Efficacy and Safety of First-Line Nivolumab Plus Ipilimumab in Patients with Postoperative Recurrent and Inoperable Non-Small Cell Lung Cancer: A Real-World Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Efficacy and Evaluation of AEs
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Treatment Response and Survival
3.3. Prognostic Factors
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| AL | Anaplastic lymphoma |
| BMI | Body mass index |
| CI | Confidence interval |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| EGFR | Epidermal growth factor receptor |
| ECOG-PS | Eastern Cooperative Oncology Group performance status |
| ICI | Immune checkpoint inhibitor |
| HR | Hazard ratio |
| NCCN | National Comprehensive Cancer Network |
| NE | Not evaluable |
| NSCLC | Non-small cell lung cancer |
| Nivo-Ipi | Nivolumab plus ipilimumab |
| OS | Overall survival |
| ORR | Objective response rate |
| PD | Progressive disease |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| PR | Partial response |
| RFS | Relapse-free survival |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SD | Stable disease |
| TNM | Tumor–node–metastasis |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Fossati, R.; Torri, V.; Crinò, L.; Giaccone, G.; Silvano, G.; Martelli, M.; Clerici, M.; Cognetti, F.; Tonato, M.; et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J. Natl. Cancer Inst. 2003, 95, 1453–1461. [Google Scholar] [CrossRef]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.P.; Vansteenkiste, J.; International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, H.; Nichols, F.C.; Yang, P.; Allen, M.S.; Cassivi, S.D.; Deschamps, C.; Williams, B.A.; Pairolero, P.C. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann. Thorac. Surg. 2007, 83, 409–417, discussion 417. [Google Scholar] [CrossRef]
- Saisho, S.; Yasuda, K.; Maeda, A.; Yukawa, T.; Okita, R.; Hirami, Y.; Shimizu, K.; Nakata, M. Post-recurrence survival of patients with non-small-cell lung cancer after curative resection with or without induction/adjuvant chemotherapy. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 166–172. [Google Scholar] [CrossRef]
- Imai, H.; Onozato, R.; Kaira, K.; Kawashima, S.; Masubuchi, K.; Tajima, K.; Minato, K. Post-progression survival highly influences overall survival in driver gene mutation/translocation negative or unknown type of non-small cell lung cancer. Oncology 2022, 100, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014, 2, 846–856. [Google Scholar] [CrossRef]
- Das, R.; Verma, R.; Sznol, M.; Boddupalli, C.S.; Gettinger, S.N.; Kluger, H.; Callahan, M.K.; Wolchok, J.D.; Halaban, R.; Dhodapkar, M.V.; et al. Combination Therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J. Immunol. 2015, 194, 950–959. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 2020, 20, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.F.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Scherpereel, A.; Cornelissen, R.; Oulkhouir, Y.; Greillier, L.; Kaplan, M.A.; Talbot, T.; Monnet, I.; Hiret, S.; Baas, P.; et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann. Oncol. 2022, 33, 488–499. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lee, J.S.; Ciuleanu, T.E.; Bernabe Caro, R.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Roach, C.; Zhang, N.; Corigliano, E.; Jansson, M.; Toland, G.; Ponto, G.; Dolled-Filhart, M.; Emancipator, K.; Stanforth, D.; Kulangara, K. Development of a Companion Diagnostic PD-L1 immunohistochemistry Assay for pembrolizumab therapy in non-small-cell lung cancer. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 392–397. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN) Guidelines. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (accessed on 25 April 2025).
- Hoang, T.; Xu, R.; Schiller, J.H.; Bonomi, P.; Johnson, D.H. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on Eastern Cooperative Oncology Group data. J. Clin. Oncol. 2005, 23, 175–183. [Google Scholar] [CrossRef]
- Yuasa, I.; Hamaji, M.; Ozasa, H.; Sakamori, Y.; Yoshida, H.; Yutaka, Y.; Menju, T.; Hirai, T.; Date, H. Outcomes of immune checkpoint inhibitors for postoperative recurrence of non-small cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Motono, N.; Mizoguchi, T.; Ishikawa, M.; Iwai, S.; Iijima, Y.; Uramoto, H. Relative efficacies of epidermal growth factor receptor-tyrosine kinase inhibitors and immune checkpoint inhibitors for treatment of recurrent non-small cell lung cancer after surgery. Oncology 2024, 102, 476–483. [Google Scholar] [CrossRef]
- Turner, N.C.; Reis-Filho, J.S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012, 13, e178–e185. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Liao, S.; Penney, B.C.; Wroblewski, K.; Zhang, H.; Simon, C.A.; Kampalath, R.; Shih, M.C.; Shimada, N.; Chen, S.; Salgia, R.; et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, K.; Matsuzawa, Y.; Kotani, K.; Keno, Y.; Kobatake, T.; Fujioka, S.; Tarui, S. Ideal body weight estimated from the body mass index with the lowest morbidity. Int. J. Obes. 1991, 15, 1–5. [Google Scholar]
- Singh, S.; Loke, Y.K. Drug safety assessment in clinical trials: Methodological challenges and opportunities. Trials 2012, 13, 138. [Google Scholar] [CrossRef]
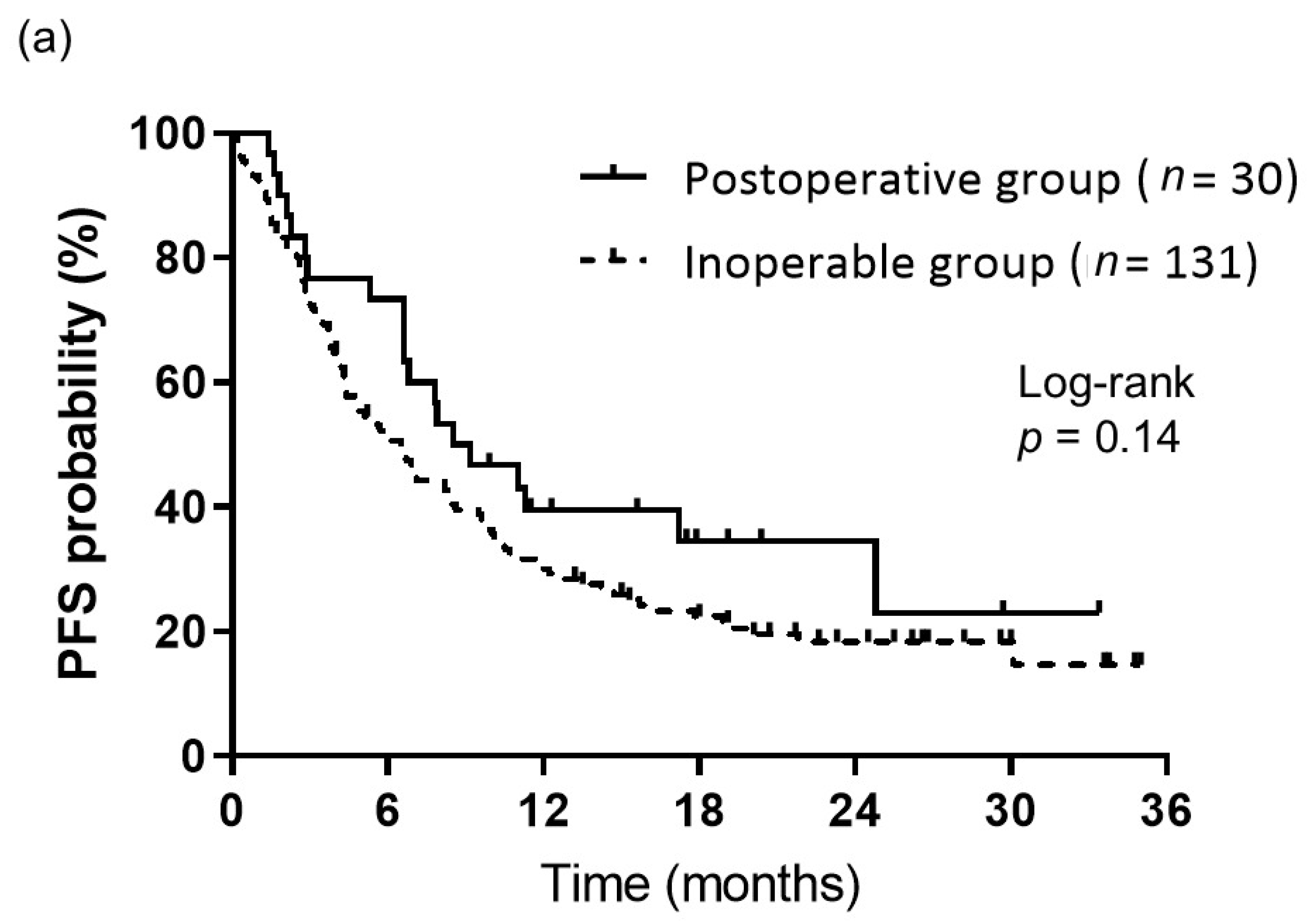
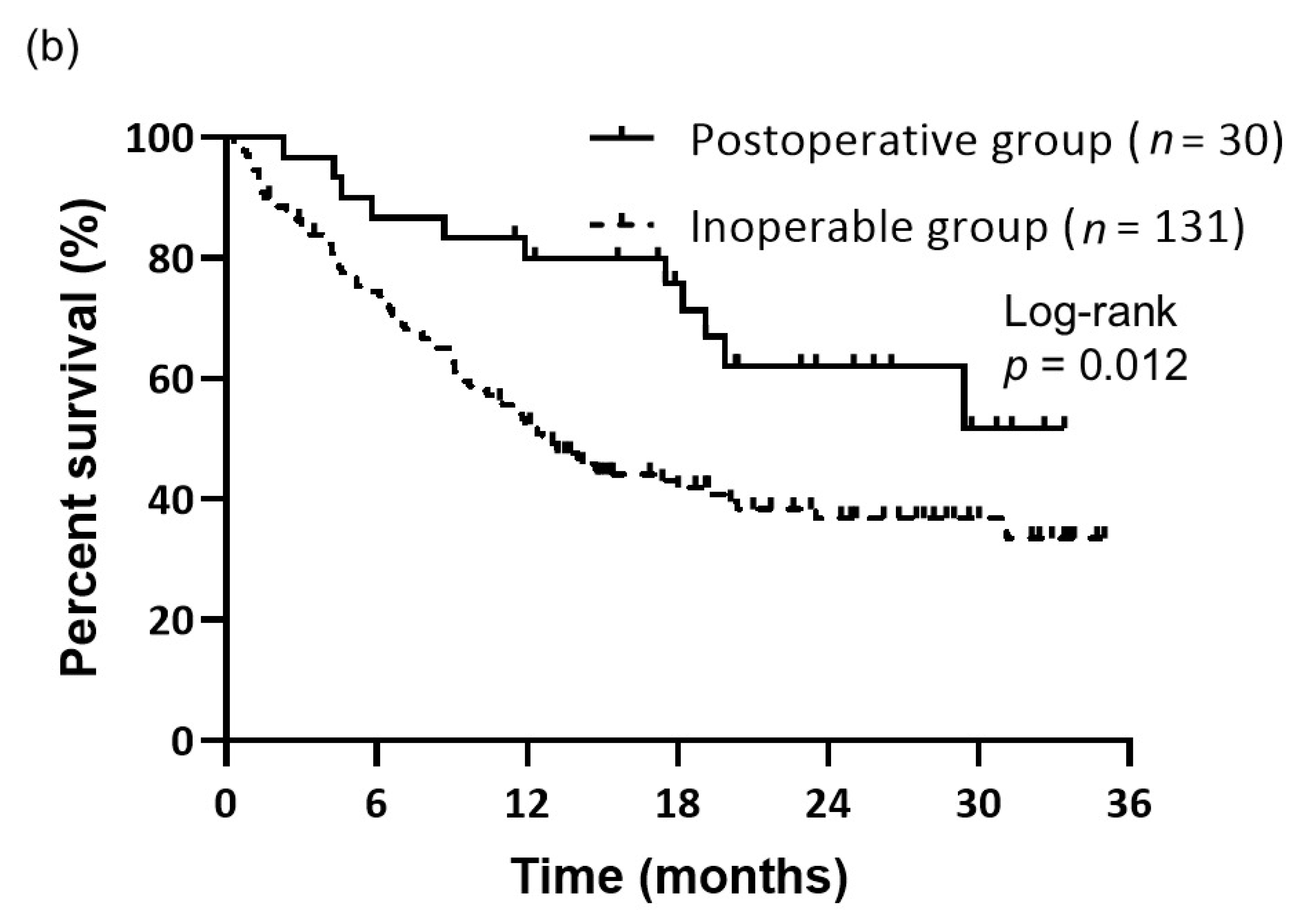
| Characteristics | Total (n = 161) (%) | Postoperative Group (n = 30) (%) | Inoperable Group (n = 131) (%) | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 128 (79.5) | 25 (83.3) | 103 (78.7) | 0.63 |
| Female | 33 (20.5) | 5 (16.7) | 28 (21.3) | |
| Median age at treatment (years) [range] | 72 (46–85) | 74 (46–82) | 72 (46–85) | 0.20 * |
| Performance status (PS) | ||||
| 0 | 51 (33.2) | 20 (66.7) | 31 (23.7) | 0.003 |
| 1 | 81 (50.5) | 10 (33.3) | 71 (54.2) | |
| ≥2 | 29 (16.3) | 0 | 29 (22.1) | |
| Smoking history | ||||
| Yes | 143 (88.8) | 26 (86.7) | 117 (89.3) | 0.75 |
| No | 18 (11.2) | 4 (13.3) | 14 (10.7) | |
| Clinical stage at diagnosis | ||||
| I | 13 (8.1) | 13 (43.3) | - | - |
| II | 5 (3.1) | 5 (16.7) | - | |
| III | 23 (14.3) | 12 (40.0) | 11 (8.4) | |
| IV | 120 (74.5) | - | 120 (91.6) | |
| Histology | ||||
| Adenocarcinoma | 95 (59.0) | 20 (66.7) | 75 (57.3) | 0.11 |
| Squamous cell carcinoma | 45 (28.0) | 10 (33.3) | 35 (26.7) | |
| Carcinoma | 6 (3.7) | 0 | 6 (4.6) | |
| Not otherwise specified | 16 (9.9) | 0 | 16 (12.2) | |
| PD-L1 TPS | ||||
| <1 | 76 (47.2) | 12 (40) | 64 (48.9) | 0.57 |
| 1–49 | 61 (37.9) | 15 (50) | 46 (35.1) | |
| ≥50 | 15 (9.3) | 2 (6.7) | 13 (9.9) | |
| Unknown | 9 (5.6) | 1 (3.3) | 8 (6.1) | |
| Metastatic site | ||||
| Brain | 24 (14.9) | 7 (23.3) | 17 (13.0) | 0.16 |
| Liver | 19 (11.8) | 2 (6.7) | 17 (13.0) | 0.53 |
| Bone | 53 (32.9) | 5 (16.7) | 48 (36.6) | 0.05 |
| Body fluids (pleural effusion or ascites) | 45 (28.0) | 4 (13.3) | 41 (31.3) | 0.07 |
| BMI (kg/m2) | ||||
| Median [range] | 22.0 (13.4–42.0) | 23.4 (18.6–42.0) | 21.6 (13.4–30.5) | 0.001 * |
| Number of cycles nivolumab plus ipilimumab administered | ||||
| Median (range) | 2 (1–20) | 5 (1–13) | 2 (1–20) | 0.0088 * |
| Continuing administration of nivolumab plus ipilimumab at data cutoff | ||||
| Yes | 26 | 6 | 20 | 0.58 |
| No | 135 | 24 | 111 | |
| Survival status at data cut-off | ||||
| Death | 89 | 11 | 78 | 0.0264 |
| Alive | 72 | 19 | 53 |
| Total (n = 161) | Postoperative Group (n = 30) | Inoperable Group (n = 131) | p-Value # | |
|---|---|---|---|---|
| Response | ||||
| Complete response | 1 | 1 | 0 | |
| Partial response | 52 | 10 | 42 | |
| Stable disease | 55 | 11 | 44 | |
| Progressive disease | 45 | 7 | 38 | |
| Not evaluated | 8 | 1 | 7 | |
| Response rate (%) (95% CI) | 32.9 (26.1–40.5) | 36.7 (21.8–54.5) | 32.1 (24.6–40.4) | 0.67 |
| Disease control rate (%) (95% CI) | 67.1 (59.5–73.9) | 73.3 (55.3–86.0) | 65.7 (57.2–73.2) | 0.52 |
| Variables | Median PFS | Univariate Analysis | Multivariate Analysis | Median OS | Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Months) | HR | 95% CI | p-Value | HR | 95% CI | p-Value | (Months) | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex | ||||||||||||||
| Male (n = 25)/female (n = 5) | 7.9/17.2 | 1.54 | 0.52–4.49 | 0.48 | 29.4/26.2 | 2.12 | 0.44–10.05 | 0.49 | ||||||
| Age at the start of Nivo-Ipi (years) | ||||||||||||||
| <75 (n = 17)/≥75 (n = 13) | 7.9/17.2 | 1.2 | 0.49–2.91 | 0.67 | NR/NR | 0.59 | 0.16–2.07 | 0.35 | ||||||
| Histology | ||||||||||||||
| Adenocarcinoma (n = 20)/non-adenocarcinoma (n = 10) | 8.5/9.9 | 1.1 | 0.43–2.82 | 0.83 | NR/NR | 0.75 | 0.20–2.74 | 0.65 | ||||||
| Smoking history | ||||||||||||||
| Yes (n = 26)/no (n = 4) | 8.2/17.2 | 1.83 | 0.56–5.89 | 0.70 | 29.4/NR | - | −1.0–1.0 | 0.19 | ||||||
| PD-L1 TPS (%) | ||||||||||||||
| <1% or unknown (n = 13)/≥1% (n = 17) | NR/7.9 | 0.57 | 0.23–1.39 | 0.23 | 0.45 | 0.15–1.20 | 0.11 | 29.4/NR | 1.62 | 0.46–5.66 | 0.41 | 2.06 | 0.56–7.31 | 0.26 |
| Postoperative adjuvant chemotherapy | ||||||||||||||
| Yes (n = 12)/no (n = 18) | 19.4/8.1 | 0.57 | 0.23–1.38 | 0.23 | NR/29.4 | 0.63 | 0.18–2.14 | 0.49 | ||||||
| Intracranial metastases at the start of Nivo-Ipi | ||||||||||||||
| Yes (n = 6)/no (n = 24) | 8.5/9.9 | 1.48 | 0.47–4.60 | 0.42 | NR/29.4 | 0.71 | 0.17–2.88 | 0.66 | ||||||
| Liver metastases at the start of Nivo-Ipi | ||||||||||||||
| Yes (n = 2)/no (n = 28) | 19.2/NR | 1.25 | 0.13–12.12 | 0.82 | 19.2/NR | 1.28 | 0.13–12.58 | 0.81 | ||||||
| Bone metastases at the start of Nivo-Ipi | ||||||||||||||
| Yes (n = 5)/no (n = 25) | 6.8/9.2 | 1.39 | 0.41–4.71 | 0.54 | NR/NR | 1.51 | 0.25–8.90 | 0.58 | ||||||
| Body fluids (pleural effusion or ascites) at the start of Nivo-Ipi | ||||||||||||||
| Yes (n = 3)/no (n = 27) | 5.3/9.2 | 1.12 | 0.24–5.19 | 0.87 | 19.1/NR | 2.02 | 0.27–14.60 | 0.35 | ||||||
| Relapse-free survival | ||||||||||||||
| <12 months (n = 10)/≥12 months (n = 20) | 2.85/11.0 | 1.93 | 0.66–5.60 | 0.14 | 1.94 | 0.68–5.25 | 0.20 | 17.5/NR | 2.67 | 0.66–10.73 | 0.08 | 2.3 | 0.63–8.12 | 0.19 |
| BMI, kg/m2 | ||||||||||||||
| <22 (n = 9)/≥22 (n = 21) | NR/6.6 | 0.23 | 0.09–0.56 | 0.0091 | 0.24 | 0.05–0.75 | 0.01 | NR/29.4 | 0.17 | 0.05–0.59 | 0.05 | 0.18 | 0.009–1.01 | 0.05 |
| All Patients (n = 161) | Postoperative Group (n = 30) | Inoperable Group (n = 131) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Event | Any Grade | % | Grade ≥ 3 | % | Any Grade | % | Grade ≥ 3 | % | Any Grade | % | Grade ≥ 3 | % | p-Value # |
| Resulted in discontinuation | 40 | 24.8 | 26 | 16.1 | 7 | 23.3 | 4 | 13.3 | 33 | 25.2 | 22 | 16.8 | >0.99 |
| Resulted in death | - | - | 1 | 0.6 | - | - | 0 | 0 | - | - | 1 | 0.8 | >0.99 |
| Treatment-related | |||||||||||||
| Skin rash | 61 | 37.9 | 5 | 3.1 | 14 | 45.2 | 1 | 3.3 | 47 | 35.9 | 4 | 3.1 | 0.30 |
| Liver dysfunction | 48 | 29.8 | 15 | 9.3 | 11 | 36.7 | 3 | 10.0 | 37 | 28.2 | 12 | 9.2 | 0.38 |
| Thyroid dysfunction | 35 | 21.7 | 0 | 0 | 10 | 33.3 | 0 | 0 | 25 | 19.1 | 0 | 0 | 0.14 |
| Lung injury | 32 | 19.9 | 18 | 11.2 | 8 | 26.7 | 4 | 13.3 | 24 | 18.3 | 14 | 10.7 | 0.35 |
| Adrenal insufficiency | 31 | 19.3 | 16 | 9.9 | 6 | 20.0 | 2 | 6.7 | 25 | 19.1 | 14 | 10.7 | >0.99 |
| Elevated creatinine levels | 27 | 16.8 | 0 | 0 | 6 | 20.0 | 0 | 0 | 21 | 16.0 | 0 | 0 | 0.59 |
| Colitis | 27 | 16.8 | 11 | 6.8 | 6 | 20.0 | 2 | 6.7 | 21 | 16.0 | 9 | 6.9 | 0.59 |
| Fever | 14 | 8.7 | 1 | 0.6 | 2 | 6.7 | 0 | 0 | 12 | 9.2 | 1 | 0.8 | >0.99 |
| Myositis | 12 | 7.5 | 3 | 1.9 | 3 | 10.0 | 1 | 3.3 | 9 | 6.9 | 2 | 1.5 | 0.70 |
| Arthritis | 8 | 4.3 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 6.1 | 0 | 0 | 0.35 |
| Type 1 diabetes | 2 | 1.3 | 1 | 0.6 | 1 | 3.3 | 0 | 0 | 1 | 0.8 | 1 | 0.8 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurata, Y.; Mouri, A.; Imai, H.; Endo, S.; Tsukamoto, K.; Masaki, K.; Hashimoto, K.; Miura, Y.; Shiono, A.; Yamaguchi, O.; et al. Efficacy and Safety of First-Line Nivolumab Plus Ipilimumab in Patients with Postoperative Recurrent and Inoperable Non-Small Cell Lung Cancer: A Real-World Retrospective Observational Study. Medicina 2025, 61, 994. https://doi.org/10.3390/medicina61060994
Kurata Y, Mouri A, Imai H, Endo S, Tsukamoto K, Masaki K, Hashimoto K, Miura Y, Shiono A, Yamaguchi O, et al. Efficacy and Safety of First-Line Nivolumab Plus Ipilimumab in Patients with Postoperative Recurrent and Inoperable Non-Small Cell Lung Cancer: A Real-World Retrospective Observational Study. Medicina. 2025; 61(6):994. https://doi.org/10.3390/medicina61060994
Chicago/Turabian StyleKurata, Yuhei, Atsuto Mouri, Hisao Imai, Satoshi Endo, Kasumi Tsukamoto, Kenji Masaki, Kosuke Hashimoto, Yu Miura, Ayako Shiono, Ou Yamaguchi, and et al. 2025. "Efficacy and Safety of First-Line Nivolumab Plus Ipilimumab in Patients with Postoperative Recurrent and Inoperable Non-Small Cell Lung Cancer: A Real-World Retrospective Observational Study" Medicina 61, no. 6: 994. https://doi.org/10.3390/medicina61060994
APA StyleKurata, Y., Mouri, A., Imai, H., Endo, S., Tsukamoto, K., Masaki, K., Hashimoto, K., Miura, Y., Shiono, A., Yamaguchi, O., Nakagawa, J., Kaira, K., Kobayashi, K., & Kagamu, H. (2025). Efficacy and Safety of First-Line Nivolumab Plus Ipilimumab in Patients with Postoperative Recurrent and Inoperable Non-Small Cell Lung Cancer: A Real-World Retrospective Observational Study. Medicina, 61(6), 994. https://doi.org/10.3390/medicina61060994







