Hepatitis Management in Saudi Arabia: Trends, Prevention, and Key Interventions (2016–2025)
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Epidemiology of Hepatitis in Saudi Arabia
3.1.1. Hepatitis A
3.1.2. Hepatitis B
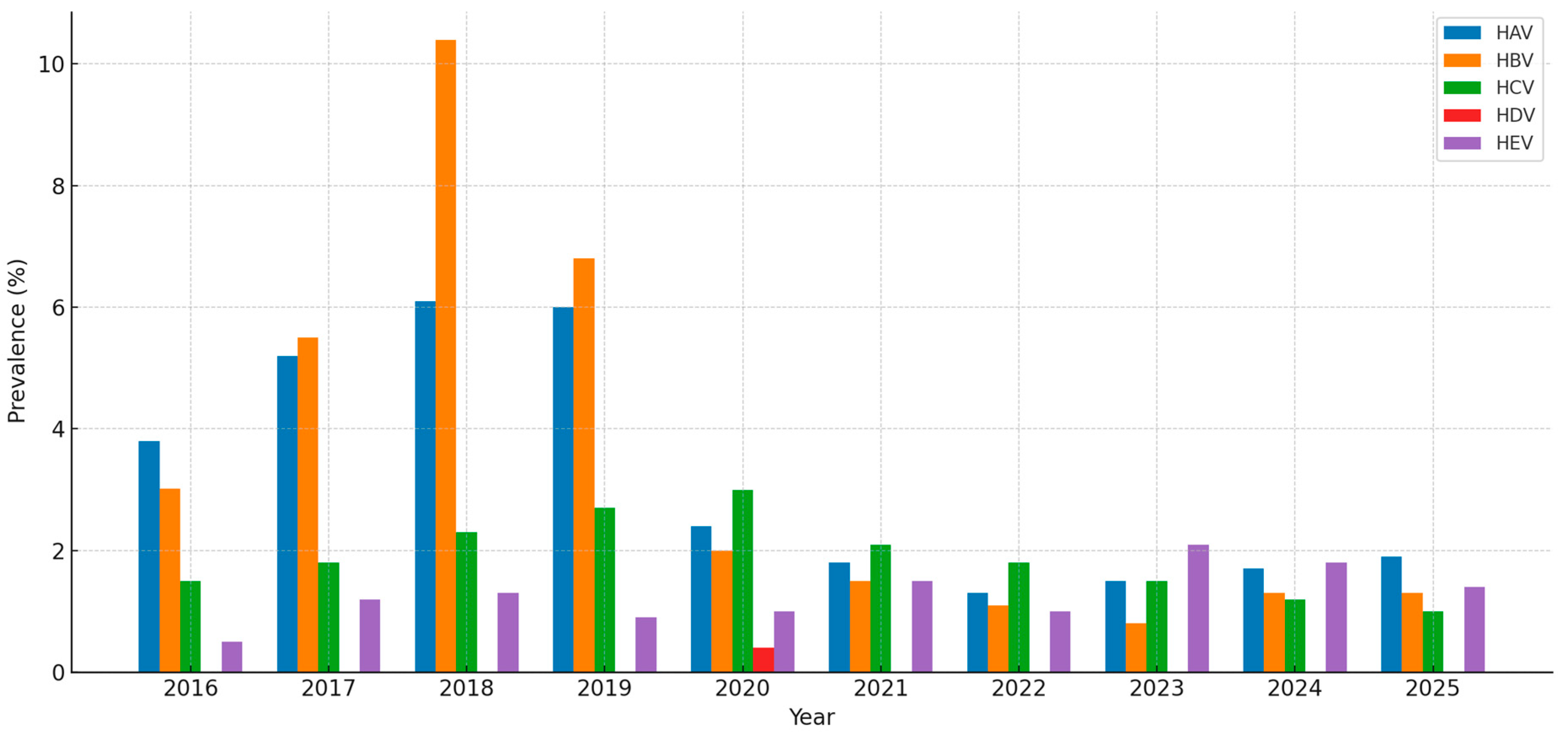
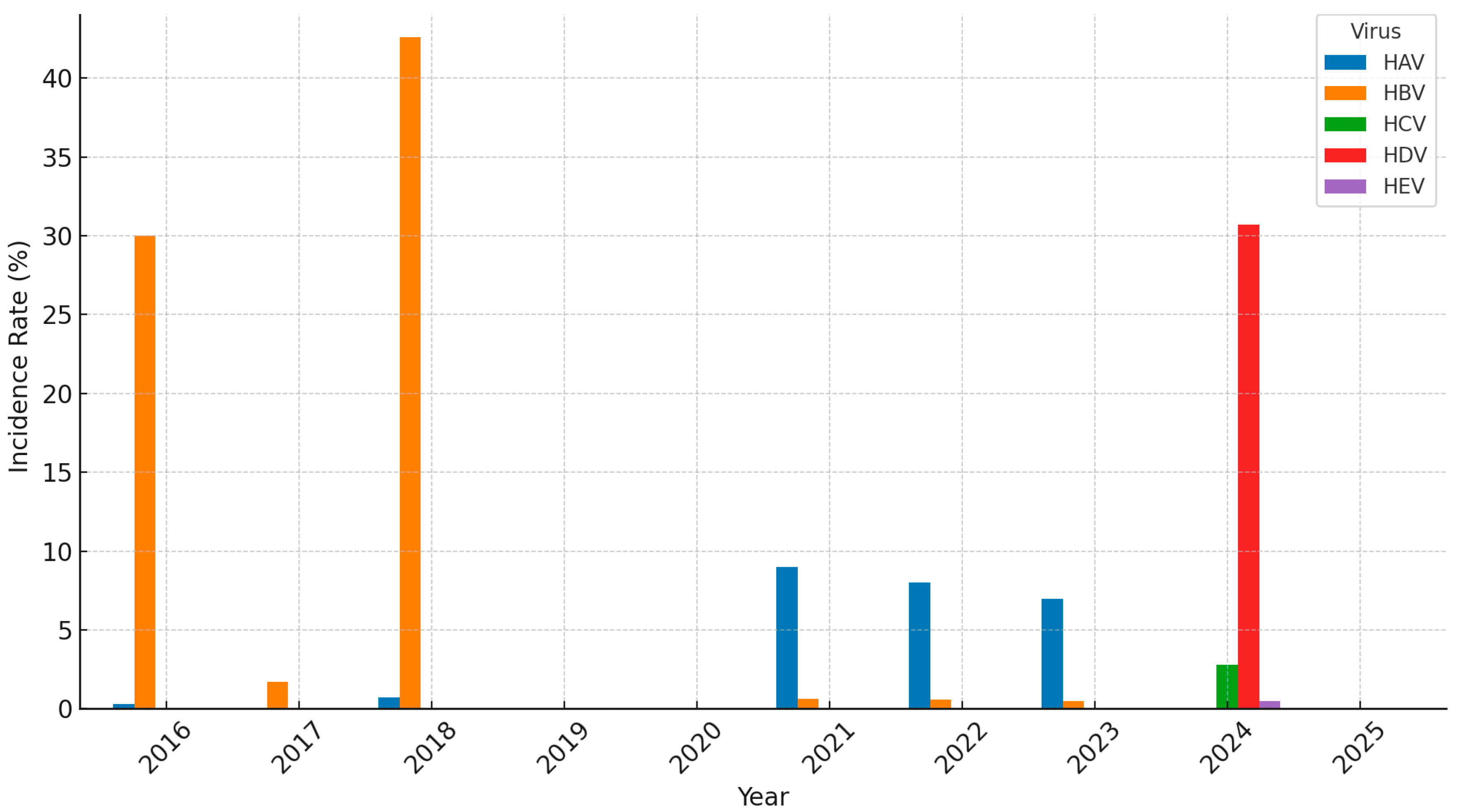
3.1.3. Hepatitis C
3.1.4. Other Hepatitis Types
3.2. Risk Factors of Hepatitis in Saudi Arabia
3.2.1. Socioeconomic Factors
3.2.2. Behavioral Factors
3.3. Hepatitis Treatment and Management in Saudi Arabia
3.3.1. Hepatitis A
3.3.2. Hepatitis B
3.3.3. Hepatitis C
3.3.4. Hepatitis D
3.3.5. Hepatitis E
3.3.6. Government and Non-Governmental Organizations (NGOs) Initiatives
3.4. Successful Policies and Interventions to Prevent and Control Hepatitis in Saudi Arabia
3.4.1. Vaccination Programs
3.4.2. Screening and Early Diagnosis Programs
3.4.3. Knowledge and Awareness Gaps
3.5. Challenges and Barriers
3.5.1. Social Stigma in Patients
3.5.2. Economic Impact of Antiviral Therapy for HBV
3.6. Future Directions to Control and Prevent Hepatitis in Saudi Arabia
3.6.1. Enhancing Viral Hepatitis Prevention and Control Strategies
3.6.2. Future Research and International Collaboration Priorities
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SNHP | Saudi National Hepatitis Program |
| HAV | Hepatitis A virus |
| HBV | Hepatitis B virus |
| HDV | Hepatitis D virus |
| HEV | Hepatitis E virus |
| HCC | Hepatocellular carcinoma |
| IDU | Intravenous drug use |
| RCTs | Randomized controlled trials |
| WHO | World Health Organization |
| UAE | United Arab Emirates |
| HBsAg | Hepatitis B surface antigen |
| GCC | Gulf Cooperation Council |
| CHB | Chronic hepatitis B |
| DAAs | Direct-acting antivirals |
| PEG-IFNα | Pegylated interferon-alpha |
| PEG-IFN | Pegylated interferon |
| NGO | Non-Governmental Organizations |
| CDC | Centers for Disease Control and Prevention |
| HBeAg | Hepatitis B e-antigen |
| PWID | People who inject drugs |
| TAF | Tenofovir alafenamide |
References
- Gan, C.; Yuan, Y.; Shen, H.; Gao, J.; Kong, X.; Che, Z.; Guo, Y.; Wang, H.; Dong, E.; Xiao, J. Liver diseases: Epidemiology, causes, trends and predictions. Signal Transduct. Target. Ther. 2025, 10, 33. [Google Scholar] [CrossRef]
- World Health Organization. WHO position paper on hepatitis A vaccines–June 2012. Wkly. Epidemiol. Rec. 2012, 87, 261–276. [Google Scholar]
- Khalil, M.; Al-Mazrou, Y.; Al-Jeffri, M.M.; Al-Howasi, M. Childhood epidemiology of hepatitis A virus in Riyadh, Saudi Arabia. Ann. Saudi Med. 1998, 18, 18–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atkinson, W. Hepatitis A, Epidemiology and Prevention of Vaccine-Preventable Diseases. In Centers for Disease Control and Prevention, 8th ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2005; pp. 177–189. [Google Scholar]
- Aljumah, A.; Babatin, M.; Hashim, A.; Sanai, F.; Abaalkhail, F.; Bassil, N.; Safwat, M. Hepatitis B care pathway in Saudi Arabia: Current situation, gaps and actions. Saudi J. Gastroenterol. 2019, 25, 73–80. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Sanai, F.M.; Banama, M.A.; Alghamdi, M.Y.; Altarrah, M.Y.; Abaalkhail, F.A. Multisociety consensus recommendations on hepatitis delta virus infection. Saudi J. Gastroenterol. 2025, 31, 5–13. [Google Scholar] [CrossRef]
- Madani, T.A. Hepatitis C virus infections reported over 11 years of surveillance in Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 132–136. [Google Scholar] [CrossRef]
- Abdo, A.; Sanai, F.M.; Al-Faleh, F.Z. Epidemiology of viral hepatitis in Saudi Arabia: Are we off the hook? Saudi J. Gastroenterol. 2012, 18, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Saudi Ministry of Health (MOH). Annual Health Statistics Booklet 2024; Ministry of Health: Riyadh, Saudi Arabia, 2024.
- Ghasemian, A. Prevalence of hepatitis A across various countries in the Middle East, African and Eastern European countries. Casp. J. Intern. Med. 2016, 7, 302–303. [Google Scholar]
- Al-Harbi, R.A. Viral hepatitis trend in Majmaah City, Riyadh Region, Saudi Arabia: A 3-year retrospective study (2017–2019). Int. J. Med. Dev. Ctries. 2020, 4, 975–980. [Google Scholar] [CrossRef]
- Alhaydar, N.; Abusaris, R.; Alwatban, N. Prevalence and Factors Associated With Hepatitis C Virus in Riyadh, Saudi Arabia: A Large Cross-Sectional Study. J. Epidemiol. Glob. Health 2025, 15, 42. [Google Scholar] [CrossRef]
- Arif, M. Enterically transmitted hepatitis in Saudi Arabia: An epidemiological study. Ann. Trop. Med. Parasitol. 1996, 90, 197–201. [Google Scholar] [CrossRef]
- Arimi, E.M.; Solhi, M.; Eghdami, S.; Moghadam, S.M.K.; Mohammadi, M.; Fathi, M.; Kachuei, M. A cross-sectional study of patient satisfaction among immigrants in the Pediatric Outpatient Clinic of Firoozabadi Hospital. J. Patient Exp. 2024, 11, 23743735241272175. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Bello, K.E.; Radwan, Z.; Hassouneh, A.K.; Alrasheed, H.A.; Alotaibi, J.; Basrana, B.; Zaidan, A.A.; Garout, M.A.; Zaidan, T.I.; et al. The Dual Burden of Hepatitis B and C Among Drug Users in Asia: The First Systematic Review and Meta-Analysis. Pathogens 2025, 14, 360. [Google Scholar] [CrossRef]
- World Health Organization. Web Annex A, Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2017. In Global Hepatitis Report 2017; London School of Hygiene and Tropical Medicine: London, UK, 2017. [Google Scholar]
- Memish, Z.A.; Al Knawy, B.; El-Saed, A. Incidence trends of viral hepatitis A, B, and C seropositivity over eight years of surveillance in Saudi Arabia. Int. J. Infect. Dis. 2010, 14, e115–e120. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030; World Health Organization: Geneva, Switzerland, 2016. Available online: https://iris.who.int/bitstream/handle/10665/206453/WHO_HIV_2016.04_eng.pdf?sequence=1 (accessed on 18 April 2024).
- World Health Organization. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 20 April 2024).
- Al-Faleh, F.Z.; Al-Jeffri, M.H.; Al-Rashed, R.S.; Aref, M. Efficacy of hepatitis B vaccine in a cohortcommunity-based study in Riyadh and Hail regions of Saudi Arabia. Saudi J. Gastroenterol. 2002, 8, 81–84. [Google Scholar] [PubMed][Green Version]
- Minshawi, F.; A Abdulshakoor, A.; Alwakil, E.M.; Basfar, G.T.; Kabrah, S.; Aslam, A.; Almasmoum, H.; Mujalli, A.; Moaminah, R.H.; A Almoalad, G.; et al. Seroprevalence of transfusion-transmitted infections among blood donors in Makkah, Saudi Arabia. J. Infect. Dev. Ctries. 2024, 18, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Sanai, F.; Alkhatry, M.; Alzanbagi, A.; Kumar, S. Hepatitis B virus infection in Saudi Arabia and the UAE: Public health challenges and their remedial measures. J. Infect. Public Health 2023, 16, 1410–1417. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Abaalkhail, F.; Alghamdi, S.; Bzeizi, K.; Al-Hamoudi, W.K.; Paik, J.M.; Henry, L.; Al-Judaibi, B.; Sanai, F.M.; Younossi, Z.M. The burden of metabolic dysfunction-associated steatotic liver disease and viral hepatitis in Saudi Arabia. Saudi J. Gastroenterol. 2024, 30, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.; Alghamdi, H.; Alserehi, H.A.; Babatin, M.A.; Alswat, K.A.; Alghamdi, M.; AlQutub, A.; Abaalkhail, F.; Altraif, I.; Alfaleh, F.Z.; et al. SASLT guidelines: Update in treatment of hepatitis C virus infection. Saudi J. Gastroenterol. 2024, 30, S1–S42. [Google Scholar] [CrossRef]
- Khalid, S.S.; Alswat, K. Genetic susceptibility of Saudi population to Hepatitis B Virus (HBV) infection and the predicted functional consequences. medRxiv 2025. [Google Scholar] [CrossRef]
- Alshabi, A.; Fatima, N.; Marwan, A.; Oraibi, K.G.; Qubaisi, E.A.; Arif, H.O.; Daghriri, E.M.; Zelai, N.A.; Khan, I.A. Epidemiology, Screening, and Genotyping Analysis for Hepatitis B Virus in Southwestern Region of Saudi Arabia. J. Infect. Public Health 2021, 14, 187–192. [Google Scholar] [CrossRef]
- Toumi, M.; Gantumur, Z.; Nguyen, M.H.; Tran, T.T.; Zoulim, F.; Kim, W.R.; Han, S.H.B.; Loomba, R.; Chayama, K.; Lim, Y.S.; et al. Experience and Impact of Stigma in People with Chronic Hepatitis B: A Qualitative Study in Asia, Europe, and the United States. BMC Public Health 2024, 24, 611–614. [Google Scholar] [CrossRef]
- Alzahrani, F.M.; Muzaheed, A.; Shaikh, S.S.; Alomar, A.I.; Acharya, S.; Elhadi, N. Prevalence of Hepatitis B Virus (HBV) Among Blood Donors in Eastern Saudi Arabia: Results From a Five-Year Retrospective Study of HBV Seromarkers. Ann. Lab. Med. 2019, 39, 81–85. [Google Scholar] [CrossRef]
- Alghamdi, I.G.; Alghamdi, R.M.; Alghamdi, M.S.; Alghamdi, A.M.; Alghamdi, M.; Alghamdi, Z.; Alghamdi, K.S. Epidemiology of Hepatitis B in Saudi Arabia from 2006 to 2021. Hepatic Med. Evid. Res. 2023, 15, 233–247. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Hepatitis C|Fact Sheet [Internet]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 5 July 2024).
- Altraif, I. Can hepatitis C virus be eliminated by 2030? Saudi Arabia as an example. Saudi Med. J. 2018, 39, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Alsughayyir, J.; Almalki, Y.; Alburayk, I.; Alalshaik, M.; Aljoni, I.; Kandel, M.; Alfhili, M.A.; Alabdullateef, A.A. Prevalence of transfusion-transmitted infections in Saudi Arabia blood donors: A nationwide, cross-sectional study. Saudi Med. J. 2022, 43, 1363. [Google Scholar] [CrossRef] [PubMed]
- Hiebert-Suwondo, L.; Manning, J.; Tohme, R.A.; Buti, M.; Kondili, L.A.; Spearman, C.W.; Prabdial-Sing, N.; Turnier, V.; Lazarus, J.V.; Waked, I.; et al. A 2024 global report on national policy, programmes, and progress towards hepatitis B elimination: Findings from 33 hepatitis elimination profiles. Lancet Gastroenterol. Hepatol. 2025, 10, 671–684. [Google Scholar] [CrossRef]
- Almajid, A.; Albarbari, H.; Bazroon, A.; Al-Awami, H.; Aljurayyad, R.; Albadran, R.; Alkhamis, Z.; Alomair, H.; Aljishi, Y. Epidemiological Perspectives: A Four-Year Insight Into Hepatitis C Surveillance in the Kingdom of Saudi Arabia. Cureus 2024, 16, e52646. [Google Scholar] [CrossRef]
- Makhoul, M.; Mumtaz, G.R.; Ayoub, H.H.; Jamil, M.S.; Hermez, J.G.; Alaama, A.S.; Abu-Raddad, L.J. Hepatitis C Virus Transmission among People Who Inject Drugs in the Middle East and North Africa: Mathematical Modeling Analyses of Incidence and Intervention Impact. eClinicalMedicine 2025, 80, 101632. [Google Scholar] [CrossRef]
- Alzahrani, M.S.; Ayn Aldeen, A.; Almalki, R.S.; Algethami, M.B.; Altowairqi, N.F.; Alzahrani, A.; Algarni, M.A. Knowledge of and testing rate for hepatitis C infection among the general public of Saudi Arabia: A cross-sectional study. Int. J. Environ. Res. Public Health 2023, 20, 2080. [Google Scholar] [CrossRef]
- Jamjoom, G.A.; El-Daly, M.M.; Azhar, E.I.; Fallatah, H.I.; Akbar, H.O.; Babatin, M.; Alghamdi, A.S.; Dgdgi, M.I.; Hamid, M.A.; Qari, Y.A.; et al. Prevalence and molecular characterization of hepatitis D virus in Saudi Arabia: A single-center study. Saudi J. Gastroenterol. 2017, 23, 176–182. [Google Scholar] [CrossRef]
- Saade, M.C.; Haddad, G.; El Hayek, M.; Shaib, Y. The burden of hepatitis E virus in the Middle East and North Africa region: A systematic review. J. Infect. Dev. Ctries. 2022, 16, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Alhatlani, B.Y.; Aljabr, W.A.; Almarzouqi, M.S.; Alhatlani, S.M.; Alzunaydi, R.N.; Alsaykhan, A.S.; Almaiman, S.H.; Aleid, A.A.; Alsughayir, A.H.; Bishawri, Y.E.; et al. Seroprevalence of the hepatitis E virus antibodies among blood donors in the Qassim region, Saudi Arabia. Future Virol. 2021, 16, 383–388. [Google Scholar] [CrossRef]
- El-Daly, M.M.; Al-Raddadi, R.; Alharbi, A.; Azhar, A.E.; Khallaf, A.M.; Hassan, A.M.; Alwafi, O.M.; Shabouni, O.I.; Alandijany, T.A.; Li, T.-C.; et al. Hepatitis E virus (HEV) in Makkah, Saudi Arabia: A population-based seroprevalence study. Viruses 2023, 15, 484. [Google Scholar] [CrossRef]
- El-Kafrawy, S.A.; Hassan, A.M.; El-Daly, M.M.; Al-Hajri, M.; Farag, E.; Elnour, F.A.; Khan, A.; Tolah, A.M.; Alandijany, T.A.; Othman, N.A.; et al. Genetic Diversity of Hepatitis E Virus (HEV) in Imported and Domestic Camels in Saudi Arabia. Sci. Rep. 2022, 12, 7005. [Google Scholar] [CrossRef] [PubMed]
- Masood, U.; John, S. Hepatitis D. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470436/ (accessed on 12 September 2022).
- Ramos Flores, C.; Echeagaray, E.; Castañeda, G.; Vargas, M.d.L.; Montes-González, R.; Luna, S.; Díaz, L.; Torres, O. Linking hepatitis C virus infection to pre-1994 blood transfusions in female patients [Asociación del antecedente transfusional antes de 1994 con hepatitis vírica tipo C en mujeres]. Medwave 2017, 17, e6886. [Google Scholar] [CrossRef]
- Abdullah, S.M. Prevalence of Hepatitis B and C virus infection and their co-relation with hematological and hepatic parameters in subjects undergoing Premarital Screening in the Jazan Region, Kingdom of Saudi Arabia. Pak. J. Med. Sci. 2018, 34, 316–321. [Google Scholar] [CrossRef]
- Alshomrani, A.T. Prevalence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus infection among heroin injectors in the central region of Saudi Arabia. Saudi Med. J. 2015, 36, 802. [Google Scholar] [CrossRef]
- Khafagy, A.; AlJahdaly, I.; Goweda, R. Hepatitis B Vaccine: Assessment of Immunologic Response, Coverage Rate, and Factors Influencing Seroreactivity. Clin. Lab. 2020, 66, 1351–1356. [Google Scholar] [CrossRef]
- Al-Qahtani, S.G.; Alsulami, B.A. Prevalence and predictors of use of cupping among patients attending a primary care center in Riyadh, Saudi Arabia. J. Fam. Med. Prim. Care 2023, 12, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.A.; Al-Hemiary, N. Patterns and sociodemographic characteristics of substance abuse among hepatitis C virus-infected patients in Iraq: A cross-sectional study. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 94. [Google Scholar] [CrossRef]
- Viral Hepatitis: Types and Tips. Available online: https://www.moh.gov.sa/en/HealthAwareness/EducationalContent/Diseases/Otherdiseases/Pages/003.aspx (accessed on 12 September 2022).
- Saudi Arabia Hepatitis Country Profile 2017. Available online: https://www.emro.who.int/images/stories/asd/hepatitis_profiles/ksa_hepatitis_profile.pdf (accessed on 12 September 2022).
- Al-Dharrab, A.A.; Al-Samadani, K.H. Assessment of hepatitis B vaccination and compliance with infection control among dentists in Saudi Arabia. Saudi Med. J. 2012, 33, 1205–1210. [Google Scholar] [PubMed]
- IQVIA; Shinagawa City, Tokyo, Japan. Local and MIDAS Sales Data MAT Q4. Proprietary report. 2016. [Google Scholar]
- Wedemeyer, H.; Aleman, S.; Brunetto, M.R.; Blank, A.; Andreone, P.; Bogomolov, P.; Chulanov, V.; Mamonova, N.; Geyvandova, N.; Morozov, V.; et al. A phase 3, randomized trial of bulevirtide in chronic hepatitis D. N. Engl. J. Med. 2023, 389, 22–32. [Google Scholar] [CrossRef]
- Wranke, A.; Hardtke, S.; Heidrich, B.; Dalekos, G.; Yalçin, K.; Tabak, F.; Gürel, S.; Çakaloğlu, Y.; Akarca, U.S.; Lammert, F.; et al. Ten-year follow-up of a randomized controlled clinical trial in chronic hepatitis delta. J. Viral Hepatol. 2020, 27, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.; Westman, G.; Falconer, K.; Duberg, A.; Weiland, O.; Haverinen, S.; Wejstål, R.; Carlsson, T.; Kampmann, C.; Larsson, S.B.; et al. Long-term study of hepatitis delta virus infection at secondary care centers: The impact of viremia on liver-related outcomes. Hepatology 2020, 72, 1177–1190. [Google Scholar] [CrossRef]
- Abbas, Z.; Afzal, R. Hepatitis E: When to treat and how to treat. Antivir. Ther. 2014, 19, 125–131. [Google Scholar] [CrossRef]
- Abdelaal, M.; Rowbottom, D.; Zawawi, T.; Scott, T.; Gilpin, C. Epidemiology of hepatitis C virus: A study of male blood donors in Saudi Arabia. Transfusion 1994, 34, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Ai-Faleh, F.Z.; Ai-Jeffri, M.; Ramia, S.; Ai-Rashed, R.; Arif, M.; Rezeig, M.; Ai-Toraif, I.; Bakhsh, M.; Mishkkhas, A.; Makki, O.; et al. Seroepidemiology of hepatitis B virus infection in Saudi children 8 years after a mass hepatitis B vaccination programme. J. Infect. 1999, 38, 167–170. [Google Scholar] [CrossRef]
- Ministry of Health, Kingdom of Saudi Arabia. Guidelines on Blood Donation in Ramadan. Available online: https://www.moh.gov.sa/en/awarenessplateform/SeasonalAndFestivalHealth/Educational-Content/Pages/Blood-donation.aspx (accessed on 12 September 2022).
- KSA MOH Statistics Between 2007 and 2016; Ministry of Health: Riyadh, Saudi Arabia. 2017. Available online: https://www.moh.gov.sa/en/Ministry/Statistics/book/Pages/default.aspx (accessed on 12 September 2022).
- Badur, S.; Öztürk, S.; Ozakay, A.; Khalaf, M.; Saha, D.; Van Damme, P. A review of the experience of childhood hepatitis A vaccination in Saudi Arabia and Turkey: Implications for hepatitis A control and prevention in the Middle East and North African region. Hum. Vaccines Immunother. 2021, 17, 3710–3728. [Google Scholar] [CrossRef]
- Sanai, F.M.; Alghamdi, M.; Dugan, E.; Alalwan, A.; Al-Hamoudi, W.; Abaalkhail, F.; AlMasri, N.; Razavi-Shearer, D.; Razavi, H.; Schmelzer, J.; et al. A tool to measure the economic impact of hepatitis B elimination: A case study in Saudi Arabia. J. Infect. Public Health 2023, 13, 1715–1723. [Google Scholar] [CrossRef]
- Wedhaya, M.A.; Kurban, M.A.; Abyadh, D.A.; Alshamrani, A.S.R.; Alzahrani, G.S.; Alhabi, H.A.; Alrehaily, S.S.; Alqahtani, A.M.; Mansi, M.H.; Mofareh, A.S. Assessment of knowledge, attitude and practice towards Hepatitis B among healthy population in Saudi Arabia. Egypt. J. Hosp. Med. 2017, 69, 1973–1977. [Google Scholar] [CrossRef]
- Elbur, A. knowledge, attitude and practice on Hepatitis B: A survey among the internet users in Taif, Kingdom of Saudi Arabia. J. Infect. Dis. Epidemiol. 2017, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Abukaram, T.M.; Alwan, M.; Alanazi, A.K.; Habra, S.M.; Almalik, A.M.; Alanazi, S.S.; Alali, N.M.; Alnowaisser, L.; Alotaibi, R.; Farooqi, W.A. Awareness of Hepatitis B Among the General Population in Riyadh, Saudi Arabia. Cureus 2025, 17, e77437. [Google Scholar] [CrossRef] [PubMed]
- Agwa, R.; Elwan, T.H.; Alghamdi, H.A.; Alghamdi, A.A.; Altaweel, F.I.; Alghamdi, A.A.; Alhussain, H.A.; Alsawlihah, K.M.; Alzahrani, F.A. Awareness of Hepatitis B Virus (HBV) Screening Before Marriage and Pregnancy Among Adults in the Al-Baha Region, Saudi Arabia. Cureus 2024, 16, e52057. [Google Scholar] [CrossRef] [PubMed]
- Barqawi, H.J.; Samara, K.A.; Al Ansari, M.M.; Al Moukdad, A.M.; Obied, S.H.; Abu-Gharbieh, E. Hepatitis B knowledge and stigma in the United Arab Emirates. Sci. Rep. 2025, 15, 10412. [Google Scholar] [CrossRef]
- Dai, S.; Wang, Z.; Guo, Q.; Tang, G.; Guo, Q.; Zhang, J.; Fan, Y. Awareness of hepatitis C prevention and treatment and high-risk behaviors among the general population in Anhui Province: A cross-sectional study. Front. Public Health 2025, 13, 1534169. [Google Scholar] [CrossRef]
- Ha, S.; Timmerman, K. Awareness and knowledge of hepatitis C among health care providers and the public: A scoping review. Can. Commun. Dis. Rep. 2018, 44, 157. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Sperle, I.; Matii, M.; Wiessing, L. A systematic review of Hepatitis C virus treatment uptake among people who inject drugs in the European Region. BMC Infect. Dis. 2014, 14, S16. [Google Scholar] [CrossRef]
- Adjei, C.; Asamoah, R.; Atibila, F.; Ti-Enkawol, G.N.; Ansah-Nyarko, M. Mother-to-child transmission of hepatitis B: Extent of knowledge of physicians and midwives in Eastern region of Ghana. BMC Public Health 2016, 16, 615. [Google Scholar] [CrossRef]
- Bytyi, A.; Rashiti, P.; Kadiri, F.; Svara, L.; Xani, A. Knowledge of Hepatitis C among General Population in Kosovo. Bangladesh J. Med. Sci. 2022, 21, 1009–1017. [Google Scholar] [CrossRef]
- Jorgensen, C.; Lewis, C.; Liu, J. An Analysis of Hepatitis C Virus-Related Public Inquiries from Health Professionals: 2009–2010. Clin. Infect. Dis. 2012, 54, 1032–1039. [Google Scholar] [CrossRef]
- Korkmaz, P.; Kara, E.; Çelik, H.; Öztürk, F.; Yalçınkaya, F. Awareness of Hepatitis B Virus Reactivation Among Physicians Administering Immunosuppressive Treatment and Related Clinical Practices. Klimik Derg./Klimik J. 2019, 32, 101–108. [Google Scholar] [CrossRef]
- Patel, A.A.; Evon, D.M.; McGowan, C.E.; Sulkowski, M.S.; Spradling, P.R. Primary Care Teams in Veterans Health Administration Have High Knowledge of Universal HCV Screening But Favor System-Level Interventions to Close Care Gaps. Open Forum Infect. Dis. 2022, 9, ofac492.1078. [Google Scholar] [CrossRef]
- Al-Abd, N.; Al-Rawahi, A.; Al-Jabri, R.; Al-Mahdi, H. Prevalence and Knowledge, Attitude, and Practices of Hepatitis B Virus Among Dental Students at the University of Science and Technology, Aden, Yemen. Yemeni J. Med. Sci. 2025, 19, 2902. [Google Scholar] [CrossRef]
- Nimbvikar, A.A.; Gandhi, J. Knowledge, Attitude and Practices About Hepatitis B in Paramedical and Supporting Staff at a Tertiary Care Hospital: A Cross Sectional Study. Int. J. Community Med. Public Health 2022, 9, 3562–3568. [Google Scholar] [CrossRef]
- Almalki, F.; Alraffah, Y.M.; Alasiri, R.A.; Dhafar, M.; Albogami, F.; Alhazmi, M.; Alyazidi, A.; Alharbi, L.; Alotaibi, M. Knowledge, Attitude and Practice Towards Hepatitis B Infection and HBV Vaccine Among the Healthy Population in Makkah, Saudi Arabia. Infect. Drug Resist. 2025, 18, 2153–2164. [Google Scholar] [CrossRef]
- Nair, K.S.; Mughal, Y.H.; Albejaidi, F.; Alharbi, A.H. Healthcare Financing in Saudi Arabia: A Comprehensive Review. Healthcare 2024, 12, 2544. [Google Scholar] [CrossRef] [PubMed]
- Kushner, T.; Chappell, C.A.; Kim, A.Y. Testing for Hepatitis C in Pregnancy: The Time has Come for Routine Rather than Risk-based. Curr. Hepatol. Rep. 2019, 18, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.; Kamel, S.; Waked, I.; Fort, M. Egypt’s Ambitious Strategy to Eliminate Hepatitis C Virus: A Case Study. Glob. Health Sci. Pract. 2021, 9, 187–200. [Google Scholar] [CrossRef]
| Virus | Transmission | Regional Trends | Risk Factors | High-Risk Groups | Treatment | Prevention | References |
|---|---|---|---|---|---|---|---|
| HAV | Fecal–oral | Intermediate endemicity; higher in less developed regions | Contaminated water/food, poor sanitation, and travel | Children, adolescents, and travelers | Supportive care: oral/IV rehydration, liver function monitoring | HAV vaccine; school-based campaigns | [1,2] |
| HBV | Blood-borne, vertical, and sexual | Declining nationally; regional hotspots (Qunfudah, Jeddah) | Blood transfusions, unsafe injections, and vertical transmission | Neonates of HBV-positive mothers, healthcare workers, and migrants | Antivirals: tenofovir, entecavir; monitoring inactive carriers | Universal infant vaccination; prenatal and premarital screening | [3,4,5,6,7,9,10] |
| HCV | Blood-borne, IVDU, and unsafe procedures | Regional variation; Jeddah highest burden | Unsafe injections, IVDU, and hemodialysis | Adults ≥ 45, males, PWID, and migrants | DAAs: Glecaprevir/Pibrentasvir, Sofosbuvir/Velpatasvir; >95% SVR | No vaccine; national screening and micro-elimination programs | [1,8,11] |
| HDV | Requires HBV co-infection | Limited data; underdiagnosed | HBV co-infection, blood/body fluids, and sexual contact | Chronic HBV carriers, migrants from endemic areas | PEG-IFNα (29% SVR), Bulevirtide (71–76% efficacy) | Indirect via HBV vaccination; HDV testing for HBV patients | [3,7] |
| HEV | Fecal–oral | Outbreaks during mass gatherings; fluctuating prevalence | Contaminated water, poor sanitation, and mass gatherings | Pregnant women, immunocompromised | Supportive care; ribavirin for chronic cases (78–83% SVR) | No widespread vaccine; hygiene, water sanitation | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryani, M.A. Hepatitis Management in Saudi Arabia: Trends, Prevention, and Key Interventions (2016–2025). Medicina 2025, 61, 1509. https://doi.org/10.3390/medicina61091509
Ryani MA. Hepatitis Management in Saudi Arabia: Trends, Prevention, and Key Interventions (2016–2025). Medicina. 2025; 61(9):1509. https://doi.org/10.3390/medicina61091509
Chicago/Turabian StyleRyani, Majed A. 2025. "Hepatitis Management in Saudi Arabia: Trends, Prevention, and Key Interventions (2016–2025)" Medicina 61, no. 9: 1509. https://doi.org/10.3390/medicina61091509
APA StyleRyani, M. A. (2025). Hepatitis Management in Saudi Arabia: Trends, Prevention, and Key Interventions (2016–2025). Medicina, 61(9), 1509. https://doi.org/10.3390/medicina61091509






