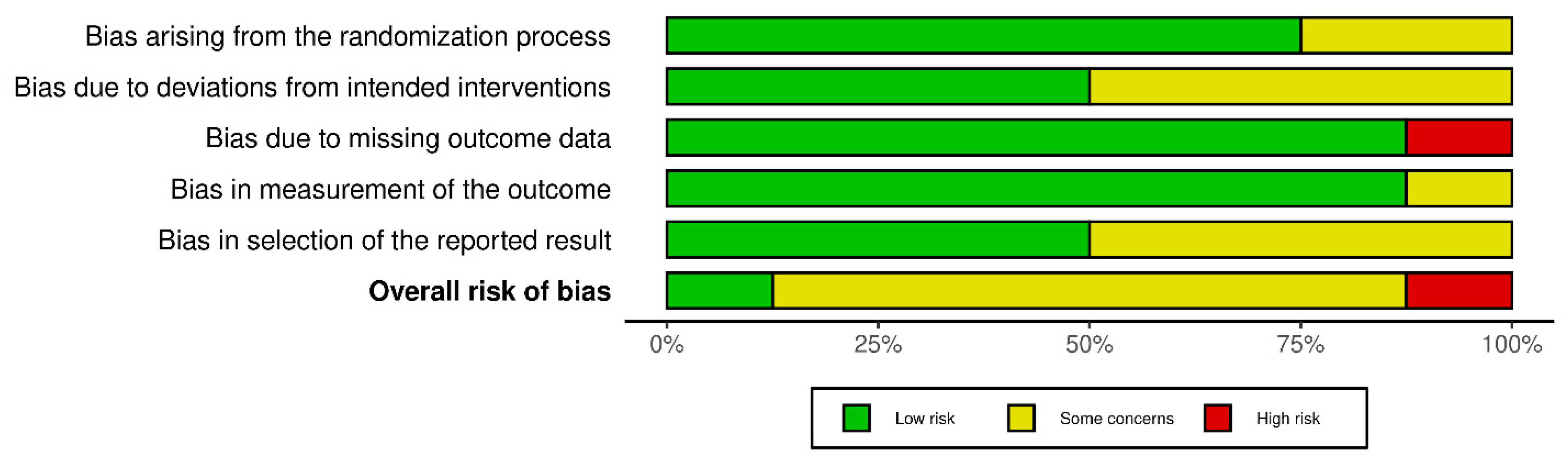
3.1. Risk of Bias
3.2. Description of Included Studies
3.3. Analysis of Included Studies
Meta-Analysis: Continuous Measures
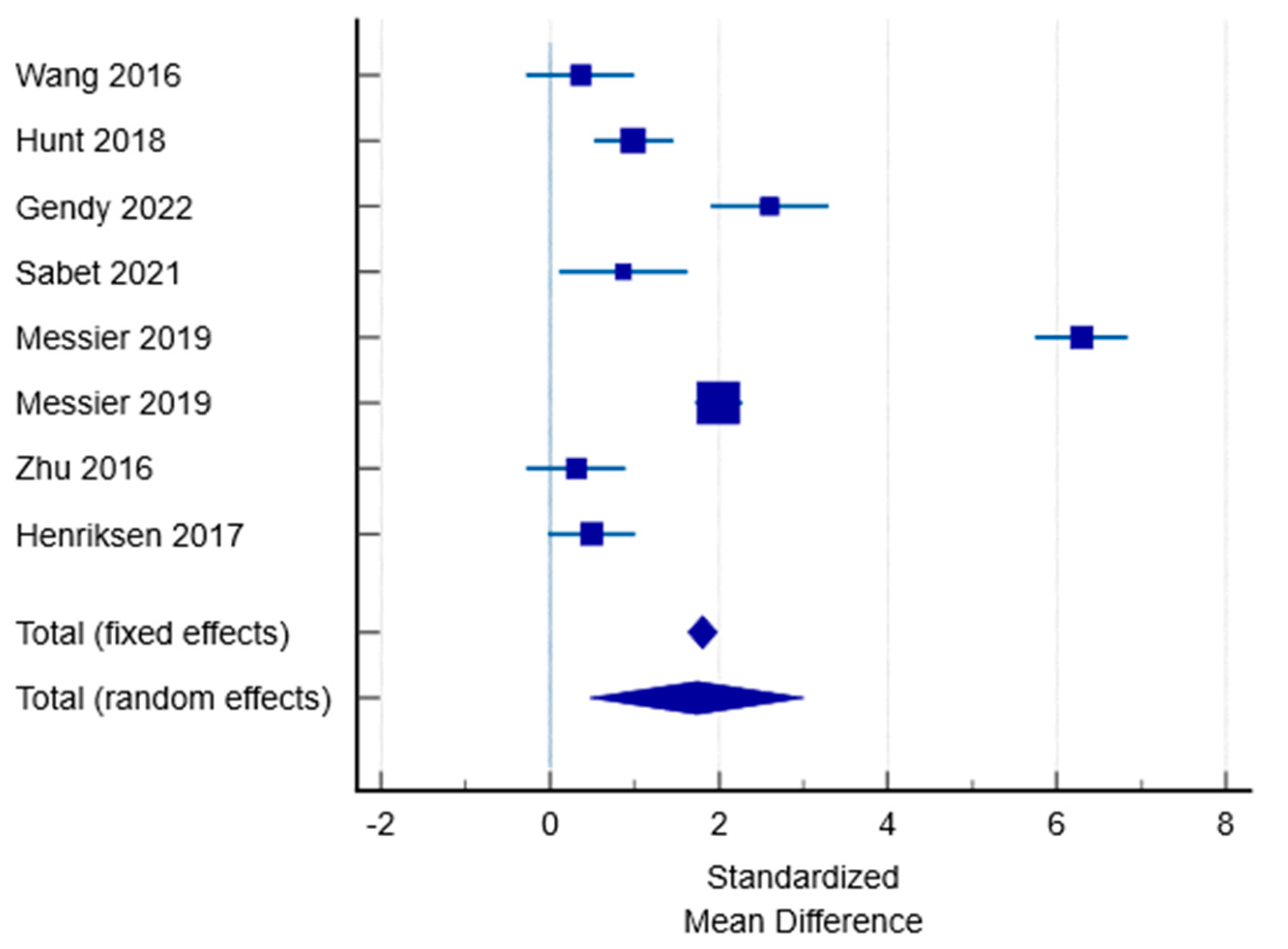
| Study | N1 | N2 | Total | SMD | SE | 95% CI | t | p | Weight (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed | Random | |||||||||
| Wang 2016 | 19 | 20 | 39 | 0.362 | 0.316 | −0.279 to 1.003 | 7.48 | 12.44 | ||
| Hunt 2018 | 40 | 39 | 79 | 0.990 | 0.236 | 0.520 to 1.461 | 13.41 | 12.60 | ||
| Gendy 2022 | 30 | 30 | 60 | 2.601 | 0.348 | 1.904 to 3.298 | 6.18 | 12.36 | ||
| Sabet 2021 | 15 | 15 | 30 | 0.866 | 0.372 | 0.103 to 1.629 | 5.40 | 12.30 | ||
| Messier 2019 | 151 | 151 | 302 | 6.291 | 0.281 | 5.739 to 6.843 | 9.52 | 12.52 | ||
| 152 | 151 | 303 | 1.995 | 0.140 | 1.719 to 2.271 | 38.03 | 12.74 | |||
| Zhu 2016 | 23 | 23 | 46 | 0.310 | 0.292 | −0.278 to 0.898 | 8.81 | 12.49 | ||
| Henriksen 2017 | 31 | 29 | 60 | 0.494 | 0.259 | −0.0248 to 1.012 | 11.17 | 12.56 | ||
| Total (fixed effects) | 461 | 458 | 919 | 1.807 | 0.0866 | 1.637 to 1.977 | 20.879 | <0.001 | 100.00 | 100.00 |
| Total (random effects) | 461 | 458 | 919 | 1.740 | 0.647 | 0.470 to 3.010 | 2.690 | 0.007 | 100.00 | 100.00 |
4. Discussion
4.1. Spatiotemporal Parameters
4.2. Kinetic Parameters
4.3. Kinematic Parameters
4.4. Future Research Directions
4.5. Strengths of This Study
4.6. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Alghadir, A.; Anwer, S. Effect of retro and forward walking on quadriceps muscle strength, pain, function, and mobility in patients with knee osteoarthritis: A protocol for a randomized controlled trial. BMC Musculoskelet. Disord. 2016, 17, 161. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, R.; Li, H.; Wan, X.; Xu, P.; Zhu, A.; Wei, P. Comparison of knee biomechanical characteristics during gait between patients with knee osteoarthritis and healthy individuals. Heliyon 2024, 10, e36931. [Google Scholar] [CrossRef]
- Richards, C.; Higginson, J.S. Knee contact force in subjects with symmetrical OA grades: Differences between OA severities. J. Biomech. 2010, 43, 2595–2600. [Google Scholar] [CrossRef]
- Cerny, K.; Perry, J.; Walker, J.M. Adaptations during the stance phase of gait for simulated flexion contractures at the knee. Orthopedics 1994, 17, 501–513. [Google Scholar] [CrossRef]
- Dobson, F.; Hinman, R.S.; Roos, E.M.; Abbott, J.H.; Stratford, P.; Davis, A.M.; Buchbinder, R.; Snyder-Mackler, L.; Henrotin, Y.; Thumboo, J.; et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Driban, J.B.; Henrotin, Y.; Hunter, D.J.; Jiang, G.-L.; Skou, S.T.; Wang, S.; Schnitzer, T. OARSI Clinical Trials Recommendations: Design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)-Development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Bruce, B.; Fries, J.F. The Health Assessment Questionnaire (HAQ). Clin. Exp. Rheumatol. 2005, 23, S14–S18. [Google Scholar]
- Feeny, D.; Furlong, W.; Boyle, M.; Torrance, G.W. Multi-Attribute Health Status Classification Systems. Health Utilities Index. Pharmacoeconomics 1995, 7, 490–502. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; Song, Z.; Shu, L.; Zeng, Q.; Huang, G.; Lin, Y. Functional Monitoring of Patients with Knee Osteoarthritis Based on Multidimensional Wearable Plantar Pressure Features: Cross-sectional study. JMIR Aging 2024, 7, e58261. [Google Scholar] [CrossRef]
- Chen, J.-L.; Dai, Y.-N.; Grimaldi, N.S.; Lin, J.-J.; Hu, B.-Y.; Wu, Y.-F.; Gao, S. Plantar Pressure-Based Insole Gait Monitoring Techniques for Diseases Monitoring and Analysis: A Review. Adv. Mater. Technol. 2021, 7, 2100566. [Google Scholar] [CrossRef]
- Bo, F.; Yerebakan, M.; Dai, Y.; Wang, W.; Li, J.; Hu, B.; Gao, S. IMU-Based Monitoring for Assistive Diagnosis and Management of IoHT: A Review. Healthcare 2022, 10, 1210. [Google Scholar] [CrossRef]
- Semwal, V.B.; Gaud, N.; Lalwani, P.; Bijalwan, V.; Alok, A. Pattern identification of different human joints for different human walking styles using inertial measurement unit (IMU) sensor. Artif. Intell. Rev. 2022, 55, 1149–1169. [Google Scholar] [CrossRef]
- Boekesteijn, R.J.; van Gerven, J.; Geurts, A.C.H.; Smulders, K. Objective gait assessment in individuals with knee osteoarthritis using inertial sensors: A systematic review and meta-analysis. Gait Posture 2022, 98, 109–120. [Google Scholar] [CrossRef]
- Taniguchi, M.; Ikezoe, T.; Tsuboyama, T.; Ito, H.; Matsuda, S.; Matsuda, F.; Ichihashi, N. Step-time variability is a specific gait characteristic associated with functional disabilities in knee osteoarthritis: The Nagahama study. Gait Posture 2025, 120, 211–216. [Google Scholar] [CrossRef]
- Purser, J.L.; Golightly, Y.M.; Feng, Q.; Helmick, C.G.; Renner, J.B.; Jordan, J.M. Association of Slower Walking Speed with Incident Knee Osteoarthritis-Related Outcomes. Arthritis Care Res. 2012, 64, 1028–1035. [Google Scholar] [CrossRef]
- Fischer, M.; Vialleron, T.; Laffaye, G.; Fourcade, P.; Hussein, T.; Chèze, L.; Deleu, P.A.; Honeine, J.L.; Yiou, E.; Delafontaine, A. Long-Term Effects of Whole-Body Vibration on Human Gait: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 627. [Google Scholar] [CrossRef]
- Huang, X.M.; Yuan, F.Z.; Chen, Y.R.; Huang, Y.; Yang, Z.X.; Lin, L.; Yu, J.K. Physical therapy and orthopaedic equipment-induced reduction in the biomechanical risk factors related to knee osteoarthritis: A systematic review and Bayesian network meta-analysis of randomised controlled trials. BMJ Open 2022, 12, e051608. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Zotero. Your Personal Research Assistant. Available online: https://www.zotero.org/ (accessed on 14 June 2025).
- Altman, R.D. The classification of osteoarthritis. J. Rheumatol. Suppl. 1995, 43, 42–43. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, L.; Li, H.; Lei, Z.; Yang, X.; Liu, C.; Jiang, H.; Zhang, L.; Zhou, Z.; Reinhardt, J.D.; et al. Effects of whole-body vibration training with quadriceps strengthening exercise on functioning and gait parameters in patients with medial compartment knee osteoarthritis: A randomised controlled preliminary study. Physiotherapy 2016, 102, 86–92. [Google Scholar] [CrossRef]
- Zhu, Q.; Huang, L.; Wu, X.; Wang, L.; Zhang, Y.; Fang, M.; Liu, Y.; Li, J.X. Effects of Tai Ji Quan training on gait kinematics in older Chinese women with knee osteoarthritis: A randomized controlled trial. J. Sport Health Sci. 2016, 5, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Klokker, L.; Bartholdy, C.; Schjoedt-Jorgensen, T.; Bandak, E.; Bliddal, H. No effects of functional exercise therapy on walking biomechanics in patients with knee osteoarthritis: Exploratory outcome analyses from a randomised trial. BMJ Open Sport Exerc. Med. 2017, 2, e000230. [Google Scholar] [CrossRef]
- Hunt, M.A.; Charlton, J.M.; Krowchuk, N.M.; Tse, C.T.F.; Hatfield, G.L. Clinical and biomechanical changes following a 4-month toe-out gait modification program for people with medial knee osteoarthritis: A randomized controlled trial. Osteoarthr. Cartil. 2018, 26, 903–911. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E.; Gainey, J.; Gorton, G.; Cochran, G.V. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J. Orthop. Res. 1989, 7, 849–860. [Google Scholar] [CrossRef]
- Messier, S.P.; Beavers, D.P.; Mihalko, S.L.; Miller, G.D.; Lyles, M.F.; Hunter, D.J.; Carr, J.J.; Eckstein, F.; Guermazi, A.; Loeser, R.F.; et al. The effects of intensive dietary weight loss and exercise on gait in overweight and obese adults with knee osteoarthritis. The Intensive Diet and Exercise for Arthritis (IDEA) trial. J. Biomech. 2019, 98, 109477. [Google Scholar] [CrossRef]
- Messier, S.P.; Legault, C.; Mihalko, S.; Miller, G.D.; Loeser, R.F.; DeVita, P.; Lyles, M.; Eckstein, F.; Hunter, D.J.; Williamson, J.D.; et al. The Intensive Diet and Exercise for Arthritis (IDEA) Trial: Design and Rationale. BMC Musculoskelet. Disord. 2009, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Pater, M.; Beavers, D.P.; Legault, C.; Loeser, R.F.; Hunter, D.J.; DeVita, P. Influences of alignment and obesity on knee joint loading in osteoarthritic gait. Osteoarthr. Cartil. 2014, 22, 912–917. [Google Scholar] [CrossRef]
- Jegede, J.A.; Adegoke, B.O.; Olagbegi, O.M. Effects of a Twelve-Week Weight Reduction Exercise Programme on Selected Spatiotemporal Gait Parameters of Obese Individuals. J. Obes. 2017, 2017, 4193256. [Google Scholar] [CrossRef] [PubMed]
- Sabet, F.; Ebrahimipour, E.; Mohammadipour, F.; Daneshjoo, A.; Jafarnezhadgero, A. Effects of Swedish massage on gait spatiotemporal parameters in adult women with medial knee osteoarthritis: A randomized controlled trial. J. Bodyw. Mov. Ther. 2021, 28, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Gendy, M.H.; Zalabia, M.M.; Moharram, A.N.; Abdelhay, M.I. Efficacy of rectus femoris stretching on pain, range of motion and spatiotemporal gait parameters in patients with knee osteoarthritis: A randomised controlled trial. BMJ Open Sport Exerc. Med. 2022, 8, e001459. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; 567p. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Tanimoto, K.; Tokuda, K.; Iwamoto, Y.; Ogata, Y.; Anan, M.; Takahashi, M.; Kito, N.; Shinkoda, K. Rear foot kinematics when wearing lateral wedge insoles and foot alignment influence the effect of knee adduction moment for medial knee osteoarthritis. Gait Posture 2017, 57, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatnezhad, P.; Shams, M.; Karimi, N.; Rahnama, L. Uphill treadmill walking plus physical therapy versus physical therapy alone in the management of individuals with knee osteoarthritis: A randomized clinical trial. Disabil. Rehabil. 2021, 43, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Varzaityte, L.; Kubilius, R.; Rapoliene, L.; Bartuseviciute, R.; Balcius, A.; Ramanauskas, K.; Nedzelskiene, I. The effect of balneotherapy and peloid therapy on changes in the functional state of patients with knee joint osteoarthritis: A randomized, controlled, single-blind pilot study. Int. J. Biometeorol. 2020, 64, 955–964. [Google Scholar] [CrossRef]
- Nasui, B.A.; Talaba, P.; Nasui, G.A.; Sirbu, D.M.; Borda, I.M.; Pop, A.L.; Ciortea, V.M.; Irsay, L.; Purcar-Popescu, A.I.; Cinteza, D.; et al. The influence of diet and physical activity on oxidative stress in Romanian females with osteoarthritis. Nutrients 2022, 14, 4159. [Google Scholar] [CrossRef]
- Irsay, L.; Ungur, R.A.; Borda, I.M.; Tica, I.; Iliescu, M.G.; Ciubean, A.D.; Popa, T.; Cinteza, D.; Popa, F.L.; Bondor, C.I.; et al. Safety of Electrotherapy Treatment in Patients with Knee Osteoarthritis and Cardiac Diseases. Life 2022, 12, 1690. [Google Scholar] [CrossRef]
- Moseng, T.; Vliet Vlieland, T.P.M.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef]
- Budarick, A.R.; Hubley-Kozey, C.L.; Theou, O.; Stanish, W.D.; Hannigan, M.; Moyer, R.F. The effect of walking interventions on biomechanical knee osteoarthritis outcomes: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2025, 73, 152755. [Google Scholar] [CrossRef]
| Term Combinations Used | PubMed | Scopus | Web of Science | Cochrane Library | PEDro | Number of Studies |
|---|---|---|---|---|---|---|
| knee osteoarthritis rehabilitation AND computerised gait analysis | 0 | 1 | 3 | 2 | 0 | 6 |
| knee osteoarthritis rehabilitation AND gait assessment | 24 | 156 | 220 | 114 | 2 | 516 |
| knee osteoarthritis rehabilitation AND spatiotemporal gait | 3 | 26 | 0 | 13 | 2 | 44 |
| knee osteoarthritis rehabilitation AND walking assessment | 36 | 203 | 0 | 266 | 4 | 509 |
| knee osteoarthritis rehabilitation AND gait inertial sensors | 0 | 18 | 0 | 1 | 0 | 19 |
| knee osteoarthritis physical therapy AND computerised gait analysis | 0 | 4 | 0 | 1 | 0 | 5 |
| knee osteoarthritis physical therapy AND gait assessment | 19 | 78 | 75 | 80 | 2 | 254 |
| knee osteoarthritis physical therapy AND spatiotemporal gait parameters | 2 | 16 | 17 | 11 | 3 | 49 |
| knee osteoarthritis physical therapy AND walking assessment | 36 | 134 | 98 | 4 | 3 | 275 |
| knee osteoarthritis physical therapy AND gait inertial sensors | 0 | 1 | 12 | 0 | 0 | 13 |
| knee osteoarthritis exercises therapy AND computerised gait analysis | 0 | 1 | 0 | 1 | 0 | 2 |
| knee osteoarthritis exercises therapy AND gait assessment | 14 | 57 | 20 | 82 | 1 | 174 |
| knee osteoarthritis exercises therapy AND spatiotemporal gait parameters | 0 | 7 | 3 | 11 | 2 | 23 |
| knee osteoarthritis exercises therapy AND walking assessment | 24 | 111 | 29 | 4 | 1 | 169 |
| knee osteoarthritis exercises therapy AND gait inertial sensors | 0 | 0 | 0 | 0 | 0 | 0 |
| knee osteoarthritis AND advanced technology in gait analysis | 7 | 3 | 21 | 1 | 0 | 32 |
| knee osteoarthritis AND technology in gait analysis AND rehabilitation treatment | 3 | 7 | 13 | 3 | 0 | 26 |
| knee osteoarthritis rehabilitation AND advanced technology in gait analysis | 0 | 7 | 13 | 1 | 0 | 21 |
| knee osteoarthritis physical therapy AND advanced technology in gait analysis | 0 | 0 | 6 | 0 | 0 | 6 |
| knee osteoarthritis exercises therapy AND advanced technology in gait analysis | 0 | 0 | 0 | 0 | 0 | 0 |
| TOTAL | 168 | 830 | 530 | 595 | 20 | 2143 |
| Databases | PubMed, National Institutes of Health (NIH) | Scopus, Elsevier | Web of Science | Cochrane Library | PEDro |
|---|---|---|---|---|---|
| Search filters | 2015–present All types of articles English Humans | 2015–present All types of articles English Humans All source types and titles of journals | 2015–present All types of articles English | 2015–present All types of articles English | 2015–present All types of articles English |
| Number of articles | 168 | 830 | 530 | 595 | 20 |
| Study/Country | RCT Type | No P | No S | No C | Age S + SD | Age C + SD | BMI S + SD | BMI C + SD | K-L | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Wang at al. (2016) China | Assessor-blinded | 39 | 19 | 20 | 61.1 (7.1) | 61.5 (7.3) | 27.8 (3.1) | 6.2 (2.7) | II–III |
| 2. | Zhu et al. (2016) China | Assessor-blinded | 46 | 23 | 23 | 64.61 (3.4) | 64.53 (3.43) | 25.23 (3.46) | 25.05 (3.42) | I–III |
| 3. | Henriksen et al. (2017) Danmark | Assessor-blinded | 60 | 31 | 29 | 65.9 (8.5) | 61.3 (7.1) | 28.7 (4.2) | 28.1 (4.5) | I–III |
| 4. | Hunt et al. (2018)- Canada | Single- blinded | 79 | 40 | 39 | 64.6 (7.6) | 65.4 (9.6) | 27.3 (3.5) | 27.4 (3.5) | II–IV |
| 5. | Messier et al. (2019)Sub-study 1 United States of America | Assessor-blinded | 302 | 151 | 151 | 66.1 (6.2) (D + E) | 66.2 (6.1) (E) | 33.6 (3.7) (D + E) | 33.5 (3.8) (E) | I–III |
| Messier et al. (2019)Sub-study 2 United States of America | Assessor-blinded | 303 | 152 | 151 | 65.9 (6.3) (D) | 66.2 (6.1) (E) | 33.7 (3.6) (D) | 33.5 (3.8) (E) | I–III | |
| 6. | Sabet et al. (2021)- Iran | Double- blinded | 30 | 15 | 15 | 52.60 (6.72) | 52.40 (6.71) | 29.9 (3.37) | 29.55 (4.08) | II–IV |
| 7. | Gendy et al. (2022) Egypt | Single- blinded | 60 | 30 | 30 | 53.63 (6.04) | 53.13 (5.94) | 53.13 (5.94) | 32.06 (0.69) | III |
| Study | Intervention Therapy | Control Therapy | Device for Gait Analysis | Outcome | Treatment Duration | Results | Ref. |
|---|---|---|---|---|---|---|---|
| Wang et al. (2016), China | WBVT + STRETCHING | STRETCHING | System Nexus+ force platforms | VAS, WOMAC Temporospatial parameters Kinetic parameters Kinematic parameters | 16 weeks | WBVT + STRETCHING/STRETCHING Improvement in temp-spatial parameters No benefit in kinematics/kinetics Cadence only—better interventional/control STRETCHING Improvements in temp-spat/kinetic/kinematic BOTH GROUPS Improvement—pain/stiffness/function—no diff. | 35 |
| Zhu et al. (2016), China | TAI JI QUAN 60 min 3/week | EDUCATIONAL SESSION 60 min biweekly | Computerised infrared motion analysis system, a 16-camera setup, and markers | WOMAC, SPPB Gait speed/step length Initial contact angle of the knee Maximal knee angle | 24 weeks | TAI JI QUAN/CONTROL Significant improvements: gait velocity/stride length/initial contact knee angle/maximal angle of the knee/WOMAC (pain, stiffness, function)/SPPB | 32 |
| Henriksen et al. (2017), Denmark | FACILITY-BASED NEUROMUSCULAR EXERCISE THERAPY 3x/week | NO INTERVENTION | Six-camera 3D motion analysis system + force platforms | Joint angles Joint moments Mechanical work Gait speed/step length Cadence Ground reaction force | 12 weeks | INTERVENTIONAL/CONTROL Significant difference only in second peak knee flexor moment and second peak vertical ground reaction force No statistical difference—other gait parameters (including speed) Gait speed worsened—both groups | 10 |
| Hunt et al. (2018), Canada | TOE-OUT GUIDED WALKING ON THE TREADMILL | UNGUIDED WALKING ON THE TREADMILL | Motion capture cam Force platforms Treadmills Biomechanical analysis software Mirror + green tape +protractor device | WOMAC pain/function NRS—intervention KAM KFM Foot progression angle Gait speed | 5 months | TOE-OUT/UNGUIDED WALKING ON TREADMILL Significant improvements—knee joint loading Similar improvements—knee pain No improvement—gait speed (both groups) | 38 |
| Messier et al. (2019), Sub-study 1 USA | DIET-INDUCED WEIGHT LOSS + STRUCTURED EXERCISE(D + E) | STRUCTURED EXERCISE SESSIONS WITHOUT DIETARY INTERVENTION(E) | Motion capture system: 6-camera motion analysis system Reflective marker set Force platform, soft | Gait speed Knee joint loading Hip and ankle mechanics Muscle forces | 18 months | D + E resulted in significant lower joint loads/E Mean speed (1.35 m/s) (like healthy) No influence on KAM | 32 |
| Messier et al. (2019), Sub-study 2 USA | INTENSIVE DIETARY WEIGHT-LOSS PROGRAMME (D). | STRUCTURED EXERCISE SESSIONS WITHOUT DIETARY INTERVENTION(E) | Motion capture system: 6-camera motion analysis system, reflective marker set, force platform, software | Gait speed Knee joint loading Hip and ankle mechanics Muscle forces | 18 months | D—Lower joint loads compared with E Decreased tibiofemoral compressive force No influence on KAM Peak knee extension moment increased in all groups below normal Peak quadricep muscle force and peak knee extension increased across 3 groups | 32 |
| Sabet et al. (2021), Iran | SWEDISH MASSAGE QUADRICEPS, 20–30 min/ses, 3X/wk | KOA REGULAR TREATMENT | 3D motion analysis system: 6 infrared cameras, reflective markers, Cortex analysis software | WOMAC pain/stiffness/function Temp-spatial parameters, including gait speed | 4 weeks | SWEDISH MASSAGE/CONTROL Relieved pain Improved function/gait speed/total support time% | 36 |
| Gendy et al. (2022), Egypt | RECTUS FEMORIS STRECH + CONVENTIONAL EXERCISES | CONVENTIONAL EXERCISES | Universal goniometer, bioindex gait trainer, stopwatch | WOMAC, VAS (pain) ROM-flexion/extension Step length Gait speed | 4 weeks | INTERVENTIONAL/CONTROL Improvement—step length/speed gait Higher flexion ROM/no diff. extension ROM Lower VAS and WOMAC scores BOTH GROUPS—better pain/ROM/temp-spat | 44 |
| Reference | Treated_N1 | Treated_Mean | Treated_SD | Control_N2 | Control_Mean | Control_SD |
|---|---|---|---|---|---|---|
| Wang 2016 | 19 | 0.17 | 0.13 | 20 | 0.12 | 0.14 |
| Hunt 2018 | 40 | 0.05 | 0.02 | 39 | 0.03 | 0.02 |
| Gendy 2022 | 30 | 0.41 | 0.12 | 30 | 0.16 | 0.06 |
| Sabet 2021 | 15 | 0.12 | 0.21 | 15 | −0.05 | 0.17 |
| Messier 2019 | 151 | 0.15 | 0.009 | 151 | 0.09 | 0.01 |
| 152 | 0.11 | 0.01 | 151 | 0.09 | 0.01 | |
| Zhu 2016 | 23 | 0.045 | 0.1 | 23 | 0.015 | 0.09 |
| Henriksen 2017 | 31 | −0.03 | 0.04 | 29 | −0.05 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minea, M.; Ismail, S.; Petcu, L.C.; Nedelcu, A.-D.; Petcu, A.; Minea, A.-E.; Iliescu, M.-G. Using Computerised Gait Analysis to Assess Changes After Rehabilitation in Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Gait Speed Improvement. Medicina 2025, 61, 1540. https://doi.org/10.3390/medicina61091540
Minea M, Ismail S, Petcu LC, Nedelcu A-D, Petcu A, Minea A-E, Iliescu M-G. Using Computerised Gait Analysis to Assess Changes After Rehabilitation in Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Gait Speed Improvement. Medicina. 2025; 61(9):1540. https://doi.org/10.3390/medicina61091540
Chicago/Turabian StyleMinea, Mihaela, Sermina Ismail, Lucian Cristian Petcu, Andreea-Dalila Nedelcu, Adina Petcu, Alexandra-Elena Minea, and Mădălina-Gabriela Iliescu. 2025. "Using Computerised Gait Analysis to Assess Changes After Rehabilitation in Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Gait Speed Improvement" Medicina 61, no. 9: 1540. https://doi.org/10.3390/medicina61091540
APA StyleMinea, M., Ismail, S., Petcu, L. C., Nedelcu, A.-D., Petcu, A., Minea, A.-E., & Iliescu, M.-G. (2025). Using Computerised Gait Analysis to Assess Changes After Rehabilitation in Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Gait Speed Improvement. Medicina, 61(9), 1540. https://doi.org/10.3390/medicina61091540