Site-Specific Inflammatory Signatures in Metastatic NSCLC: Insights from Routine Blood Count Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Selection
- Histologically confirmed primary NSCLC [14];
- Radiological and/or histopathological confirmation of distant metastases present at the time of initial diagnosis, consistent with stage IV disease according to standard oncological staging systems (e.g., TNM classification);
- Availability of baseline hematological data, specifically a CBC, collected prior to the initiation of any oncologic treatment (chemotherapy, radiotherapy, or immunotherapy);
- Complete clinical documentation, including demographic information, tumor characteristics, and details regarding metastatic sites.
- Presence of concomitant hematologic malignancies such as leukemia, lymphoma, or myeloproliferative disorders, which could alter CBC values;
- Documented acute infections (bacterial, viral, or fungal) or systemic inflammatory diseases (e.g., autoimmune conditions, sepsis) at the time of blood testing;
- Use of chronic immunosuppressive therapies—including corticosteroids, biologics, or chemotherapy—prior to baseline hematologic assessment;
- Known severe bone marrow suppression caused by conditions unrelated to lung cancer, such as aplastic anemia or marrow infiltration from other malignancies.
2.3. Data Collection
2.4. Inflammatory Marker Calculation
2.5. Statistical Analysis
- -
- Family 1: all pairwise comparisons of NLR between metastatic sites.
- -
- Family 2: all pairwise comparisons of PLR between metastatic sites.
- -
- Family 3: all pairwise comparisons of LMR between metastatic sites.
- -
- Family 4: logistic regression models testing associations between individual hematologic parameters and a specific metastatic location.
2.6. Ethical Considerations
3. Results
4. Discussion
Strengths, Limitations, and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small-cell lung cancer |
| CBC | Complete blood count |
| NLR | Neutrophil-to-lymphocyte ratio |
| PLR | Platelet-to-lymphocyte ratio |
| LMR | Lymphocyte-to-monocyte ratio |
References
- Tang, F.H.; Wong, H.Y.T.; Tsang, P.S.W.; Yau, M.; Tam, S.Y.; Law, L.; Yau, K.; Wong, J.; Farah, F.H.M.; Wong, J. Recent advancements in lung cancer research: A narrative review. Transl. Lung Cancer Res. 2025, 14, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Nyen, J.E.; Booth, A.Ø.; Husby, Ø.; Bugge, C.; Engebretsen, I.; Oteiza, F.; Helland, Å.; Fjellbirkeland, L.; Brustugun, O.T.; Grønberg, B.H. Targeted treatment and survival in advanced non-squamous non-small cell lung cancer patients—A nationwide and longitudinal study. Front. Oncol. 2025, 15, 1506041. [Google Scholar] [CrossRef]
- Park, H.K.; Han, J.; Kwon, G.Y.; Yeo, M.-K.; Bae, G.E. Patterns of Extrathoracic Metastasis in Lung Cancer Patients. Curr. Oncol. 2022, 29, 8794–8801. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Qiu, B.M.; Luo, J.; Diao, Y.-F.; Hu, L.-W.; Liu, X.-L.; Shen, Y. Distant metastasis patterns among lung cancer subtypes and impact of primary tumor resection on survival in metastatic lung cancer using SEER database. Sci. Rep. 2024, 14, 22445. [Google Scholar] [CrossRef]
- Cha, H.K.; Lim, J.H.; Ryu, W.K.; Kim, L.; Ryu, J.-S. Solitary Uncommon Metastasis in Non-Small Cell Lung Cancer. Reports 2023, 6, 8. [Google Scholar] [CrossRef]
- Riaz, F.; Zhang, J.; Pan, F. Forces at play: Exploring factors affecting the cancer metastasis. Front. Immunol. 2024, 15, 1274474. [Google Scholar] [CrossRef]
- Lee, B.; Lee, T.; Lee, S.-H.; Choi, Y.-L.; Han, J. Clinicopathologic Characteristics of EGFR, KRAS, and ALK Alterations in 6595 Lung Cancers. Oncotarget 2016, 7, 23874–23884. [Google Scholar] [CrossRef]
- Wang, L.; Jia, J.; Lin, L.; Guo, J.; Ye, X.; Zheng, X.; Chen, Y. Predictive Value of Hematological Markers of Systemic Inflammation for Managing Cervical Cancer. Oncotarget 2017, 8, 44824–44832. [Google Scholar] [CrossRef]
- Rojko, L.; Megyesfalvi, Z.; Czibula, E.; Reiniger, L.; Teglasi, V.; Szegedi, Z.; Szallasi, Z.; Dome, B.; Moldvay, J. Longitudinal analysis of complete blood count parameters in advanced-stage lung cancer patients. Thorac. Cancer 2020, 11, 3193–3204. [Google Scholar] [CrossRef]
- Putzu, C.; Serra, R.; Campus, R.; Fadda, G.M.; Sini, C.; Marongiu, A.; Ginesu, G.C.; Fois, A.G.; Palmieri, G.; Zinellu, A.; et al. Complete Blood Count-Based Biomarkers as Predictors of Clinical Outcomes in Advanced Non-Small Cell Lung Cancer Patients with PD-L1 < 50% Treated with First-Line Chemoimmunotherapy. Curr. Oncol. 2024, 31, 4955–4967. [Google Scholar] [CrossRef] [PubMed]
- Zlatanova, T.; Arabadjiev, J. The prognostic role of markers of systemic inflammation in patients with metastatic lung cancer receiving immunotherapy: A comprehensive review of the literature. Pharmacia 2024, 71, 1–7. [Google Scholar] [CrossRef]
- Marschollek, K.; Kosacka, M.; Pokryszko-Dragan, A.; Brzecka-Bonnaud, A. Complete blood count parameters as potential predictive factors of brain metastases in lung cancer. Front. Oncol. 2025, 15, 1582788. [Google Scholar] [CrossRef]
- Lin, Y.J.; Wei, K.C.; Chen, P.Y.; Lim, M.; Hwang, T.L. Roles of Neutrophils in Glioma and Brain Metastases. Front. Immunol. 2021, 12, 701383. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Deshpande, C.; Lau, C.; Finley, D.; Rusch, V.; Pao, W.; Travis, W.D. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am. J. Surg. Pathol. 2009, 33, 1752–1764. [Google Scholar] [CrossRef] [PubMed]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Zhang, B.; Wu, X. Prognostic value of platelet to lymphocyte ratio in patients with cervical cancer: An updated systematic review and meta-analysis. World J. Surg. Oncol. 2025, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, H.; Chen, L.; Ma, X.; Li, X.; Gao, Y.; Zhang, Y.; Xie, Y.; Zhang, X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget 2016, 7, 31926–31942. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, H.; Jiang, S.; Wang, W. Role of tumor-associated neutrophils in lung cancer (Review). Oncol. Lett. 2022, 24, 2. [Google Scholar] [CrossRef]
- Mizuguchi, S.; Izumi, N.; Tsukioka, T.; Komatsu, H.; Nishiyama, N. Neutrophil-lymphocyte ratio predicts recurrence in patients with resected stage 1 non-small cell lung cancer. J. Cardiothorac. Surg. 2018, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Horvath, L.; Puschmann, C.; Scheiber, A.; Martowicz, A.; Sturm, G.; Trajanoski, Z.; Wolf, D.; Pircher, A.; Salcher, S. Beyond binary: Bridging neutrophil diversity to new therapeutic approaches in NSCLC. Trends Cancer 2024, 10, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Aldoori, A.; Malik, H.Z.; Al-Mukhtar, A.; Prasad, K.R.; Toogood, G.J.; Lodge, J.P.A. Elevated preoperative neutrophil-to-lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2008, 34, 55–60. [Google Scholar] [CrossRef]
- Foerster, Y.; Mayer, K.; Wasserer, S.; Dechant, M.; Verkhoturova, V.; Heyer, S.; Biedermann, T.; Persa, O.D. Elevated Neutrophil-to-Lymphocyte Ratio Correlates With Liver Metastases and Poor Immunotherapy Response in Stage IV Melanoma. Cancer Med. 2025, 14, e70631. [Google Scholar] [CrossRef]
- Eckert, C.; Klein, N.; Kornek, M.; Lukacs-Kornek, V. The complex myeloid network of the liver with diverse functional capacity at steady state and in inflammation. Front. Immunol. 2015, 6, 179. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, C.; Zhang, H. Targeting neutrophil extracellular traps in cancer progression and metastasis. Theranostics 2025, 15, 5846–5869. [Google Scholar] [CrossRef]
- Yan, B.; Wei, J.J.; Yuan, Y.; Sun, R.; Li, D.; Luo, J.; Liao, S.J.; Zhou, Y.H.; Shu, Y.; Wang, Q.; et al. IL-6 cooperates with G-CSF to induce protumor function of neutrophils in bone marrow by enhancing STAT3 activation. J. Immunol. 2013, 190, 5882–5893. [Google Scholar] [CrossRef]
- Clohisy, D.R.; Palkert, D.; Ramnaraine, M.L.; Pekurovsky, I.; Oursler, M.J. Human breast cancer induces osteoclast activation and increases the number of osteoclasts at sites of tumor osteolysis. J. Orthop. Res. 1996, 14, 396–402. [Google Scholar] [CrossRef]
- Maurer, S.; Ferrari de Andrade, L. NK Cell Interaction with Platelets and Myeloid Cells in the Tumor Milieu. Front. Immunol. 2020, 11, 608849. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martín-Villar, E. Podoplanin in Inflammation and Cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef]
- Kunita, A.; Kashima, T.G.; Morishita, Y.; Fukayama, M.; Kato, Y.; Tsuruo, T.; Fujita, N. The platelet aggregation-inducing factor aggrus/podoplanin promotes pulmonary metastasis. Am. J. Pathol. 2007, 170, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yao, Q.; Long, C.; Yi, P.; Song, J.; Wu, L.; Wan, W.; Rao, X.; Lin, Y.; Wei, G.; et al. Infiltration by monocytes of the central nervous system and its role in multiple sclerosis: Reflections on therapeutic strategies. Neural Regen. Res. 2025, 20, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Ji, S.; Dingman, A.; Lee, S.Y.; Derugin, N.; Wendland, M.F.; Vexler, Z.S. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J. Neurochem. 2007, 100, 893–904. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.; Wu, J.; Zhou, J.; Wang, W.; Wang, X.; Guo, J.; Wang, Q.; Zhang, X.; Xie, J.; et al. Relationship between lymphocyte to monocyte ratio and brain metastasis in non-smalll cell lung cancer patients. Am. J. Transl. Res. 2022, 14, 3936–3945. [Google Scholar]
- Li, W.Y.; Zhao, T.T.; Xu, H.M.; Wang, Z.-N.; Xu, Y.-Y.; Han, Y.; Song, Y.-X.; Wu, J.-H.; Xu, H.; Yin, S.-C.; et al. The role of EGFR mutation as a prognostic factor in survival after diagnosis of brain metastasis in non-small cell lung cancer: A systematic review and meta-analysis. BMC Cancer 2019, 19, 145. [Google Scholar] [CrossRef]
- Wang, B.X.; Ou, W.; Mao, X.Y.; Liu, Z.; Wu, H.Q.; Wang, S.Y. Impacts of EGFR mutation and EGFR-TKIs on incidence of brain metastases in advanced non-squamous NSCLC. Clin. Neurol. Neurosurg. 2017, 160, 96–100. [Google Scholar] [CrossRef]
- Lee, J.W.; Stone, M.L.; Porrett, P.M.; Thomas, S.K.; Komar, C.A.; Li, J.H.; Delman, D.; Graham, K.; Gladney, W.L.; Hua, X.; et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 2019, 567, 249–252. [Google Scholar] [CrossRef]
- Nelson, T.A.; Wang, N. Targeting lung cancer brain metastases: A narrative review of emerging insights for anaplastic lymphoma kinase (ALK)-positive disease. Transl. Lung Cancer Res. 2023, 12, 379–392. [Google Scholar] [CrossRef]
- Skoulidis, F.; Byers, L.A.; Diao, L.; Papadimitrakopoulou, V.A.; Tong, P.; Izzo, J.; Behrens, C.; Kadara, H.; Parra, E.R.; Canales, J.R.; et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015, 5, 860–877. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal. Transduct. Target Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value (n = 302) |
|---|---|
| Age (Mean ± SD) | 60.71 ± 13.44 |
| Sex | |
| Male | 244 (80.79%) |
| Female | 58 (19.20%) |
| Environment | |
| Urban | 128 (42.38%) |
| Rural | 174 (57.61%) |
| Occupational exposure to carcinogens | 202 (66.88%) |
| Smoking or alcohol history | 196 (64.90%) |
| Histological subtype | |
| Adenocarcinoma (ADK) | 162 (53.64%) |
| Squamous cell carcinoma | 110 (36.42%) |
| Other | 30 (9.93%) |
| Parameter | Mean ± SD | Min | Max |
|---|---|---|---|
| Leukocytes (×103/µL) | 12.14 ± 8.39 | 1.71 | 56.00 |
| Neutrophils (×103/µL) | 9.47 ± 7.69 | 1.02 | 52.00 |
| Erythrocytes (×106/µL) | 4.24 ± 0.61 | 2.40 | 5.50 |
| Platelets (×103/µL) | 369.66 ± 153.84 | 52.00 | 882.00 |
| Eosinophils (×103/µL) | 0.36 ± 0.20 | 0.00 | 3.06 |
| Basophils (×103/µL) | 0.03 ± 0.02 | 0.00 | 0.19 |
| Monocytes (×103/µL) | 0.70 ± 0.44 | 0.05 | 3.00 |
| Lymphocytes (×103/µL) | 1.58 ± 0.65 | 0.43 | 3.96 |
| Metastasis Type | Number of Patients | Percentage (%) |
|---|---|---|
| PLE | 52 | 17.21 |
| OSS | 86 | 28.47 |
| HEP | 66 | 21.85 |
| BRA | 98 | 32.45 |
| Molecular Feature | Frequency in Tested Patients (%) | Most Frequent in Metastases to | p-Value |
|---|---|---|---|
| EGFR mutation | 17.8 (n = 26) | Brain (42.3%) | 0.028 * |
| KRAS mutation | 24.6 (n = 36) | Liver (38.9%) | 0.041 * |
| ALK rearrangement | 5.5 (n = 8) | Brain (62.5%), Pleura (37.5%) | 0.052 |
| TP53 alteration | 32.8 (n = 48) | Bone (31.3%), Liver (27.1%) | 0.063 |
| PD-L1 ≥ 50% | 28.1 (n = 41) | Pleura (29.3%), Brain (26.8%) | 0.271 |
| Group | Leukocytes (×103) | Neutrophils (×103) | Erythrocytes (×106) | Platelets (×103) | Eosinophils | Basophils | Monocytes | Lymphocytes |
|---|---|---|---|---|---|---|---|---|
| PLE (n = 52) | 11.86 ± 8.31 | 9.26 ± 7.27 | 4.02 ± 0.62 | 376.14 ± 154.01 | 188.24 ± 153.16 | 42.76 ± 24.76 | 710.71 ± 511.69 | 1374.52 ± 549.47 |
| Non-PLE (n = 281) | 12.44 ± 8.58 | 9.71 ± 8.23 | 4.37 ± 0.59 | 362.50 ± 155.40 | 420.26 ± 228.92 | 37.63 ± 19.54 | 686.58 ± 346.15 | 1720.32 ± 730.98 |
| Cohen’s d | −0.068 | −0.056 | −0.589 | 0.088 | −1.06 | 0.251 | 0.064 | −0.49 |
| p-value (raw) | 0.658 | 0.716 | 0.0005 | 0.568 | 0.0005 | 0.106 | 0.681 | 0.003 |
| p-value (adjusted) | 0.716 | 0.999 | 0.002 | 0.999 | 0.002 | 0.424 | 1.000 | 0.012 |
| Observed power (%) | 7 | 7 | 97 | 90 | 100 | 38 | 7 | 90 |
| OSS (n = 86) | 14.06 ± 11.73 | 11.33 ± 10.56 | 4.10 ± 0.67 | 397.12 ± 143.73 | 133.08 ± 100.14 | 33.46 ± 32.14 | 795.38 ± 561.28 | 1569.23 ± 725.48 |
| non-OSS (n = 226) | 11.21 ± 6.12 | 8.58 ± 5.76 | 4.30 ± 0.58 | 376.44 ± 158.07 | 317.04 ± 296.82 | 30.37 ± 27.69 | 652.96 ± 362.07 | 1581.48 ± 613.15 |
| Cohen’s d | 0.354 | 0.372 | −0.33 | 0.134 | −0.712 | 0.107 | 0.334 | −0.019 |
| p-value (raw) | 0.005 | 0.003 | 0.009 | 0.290 | 0.0005 | 0.400 | 0.008 | 0.885 |
| p-value (adjusted) | 0.013 | 0.120 | 0.036 | 0.999 | 0.002 | 1.000 | 0.032 | 1.000 |
| Observed power (%) | 80 | 83 | 74 | 18 | 100 | 13 | 75 | 5 |
| HEP (n = 66) | 15.33 ± 12.84 | 14.21 ± 11.68 | 4.17 ± 0.74 | 405.33 ± 163.99 | 139.52 ± 113.16 | 35.24 ± 24.23 | 843.81 ± 585.80 | 1868.10 ± 768.16 |
| non-HEP (n = 263) | 11.00 ± 5.84 | 8.50 ± 5.46 | 4.26 ± 0.57 | 356.97 ± 149.00 | 299.15 ± 175.82 | 30.00 ± 14.44 | 747.80 ± 364.87 | 1474.07 ± 570.54 |
| Cohen’s d | 0.559 | 0.800 | −0.148 | 0.318 | −0.966 | 0.311 | 0.230 | 0.641 |
| p-value (raw) | 0.0005 | 0.0005 | 0.282 | 0.021 | 0.0005 | 0.024 | 0.096 | 0.001 |
| p-value (adjusted) | 0.002 | 0.002 | 0.999 | 0.084 | 0.002 | 0.096 | 0.384 | 0.002 |
| Observed power (%) | 98 | 100 | 19 | 63 | 100 | 62 | 38 | 100 |
| BRA (n = 98) | 13.34 ± 12.27 | 11.14 ± 13.67 | 4.58 ± 0.60 | 354.64 ± 112.47 | 86.00 ± 77.16 | 19.09 ± 17.58 | 553.64 ± 289.39 | 1534.55 ± 623.83 |
| non-BRA (n = 204) | 11.95 ± 7.18 | 9.21 ± 6.39 | 4.19 ± 0.60 | 372.06 ± 159.98 | 385.51 ± 136.06 | 35.01 ± 31.98 | 722.46 ± 454.91 | 1584.35 ± 654.97 |
| Cohen’s d | 0.152 | 0.206 | 0.650 | −0.119 | −2.491 | −0.566 | −0.413 | −0.077 |
| p-value (raw) | 0.210 | 0.089 | 0.0005 | 0.331 | 0.0001 | 0.0005 | 0.0005 | 0.527 |
| p-value (adjusted) | 0.28 | 0.356 | 0.002 | 0.999 | 0.002 | 0.002 | 0.002 | 1.000 |
| Observed power (%) | 24 | 40 | 100 | 16 | 100 | 99 | 92 | 10 |
| Metastasis Type | NLR ‡ | PLR ‡ | LMR ‡ | p-Value (NLR)—Raw | p-Value (NLR)—Adjusted | p-Value (PLR)—Raw | p-Value (plr) —Adjusted | p-Value (LMR)—Raw | p-Value (LMR)—Adjusted |
|---|---|---|---|---|---|---|---|---|---|
| PLE | 6.7 | 274 | 1.9 | 0.220 | 0.880 | 0.006 | 0.0240 | 0.018 | 0.072 |
| OSS | 7.2 | 253 | 2.0 | 0.036 | 0.144 | 0.410 | 1.000 | 0.032 | 0.048 |
| HEP | 7.6 | 217 | 2.2 | 0.041 | 0.164 | 0.150 | 0.600 | 0.300 | 1.000 |
| BRA | 7.3 | 231 | 2.8 | 0.150 | 0.600 | 0.810 | 1.000 | 0.008 | 0.032 |
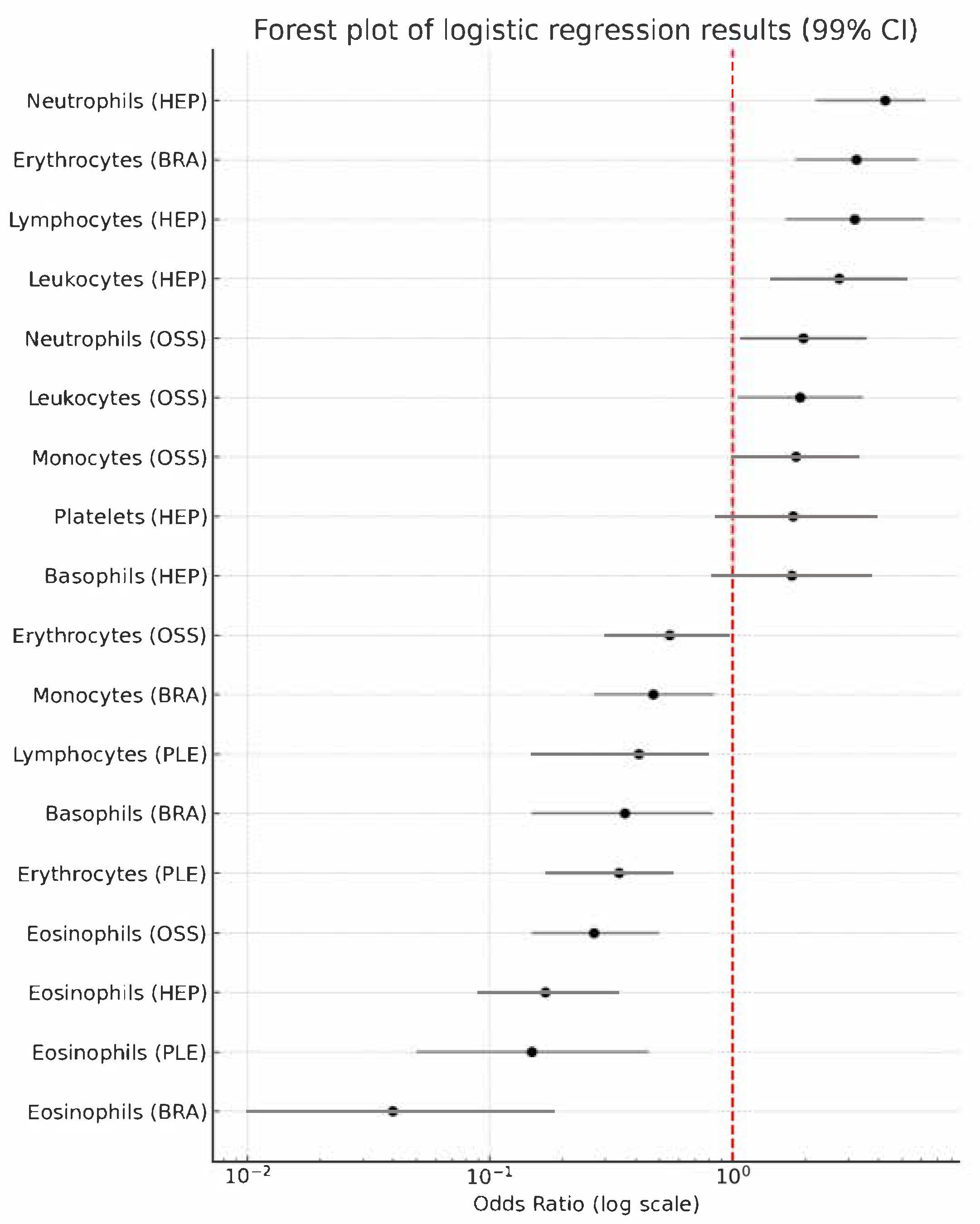
| Metastasis Type | Marker | OR 99 † | p-Value Raw | p-Value Adjusted |
|---|---|---|---|---|
| PLE | Erythrocytes | 0.34 (0.17–0.57) | 0.001 | 0.018 |
| Eosinophils | 0.15 (0.05–0.45) | 0.001 | 0.018 | |
| Lymphocytes | 0.41 (0.15–0.80) | 0.001 | 0.018 | |
| OSS | Leukocytes | 1.90 (1.05–3.45) | 0.006 | 0.018 |
| Neutrophils | 1.96 (1.08–3.56) | 0.004 | 0.072 | |
| Erythrocytes | 0.55 (0.30–0.97) | 0.010 | 0.180 | |
| Eosinophils | 0.27 (0.15–0.50) | 0.001 | 0.018 | |
| Monocytes | 1.83 (1.01–3.33) | 0.009 | 0.162 | |
| HEP | Leukocytes | 2.75 (1.44–5.28) | 0.001 | 0.018 |
| Neutrophils | 4.26 (2.20–6.25) | 0.001 | 0.018 | |
| Platelets | 1.78 (0.85–3.72) | 0.021 | 0.378 | |
| Eosinophils | 0.17 (0.09–0.34) | 0.001 | 0.018 | |
| Basophils | 1.76 (0.82–3.77) | 0.024 | 0.432 | |
| Lymphocytes | 3.20 (1.66–6.15) | 0.001 | 0.018 | |
| BRA | Erythrocytes | 3.25 (1.82–5.82) | 0.001 | 0.018 |
| Eosinophils | 0.04 (0.01–0.18) | 0.001 | 0.018 | |
| Basophils | 0.36 (0.15–0.83) | 0.001 | 0.018 | |
| Monocytes | 0.47 (0.27–0.84) | 0.001 | 0.018 |
| Age 28–50 (n = 35) | Age 51–70 (n = 206) | Age 71–78 (n = 61) | p-Value ANOVA | p-Value Age 28–50 vs. Age 51–70 | p-Value Age 28–50 vs. Age 71–78 | p-Value Age 51–70 vs. Age 71–78 | |
|---|---|---|---|---|---|---|---|
| Metastases PLE | 4 (11.11%) | 37 (18.97%) | 11 (15.49%) | 0.628 | 0.467 | 0.561 | >0.999 |
| Metastases OSS | 11 (30.55%) | 61 (31.28%) | 14 (19.71%) | 0.550 | 0.843 | 0.469 | 0.335 |
| Metastases HEP | 7 (19.44%) | 44 (22.56%) | 15 (21.21%) | 0.832 | >0.999 | 0.801 | 0.601 |
| Metastases BRA | 13 (36.11%) | 64 (32.82%) | 21 (29.57%) | 0.726 | 0.557 | 0.827 | 0.641 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vornicu, V.-N.; Negru, A.-G.; Vonica, R.C.; Cosma, A.A.; Saftescu, S.; Pasca-Fenesan, M.M.; Cimpean, A.M. Site-Specific Inflammatory Signatures in Metastatic NSCLC: Insights from Routine Blood Count Parameters. Medicina 2025, 61, 1521. https://doi.org/10.3390/medicina61091521
Vornicu V-N, Negru A-G, Vonica RC, Cosma AA, Saftescu S, Pasca-Fenesan MM, Cimpean AM. Site-Specific Inflammatory Signatures in Metastatic NSCLC: Insights from Routine Blood Count Parameters. Medicina. 2025; 61(9):1521. https://doi.org/10.3390/medicina61091521
Chicago/Turabian StyleVornicu, Vlad-Norin, Alina-Gabriela Negru, Razvan Constantin Vonica, Andrei Alexandru Cosma, Sorin Saftescu, Mihaela Maria Pasca-Fenesan, and Anca Maria Cimpean. 2025. "Site-Specific Inflammatory Signatures in Metastatic NSCLC: Insights from Routine Blood Count Parameters" Medicina 61, no. 9: 1521. https://doi.org/10.3390/medicina61091521
APA StyleVornicu, V.-N., Negru, A.-G., Vonica, R. C., Cosma, A. A., Saftescu, S., Pasca-Fenesan, M. M., & Cimpean, A. M. (2025). Site-Specific Inflammatory Signatures in Metastatic NSCLC: Insights from Routine Blood Count Parameters. Medicina, 61(9), 1521. https://doi.org/10.3390/medicina61091521








