Does Periodontal Bone Loss Play a Significant Role in Schneiderian Membrane Thickening? A Cone-Beam Computed Tomography Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Sample Size Calculation
2.3. Patient Selection
2.4. Inclusion and Exclusion Criteria
2.5. CBCT Software and Image Processing
2.6. Examiner Calibration and Reliability
2.7. Blinding and Data Entry
2.8. Classification of Demographic Variables
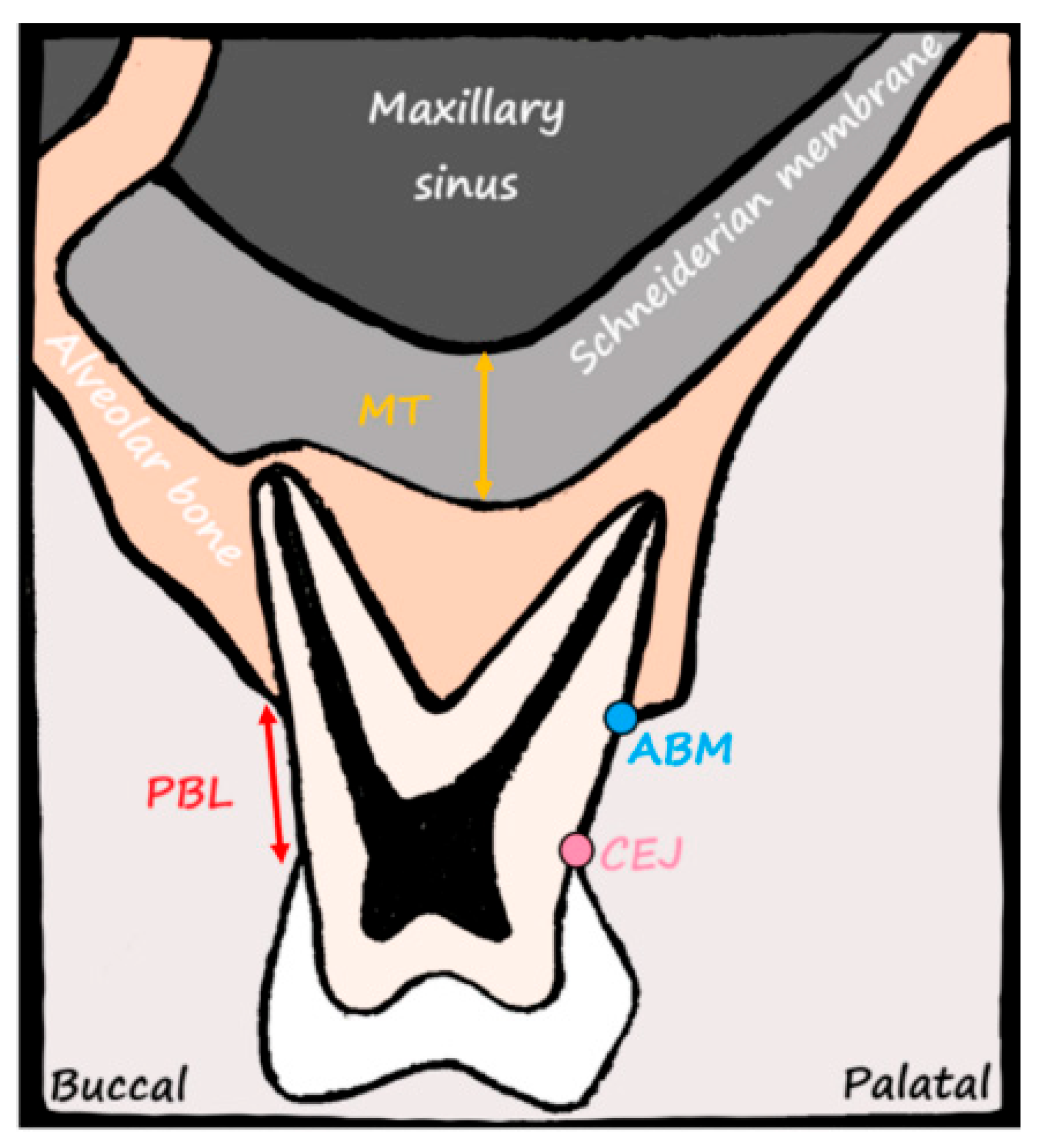
2.9. Measurement of MT
2.10. Assessment of PBL
2.11. Statistical Analysis
2.12. Assumption Checks for Regression Analysis
3. Results
3.1. Demographic Characteristics and General MT
3.2. Distribution of MT by Classification
3.3. Association Between PBL and MT
3.4. Diagnostic Accuracy of Bone Loss for Predicting Mucosal Thickening
3.5. Correlation Between Age and MT
3.6. Multiple Linear Regression Analysis
Regression Assumptions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Zhang, T.; He, Z.; Tian, H. Association between periodontal status and degree of maxillary sinus mucosal thickening: A retrospective CBCT study. BMC Oral Health 2021, 21, 392. [Google Scholar] [CrossRef]
- Avila, G.; Galindo-Moreno, P.; Soehren, S.; Misch, C.E.; Morelli, T.; Wang, H.L. A novel decision-making process for tooth retention or extraction. J. Periodontol. 2009, 80, 476–491. [Google Scholar] [CrossRef]
- Guo, Z.Z.; Liu, Y.; Qin, L.; Song, Y.L.; Xie, C.; Li, D.H. Longitudinal response of membrane thickness and ostium patency following sinus floor elevation: A prospective cohort study. Clin. Oral Implants Res. 2016, 27, 724–729. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Xu, Q.; Shu, J.; Xu, B.; Liu, L.; Chen, H.; Hu, Y.; Li, Y.; Song, L. Increased risks of maxillary sinus mucosal thickening in Chinese patients with periapical lesions. Heliyon 2023, 9, e18050. [Google Scholar] [CrossRef] [PubMed]
- White, S.C.; Pharoah, M.J. Oral Radiology: Principles and Interpretation, 6th ed.; Mosby/Elsevier: St. Louis, MO, USA, 2009; pp. 506–512. [Google Scholar]
- Phothikhun, S.; Suphanantachat, S.; Chuenchompoonut, V.; Nisapakultorn, K. Cone-beam computed tomographic evidence of the association between periodontal bone loss and mucosal thickening of the maxillary sinus. J. Periodontol. 2012, 83, 557–564. [Google Scholar] [CrossRef]
- Goller-Bulut, D.; Sekerci, A.E.; Köse, E.; Sisman, Y. Cone beam computed tomographic analysis of maxillary premolars and molars to detect the relationship between periapical and marginal bone loss and mucosal thickness of maxillary sinus. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e572–e579. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Z.; Zhang, L.; Zhou, X.; Zheng, Q.; Duan, X.; Zheng, G.; Wang, H.; Huang, D. Associations between maxillary sinus mucosal thickening and apical periodontitis using cone-beam computed tomography scanning: A retrospective study. J. Endod. 2012, 38, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Rancitelli, D.; Borgonovo, A.E.; Cicciù, M.; Re, D.; Rizza, F.; Frigo, A.C.; Maiorana, C. Maxillary sinus septa and anatomic correlation with the Schneiderian membrane. J. Craniofac. Surg. 2015, 26, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Schiebel, V.; Hof, M.; Ulm, C.; Watzek, G.; Pommer, B. Risk Factors of Membrane Perforation and Postoperative Complications in Sinus Floor Elevation Surgery: Review of 407 Augmentation Procedures. J. Oral Maxillofac. Surg. 2015, 73, 1275–1282. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Ataman-Duruel, E.T.; Duruel, O.; Tözüm, M.D.; Yıldırım, T.T.; Tözüm, T.F. Effects of local clinical factors on Schneiderian membrane thickness and morphology: A retrospective clinical study. Clin. Implant Dent. Relat. Res. 2019, 21, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Mozzo, P.; Procacci, C.; Tacconi, A.; Martini, P.T.; Andreis, I.A. A new volumetric CT machine for dental imaging based on the cone-beam technique: Preliminary results. Eur. Radiol. 1998, 8, 1558–1564. [Google Scholar] [CrossRef]
- Suomalainen, A.; Kiljunen, T.; Kaser, Y.; Peltola, J.; Kortesniemi, M. Dosimetry and image quality of four dental cone beam computed tomography scanners compared with multislice computed tomography scanners. Dentomaxillofac. Radiol. 2009, 38, 367–378. [Google Scholar] [CrossRef]
- Schiller, L.A.; Barbu, H.M.; Iancu, S.A.; Brad, S. Incidence. Size and orientation of maxillary sinus Septa-A retrospective clinical study. J. Clin. Med. 2022, 11, 2393. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Eksi, C.; Seker, B. Evaluating the relationship between periodontal bone loss in maxillary posterior teeth and Schneiderian membrane thickness. BMC Oral Health 2025, 25, 477. [Google Scholar] [CrossRef]
- Sheikhi, M.; Pozve, N.J.; Khorrami, L. Using cone beam computed tomography to detect the relationship between the periodontal bone loss and mucosal thickening of the maxillary sinus. Dent. Res J. 2014, 11, 495–501. [Google Scholar]
- Kış, H.C.; Soydan, D.; Canger, E.M. Evaluation of Relationship Between Alveolar Bone Loss of First Maxillary Molar and Maxillary Sinus Mucosal Thickening. Selcuk Dent. J. 2019, 6, 104–107. [Google Scholar]
- Brüllmann, D.D.; Schmidtmann, I.; Hornstein, S.; Schulze, R.K. Correlation of cone beam computed tomography (CBCT) findings in the maxillary sinus with dental diagnoses: A retrospective cross-sectional study. Clin. Oral Investig. 2012, 16, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, M.; Vandewoude, C.; Wyatt, J.; Jacobs, R. Comparative assessment of panoramic radiography and CBCT imaging for radiodiagnostics in the posterior maxilla. Clin. Oral Investig. 2014, 18, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Cymerman, J.J.; Cymerman, D.H.; O’Dwyer, R.S. Evaluation of odontogenic maxillary sinusitis using cone-beam computed tomography: Three case reports. J. Endod. 2011, 37, 1465–1469. [Google Scholar] [CrossRef]
- Vallo, J.; Suominen-Taipale, L.; Huumonen, S.; Soikkonen, K.; Norblad, A. Prevalence of mucosal abnormalities of the maxillary sinus and their relationship to dental disease in panoramic radiography: Results from the health 2000 health examination survey. Oral Surg. Med. Oral Pathol Oral Radiol. Endod. 2010, 109, e80–e87. [Google Scholar] [CrossRef]
- Ren, S.; Zhao, H.; Liu, J.; Wang, Q.; Pan, Y. Significance of maxillary sinus mucosal thickening in patients with periodontal disease. Int. Dent J. 2015, 65, 303–310. [Google Scholar] [CrossRef]
- Rege, I.C.; Sousa, T.O.; Leles, C.R.; Mendonça, E.F. Occurrence of maxillary sinus abnormalities detected by cone beam CT in asymptomatic patients. BMC Oral Health 2012, 12, 30. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, Y.; Cao, J.; Xu, T.; Zhen, M.; Yang, G.; Chung, K.H.; Hu, W. Association between the dimensions of the maxillary sinus membrane and molar periodontal status: A retrospective CBCT study. J. Periodontol. 2020, 91, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Janner, S.F.; Caversaccio, M.D.; Dubach, P.; Sendi, P.; Buser, D.; Bornstein, M.M. Characteristics and dimensions of the Schneiderian membrane: A radiographic analysis using cone beam computed tomography in patients referred for dental implant surgery in the posterior maxilla. Clin. Oral Implant. Res. 2011, 22, 1446–1453. [Google Scholar] [CrossRef]
- Shanbhag, S.; Karnik, P.; Shirke, P.; Shanbhag, V. Association between periapical lesions and maxillary sinus mucosal thickening: A retrospective cone-beam computed tomographic study. J. Endod. 2013, 39, 853–857. [Google Scholar] [CrossRef]
- Pazera, P.; Bornstein, M.M.; Pazera, A.; Sendi, P.; Katsaros, C. Incidental maxillary sinus findings in orthodontic patients: A radiographic analysis using cone-beam computed tomography (CBCT). Orthod. Craniofac. Res. 2011, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ritter, L.; Lutz, J.; Neugebauer, J.; Scheer, M.; Dreiseidler, T.; Zinser, M.J.; Rothamel, D.; Mischkowski, R.A. Prevalence of pathologic findings in the maxillary sinus in cone-beam computerized tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 634–640. [Google Scholar] [CrossRef]
- Graves, D.T.; Fine, D.; Teng, Y.T.; Van Dyke, T.E.; Hajishengallis, G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008, 35, 89–105. [Google Scholar] [CrossRef]
- Seymour, G.J.; Gemmell, E. Cytokines in periodontal disease: Where to from here? Acta Odontol. Scand. 2001, 59, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Sinusitis of odontogenic origin. Otolaryngol. Head Neck Surg. 2006, 135, 349–355. [Google Scholar] [CrossRef]
- Pommer, B.; Ulm, C.; Lorenzoni, M.; Palmer, R.; Watzek, G.; Zechner, W. Prevalence, location and morphology of maxillary sinus septa: Systematic review and meta-analysis. J. Clin. Periodontol. 2012, 39, 769–773. [Google Scholar] [CrossRef]
- Mealey, B.L.; Oates, T.W.; American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef] [PubMed]



| Gender | n (%) | Mean ± SD (mm) | Median (Min–Max) (mm) | p-Value 1 |
|---|---|---|---|---|
| Male | 157 (51.6) | 3.77 ± 1.94 | 3.65 (0.65–12.40) | |
| Female | 147 (48.4) | 4.13 ± 1.91 | 3.91 (0.47–9.56) | 0.063 |
| MT | n | % | Mean ± SD | Median (Min–Max) |
|---|---|---|---|---|
| Class I (≤1 mm) | 15 | 4.93 | 0.77 ± 0.17 | 0.79 (0.47–0.98) |
| Class II (1–2 mm) | 39 | 12.83 | 1.67 ± 0.23 | 1.73 (1.12–1.98) |
| Class III (2–4 mm) | 124 | 40.79 | 3.19 ± 0.57 | 3.26 (2.01–3.98) |
| Class IV (4–10 mm) | 125 | 41.12 | 5.73 ± 1.28 | 5.54 (4.01–9.56) |
| Class V (>10 mm) | 1 | 0.33 | 12.40 | 12.40 |
| Bone Loss | n | % | Mean ± SD | Median (Min–Max) |
|---|---|---|---|---|
| None | 12 | 3.9 | 1.76 ± 0.40 | 1.73 (0.65–2.57) |
| Mild | 41 | 13.5 | 1.57 ± 0.56 | 1.62 (0.47–2.57) |
| Moderate | 130 | 42.8 | 3.36 ± 0.97 | 3.37 (0.67–6.24) |
| Severe | 121 | 39.8 | 5.61 ± 1.67 | 5.45 (0.52–12.40) |
| p = 0.000197 * |
| Age Group (Years) | n | Mean ± SD (mm) | Median (Min–Max) (mm) |
|---|---|---|---|
| 19–25 | 11 | 2.01 | 1.65 |
| 26–40 | 112 | 3.01 | 3.06 |
| 41–60 | 159 | 4.43 | 4.06 |
| >60 | 22 | 6.21 | 6.13 |
| Variable | Coefficient (β) | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| Bone Loss | 1.5483 | 0.1199 | 12.9162 | <0.001 |
| Age | 0.0228 | 0.0087 | 2.6296 | 0.009 |
| Gender | 0.1895 | 0.1501 | 1.2627 | 0.208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarıbaş, E.; Kandemir, M.; Tuncer, M.C. Does Periodontal Bone Loss Play a Significant Role in Schneiderian Membrane Thickening? A Cone-Beam Computed Tomography Evaluation. Medicina 2025, 61, 1529. https://doi.org/10.3390/medicina61091529
Sarıbaş E, Kandemir M, Tuncer MC. Does Periodontal Bone Loss Play a Significant Role in Schneiderian Membrane Thickening? A Cone-Beam Computed Tomography Evaluation. Medicina. 2025; 61(9):1529. https://doi.org/10.3390/medicina61091529
Chicago/Turabian StyleSarıbaş, Ebru, Müzeyyen Kandemir, and Mehmet Cudi Tuncer. 2025. "Does Periodontal Bone Loss Play a Significant Role in Schneiderian Membrane Thickening? A Cone-Beam Computed Tomography Evaluation" Medicina 61, no. 9: 1529. https://doi.org/10.3390/medicina61091529
APA StyleSarıbaş, E., Kandemir, M., & Tuncer, M. C. (2025). Does Periodontal Bone Loss Play a Significant Role in Schneiderian Membrane Thickening? A Cone-Beam Computed Tomography Evaluation. Medicina, 61(9), 1529. https://doi.org/10.3390/medicina61091529







