Anti-Bacterial, Anti-Viral, and Anti-Inflammatory Properties of Kumazasa Extract: A Potential Strategy to Regulate Smoldering and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. KZExt
2.2. In Vitro Evaluations
2.2.1. Evaluation of the Anti-Microbial Effect of KZExt
2.2.2. Evaluation of the Anti-Influenza Virus Effect of KZExt
2.2.3. Evaluation of the Anti-Oxidant Effect of KZExt
2.2.4. Evaluation of KZExt on LPS-Stimulated IL-β Production
2.3. In Vivo Evaluations
2.3.1. Effect of KZExt in a Mouse Model with Formalin-Induced Inflammation
2.3.2. Effect of KZExt in Mice Model with LPS-Induced Inflammation
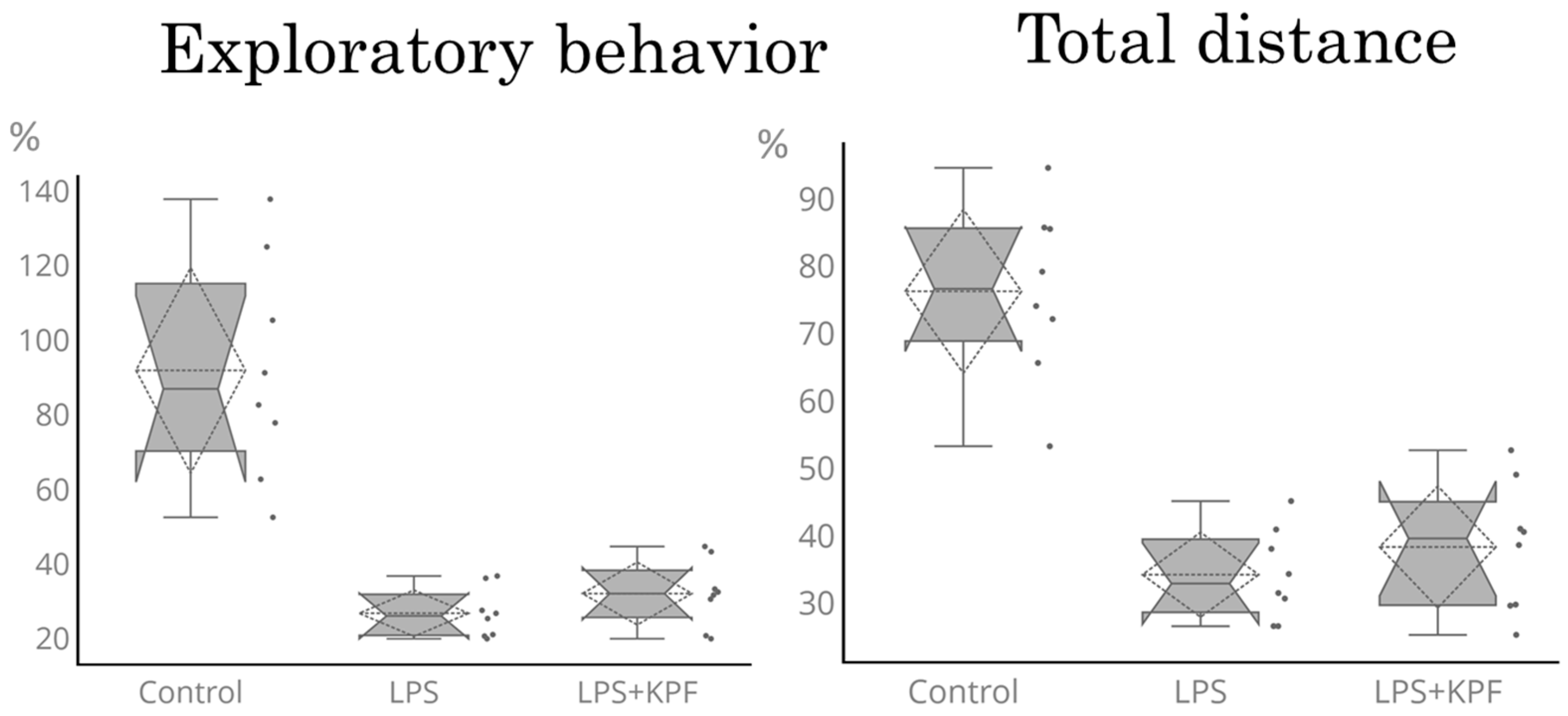
2.3.3. Open Field Test (OFT)
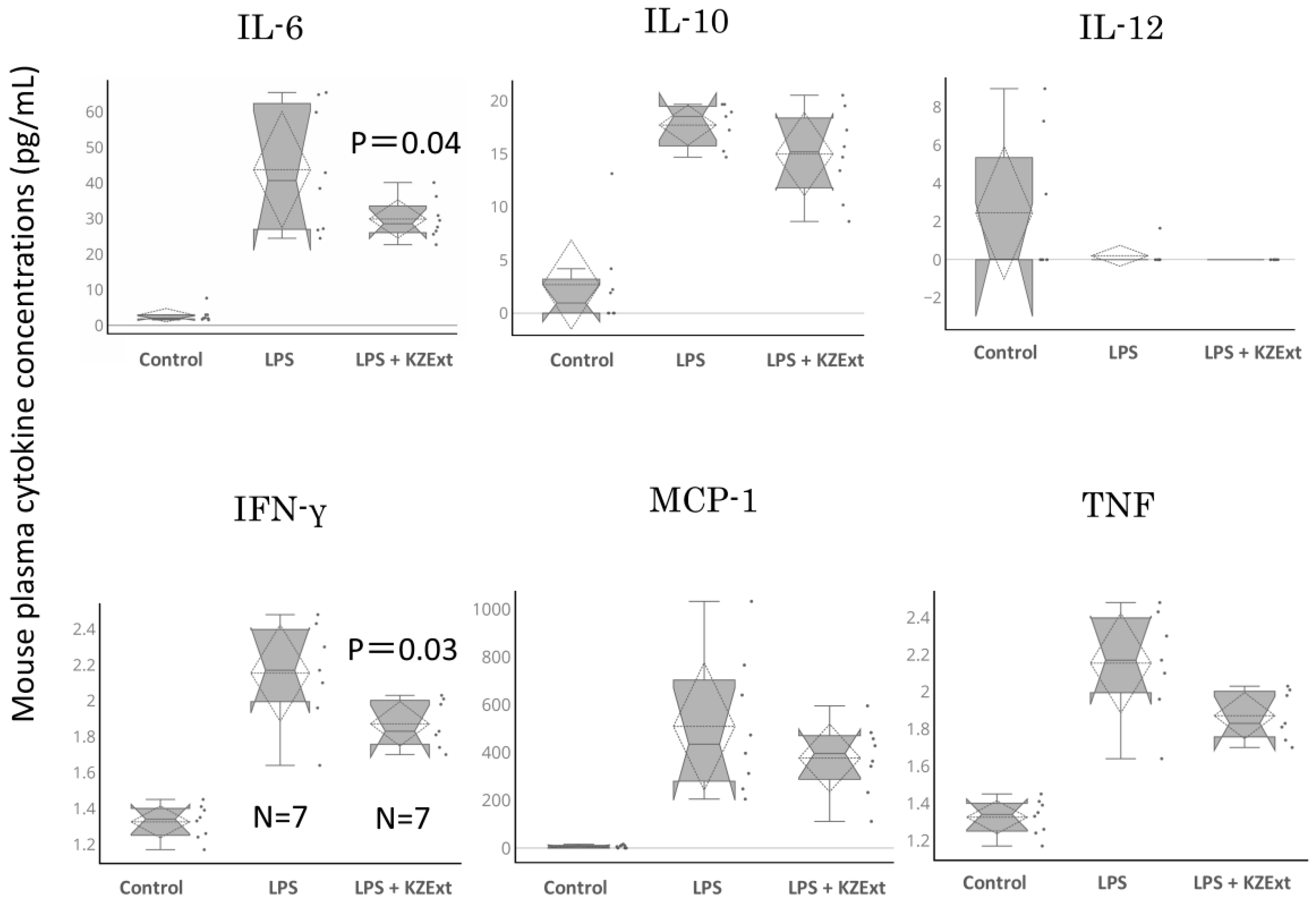
2.3.4. Quantification of Blood Cytokine Levels
2.4. Statistical Analyses
3. Results
3.1. In Vitro Evaluation
3.1.1. Anti-Microbial Effect of KZExt
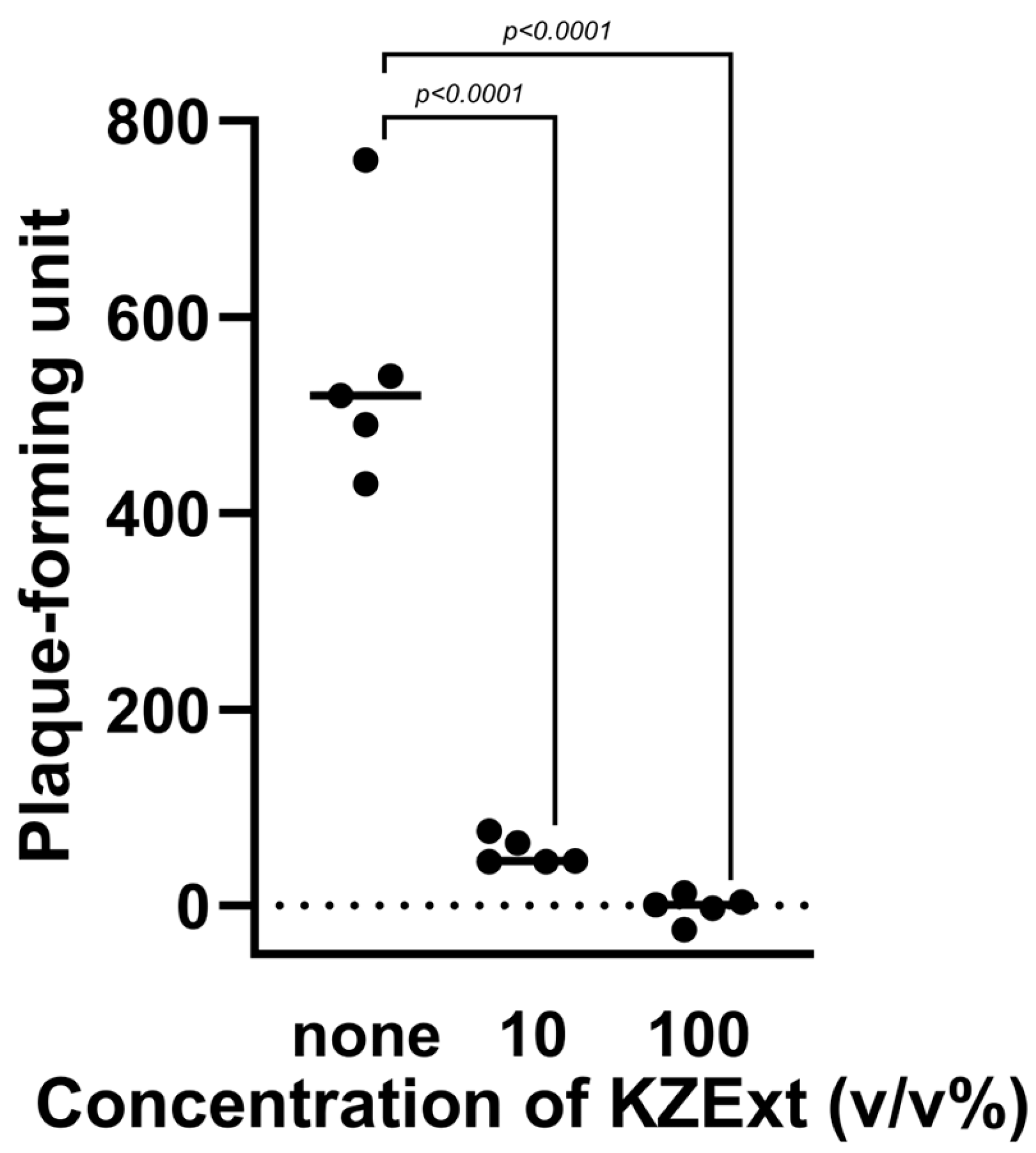
3.1.2. Anti-Influenza Virus Effect of KZExt
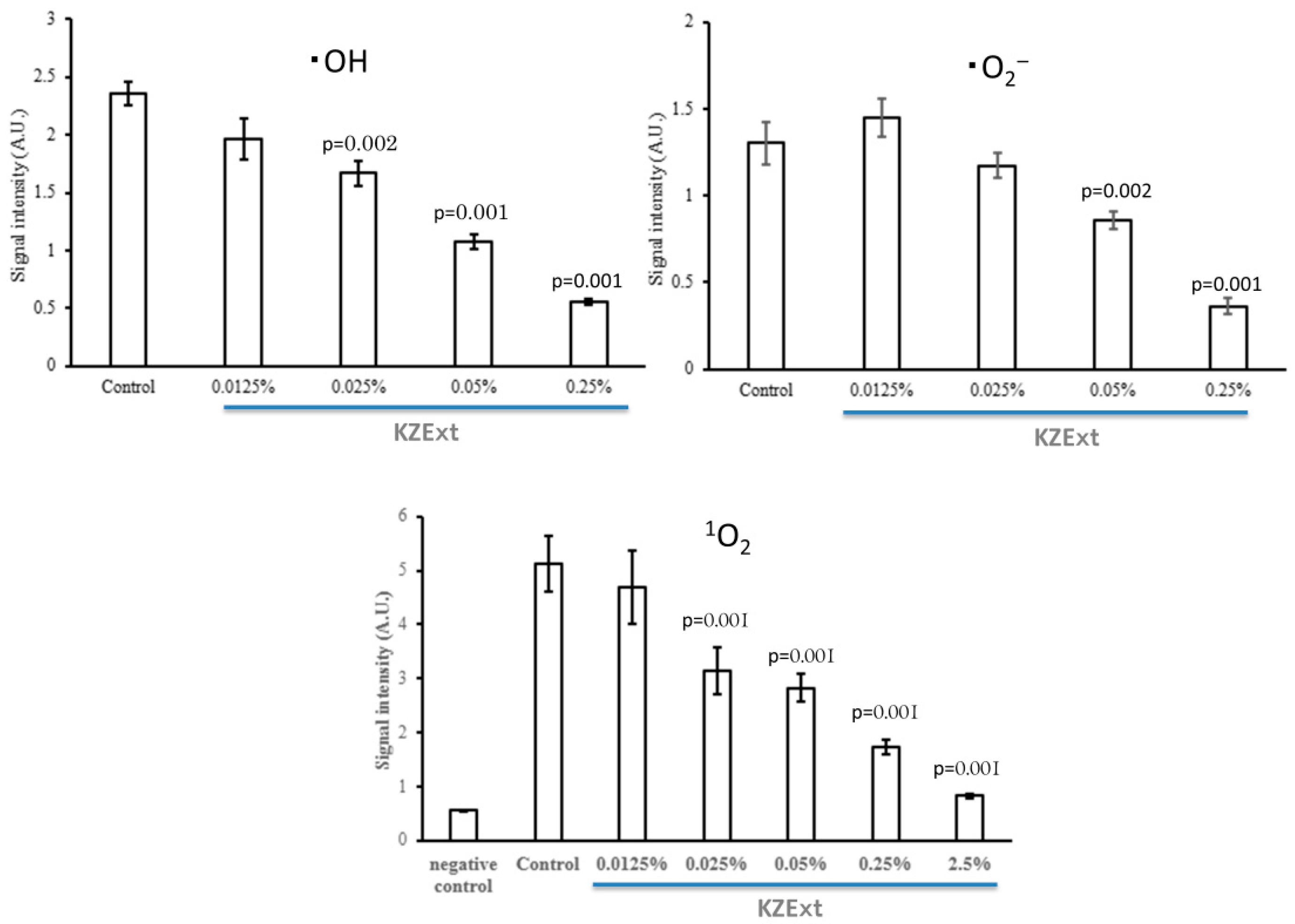
3.1.3. Anti-Oxidant Effect of KZExt
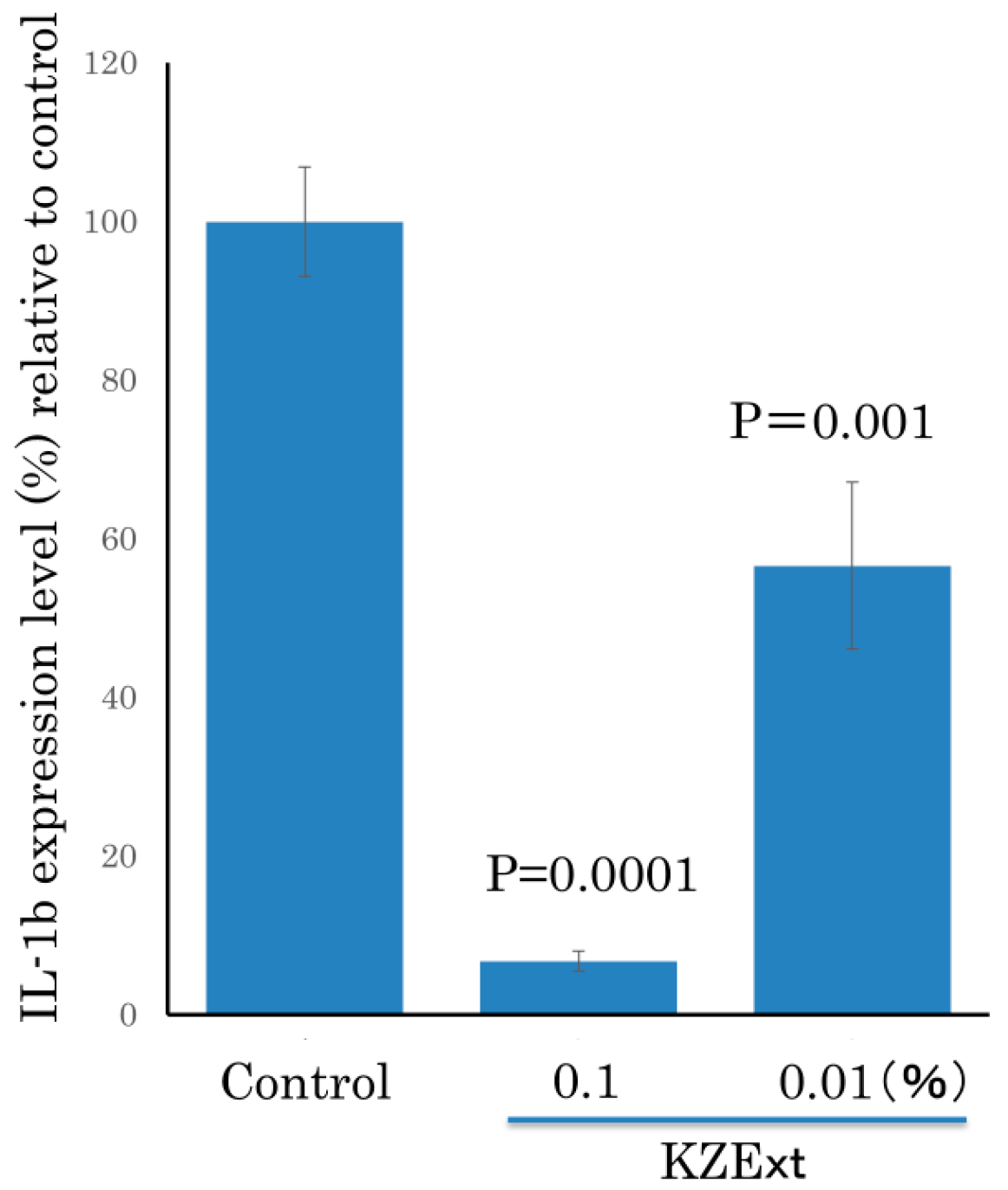
3.1.4. Effect of KZExt on LPS-Stimulated IL-β Production
3.2. In Vivo Evaluation
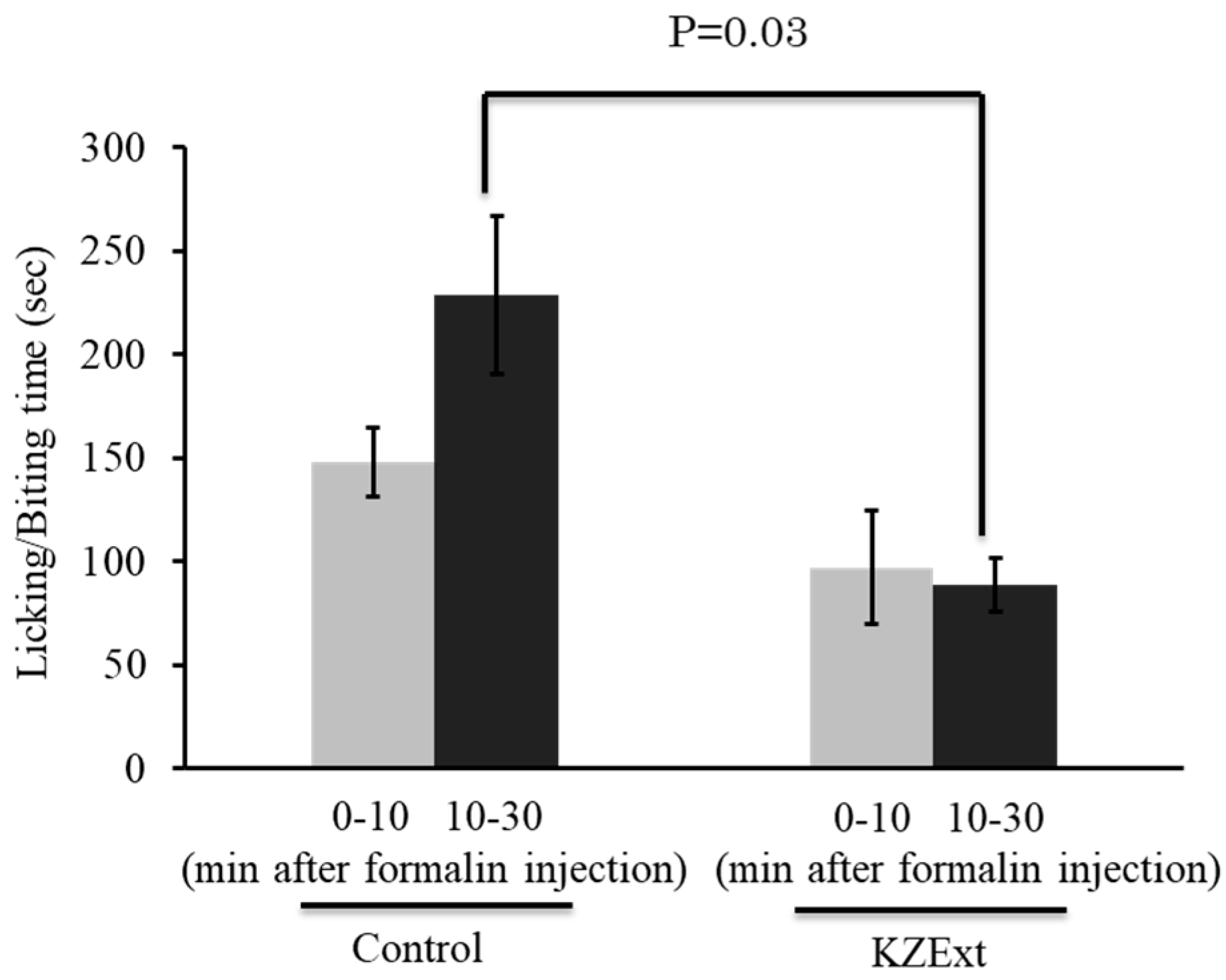
3.2.1. Effect of KZExt in Mice with Formalin-Induced Inflammation
3.2.2. Effect of KZExt in Mice with LPS-Induced Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yagi, Y.; Hara, H.; Hara, A. Food function of small molecules derived from Sasa. Food Funct. 2013, 11, 20–29. (In Japanese) [Google Scholar]
- Matsuta, T.; Sakagami, H.; Satoh, K.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Kitajima, M.; Oizumi, H.; Oizumi, T. Biological activity of luteolin glycosides and tricin from Sasa senanensis Rehder. In Vivo 2011, 25, 757–762. [Google Scholar] [PubMed]
- Matsuta, T.; Sakagami, H.; Sugiura, T.; Kitajima, M.; Oizumi, H.; Oizumi, T. Structural characterization of anti-UV components from Sasa senanensis Rehder extract. In Vivo 2013, 27, 77–83. [Google Scholar] [PubMed]
- Sakagami, H.; Amano, S.; Yasui, T.; Satoh, K.; Shioda, S.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Watanabe, K.; Sugiura, T.; et al. Biological interaction between Sasa senanensis Rehder leaf extract and toothpaste ingredients. In Vivo 2013, 27, 275–284. [Google Scholar] [PubMed]
- Sakagami, H.; Iwamoto, S.; Matsuta, T.; Satoh, K.; Shimada, C.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Morita, Y.; Ohkubo, A.; et al. Comparative study of biological activity of three commercial products of Sasa senanensis Rehder leaf extract. In Vivo 2012, 26, 259–264. [Google Scholar]
- Sakagami, H.; Tsuji, M.; Tomomura, M.; Masuda, Y.; Iwama, S.; Nakagawa, M.; Suzuki, H.; Tanaka, K.; Abe, T.; Tamura, N.; et al. Protection of differentiating neuronal cells from amyloid β peptide-induced injury by alkaline extract of leaves of Sasa senanensis Rehder. In Vivo 2018, 32, 231–239. [Google Scholar] [CrossRef]
- Zhou, L.; Hashimoto, K.; Satoh, K.; Yokote, Y.; Kitajima, M.; Oizumi, T.; Oizumi, H.; Sakagami, H. Effect of Sasa senanensis Rehder extract on NO and PGE2 production by activated mouse macrophage-like RAW264.7 cells. In Vivo 2009, 23, 773–777. [Google Scholar]
- Papikinou, M.A.; Pavlidis, K.; Cholidis, P.; Kranas, D.; Adamantidi, T.; Anastasiadou, C.; Tsoupras, A. Marine Fungi Bioactives with Anti-Inflammatory, Antithrombotic and Antioxidant Health-Promoting Properties Against Inflammation-Related Chronic Diseases. Mar. Drugs 2024, 22, 520. [Google Scholar] [CrossRef]
- Pan, X.; Lv, J.; Liu, M.; Li, Y.; Zhang, Y.; Zhang, R.; Liu, J.; Sun, C.; Guo, H. Chronic systemic inflammation predicts long-term mortality among patients with fatty liver disease: Data from the National Health and Nutrition Examination Survey 2007–2018. PLoS ONE 2024, 19, e0312877. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Jiang, M.; Wang, M.; Ji, M.; Xie, X.; Sheng, H. Chronic stress induces depression-like behavior in rats through affecting brain mitochondrial function and inflammation. Psychoneuroendocrinology 2024, 172, 107261. [Google Scholar] [CrossRef]
- Martinez Nieto, M.; De Leon Rodriguez, M.L.; Anaya Macias, R.D.C.; Lomeli Martinez, S.M. Periodontitis and chronic kidney disease: A bidirectional relationship based on inflammation and oxidative stress. World J. Clin. Cases 2024, 12, 6775–6781. [Google Scholar] [CrossRef]
- Hertis Petek, T.; Marcun Varda, N. Childhood Cardiovascular Health, Obesity, and Some Related Disorders: Insights into Chronic Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 9706. [Google Scholar] [CrossRef]
- Brzozowski, T.; Konturek, P.; Konturek, S.J.; Kwiecien, S.; Sliwowski, Z.; Pajdo, R.; Duda, A.; Ptak, A.; Hahn, E.G. Implications of reactive oxygen species and cytokines in gastroprotection against stress-induced gastric damage by nitric oxide releasing aspirin. Int. J. Color. Dis. 2003, 18, 320–329. [Google Scholar] [CrossRef]
- MohanKumar, S.M.J.; Murugan, A.; Palaniyappan, A.; MohanKumar, P.S. Role of cytokines and reactive oxygen species in brain aging. Mech. Ageing Dev. 2023, 214, 111855. [Google Scholar] [CrossRef] [PubMed]
- Llanos, P.; Palomero, J. Reactive Oxygen and Nitrogen Species (RONS) and Cytokines-Myokines Involved in Glucose Uptake and Insulin Resistance in Skeletal Muscle. Cells 2022, 11, 4008. [Google Scholar] [CrossRef] [PubMed]
- Tan-No, K.; Sakurada, T.; Yamada, T.; Sakurada, S.; Kisara, K. Involvement of opioid receptors in the antinociception produced by intracerebroventricularly administered spantide in mice. Neuropeptides 1995, 29, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Toyoda, A.; Kawase, T.; Nakamura, S.-I.; Ochiai, K. Consecutive intra-gingival injections of lipopolysaccharide and butyric acid to mice induce abnormal behavior and changes in cytokine concentrations. J. Neuroinflam. 2020, 17, 331. [Google Scholar] [CrossRef]
- Sato, I.; Maeda, T.; Katsuyama, S.; Kawase, T.; Kaku, T.; Takahashi, T.; Haniu, H.; Tsukahara, T.; Tsukahara, T.; Matsuda, Y. Anti-neuroinflammatory potential of porcine liver decomposition products in improving behavioral abnormalities: Effects on formalin- and LPS-induced inflammation. Biomed. Rep. 2025, 23, 154. [Google Scholar] [CrossRef]
- Wakasugi, D.; Kondo, S.; Ferdousi, F.; Mizuno, S.; Yada, A.; Tominaga, K.; Takahashi, S.; Isoda, H. A rare olive compound oleacein functions as a TrkB agonist and mitigates neuroinflammation both in vitro and in vivo. Cell Commun. Signal. 2024, 22, 38835076. [Google Scholar] [CrossRef]
- Hong, J.W.; Yang, G.E.; Kim, Y.B.; Eom, S.H.; Lew, J.H.; Kang, H. Anti-inflammatory activity of cinnamon water extracts in vivo and in vitro LPS-induced models. BMC Complement. Altern. Med. 2012, 12, 237. [Google Scholar] [CrossRef]
- Panda, S.R.; Meher, A.; Prusty, G.; Behera, S.; Prasad, B.R. Antibacterial properties and therapeutic potential of few medicinal plants: Current insights and challenges. Discov. Plants 2025, 2, 21. [Google Scholar] [CrossRef]
- Stephanie, A.; Boone, S.A.; Ijaz, M.K.; Bright, K.E.; Silva-Beltran, N.P.; Nims, R.W.; McKinney, J.; Gerba, C.P. Antiviral Natural Products, Their Mechanisms of Action and Potential Applications as Sanitizers and Disinfectants. Food Environ. Virol. 2023, 15, 265–280. [Google Scholar] [CrossRef]
- Sato, K.; Tatsunami, R.; Nakata, A.; Komatsu, K.I.; Harakawa, S.; Nedachi, T.; Haketa, K.; Inagawa, H.; Wakame, K. Effects of Kumaizasa (Sasa senanensis) Leaf Extract on Innate Immune Regulation in HEK293 Cells and Macrophages. Anticancer Res. 2021, 41, 4093–4100. [Google Scholar] [CrossRef]
- Honda, K.; Takano, Y. Experimental methods in pain using neuropathic and inflammatory animal models. Folia Pharmacol. Jpn. 2007, 130, 39–44. [Google Scholar] [CrossRef]
- Han, P.; Zhao, J.; Liu, S.B.; Yang, C.J.; Wang, Y.Q.; Wu, G.C.; Xu, D.M.; Mi, W.L. Interleukin-33 mediates formalin-induced inflammatory pain in mice. Neuroscience 2013, 241, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, K.; Sakagami, H.; Sugita, Y.; Takao, K.; Asai, D.; Terakubo, S.; Takemura, H.; Ohno, H.; Horiuchi, M.; Suguro, M.; et al. Quantification of the Ability of Natural Products to Prevent Herpes Virus Infection. Medicines 2020, 7, 64. [Google Scholar] [CrossRef]
- Sakagami, H.; Nakatani, S.; Enomoto, A.; Ota, S.; Kaneko, M.; Sugimoto, M.; Horiuchi, M.; Toeda, K.; Oizumi, T. Multi-Omics Analysis of Anti-Inflammatory Action of Alkaline Extract of the Leaves of Sasa sp. J. Clin. Med. 2021, 10, 2100. [Google Scholar] [CrossRef]
| Species | Strain | Culture Media | Culture Condition |
|---|---|---|---|
| Campylobacter jejuni | GTC03286 | Brain heart infusion broth | Microaerobic, 37 °C, 2 days |
| Escherichia coli | MG1655 | Muller Hinton broth with Ca and Mg | Aerobic, 37 °C, 2 days |
| Porphyromonas gingivalis | MW001 | Brucella broth with horse blood | Anaerobic, 37 °C, 6 days |
| Pseudomonas aeruginosa | NBRC13275 | Muller Hinton broth with Ca and Mg | Aerobic, 37 °C, 2 days |
| Salmonella enterica | NBRC13245T | Muller Hinton broth with Ca and Mg | Aerobic, 37 °C, 2 days |
| Staphylococcus aureus | NBRC13276 | Muller Hinton broth with Ca and Mg | Aerobic, 37 °C, 2 days |
| Streptococcus mutans | NBRC19355T | Brain heart infusion broth | Anaerobic, 37 °C, 2 days |
| Streptococcus pyogenes | KI | Muller Hinton broth with Ca and Mg | Aerobic, 37 °C, 2 days |
| Aspergillus niger | NBRC33023 | Potato dextrose broth | Aerobic, 30 °C, 3 days |
| Candida albicans | NBRC1385T | Potato dextrose broth | Aerobic, 30 °C, 2 days |
| Microbes | Minimum Inhibitory Concentration % (V/V) |
|---|---|
| Aspergillus niger | 12.5 |
| Candida albicans | 12.5 |
| Porphylomonas gingivalis | 6.3 |
| Streptococcus mutans | 6.3 |
| Salmonella enterica | 3.1 |
| Pseudomonas aeruginosa | 1.6 |
| Escherichia coli | 1.6 |
| Streptococcus pyogenes | 0.78 |
| Staphylococcus aureus | 0.2 |
| Campylobacter jejuni | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, H.; Gulam, S.; Maeda, T.; Watanabe, M.; Takajo, T.; Katsuyama, S.; Sano, H.; Tominaga, T.; Ogawa, A.; Sako, K.-i.; et al. Anti-Bacterial, Anti-Viral, and Anti-Inflammatory Properties of Kumazasa Extract: A Potential Strategy to Regulate Smoldering and Inflammation. Medicina 2025, 61, 1511. https://doi.org/10.3390/medicina61091511
Iwasaki H, Gulam S, Maeda T, Watanabe M, Takajo T, Katsuyama S, Sano H, Tominaga T, Ogawa A, Sako K-i, et al. Anti-Bacterial, Anti-Viral, and Anti-Inflammatory Properties of Kumazasa Extract: A Potential Strategy to Regulate Smoldering and Inflammation. Medicina. 2025; 61(9):1511. https://doi.org/10.3390/medicina61091511
Chicago/Turabian StyleIwasaki, Hideki, Shirol Gulam, Tomoji Maeda, Mineo Watanabe, Tokuko Takajo, Soh Katsuyama, Hiroaki Sano, Takanari Tominaga, Akio Ogawa, Ken-ichi Sako, and et al. 2025. "Anti-Bacterial, Anti-Viral, and Anti-Inflammatory Properties of Kumazasa Extract: A Potential Strategy to Regulate Smoldering and Inflammation" Medicina 61, no. 9: 1511. https://doi.org/10.3390/medicina61091511
APA StyleIwasaki, H., Gulam, S., Maeda, T., Watanabe, M., Takajo, T., Katsuyama, S., Sano, H., Tominaga, T., Ogawa, A., Sako, K.-i., Takahashi, T., Kawase, T., Tsukahara, T., & Matsuda, Y. (2025). Anti-Bacterial, Anti-Viral, and Anti-Inflammatory Properties of Kumazasa Extract: A Potential Strategy to Regulate Smoldering and Inflammation. Medicina, 61(9), 1511. https://doi.org/10.3390/medicina61091511







