Serum Endocan as a Predictor of Survival and Cardiovascular Events in Patients Without Diabetic Kidney Disease on Chronic Haemodialysis: A Prospective, Observational Study
Abstract
1. Introduction
2. Materials and Methods
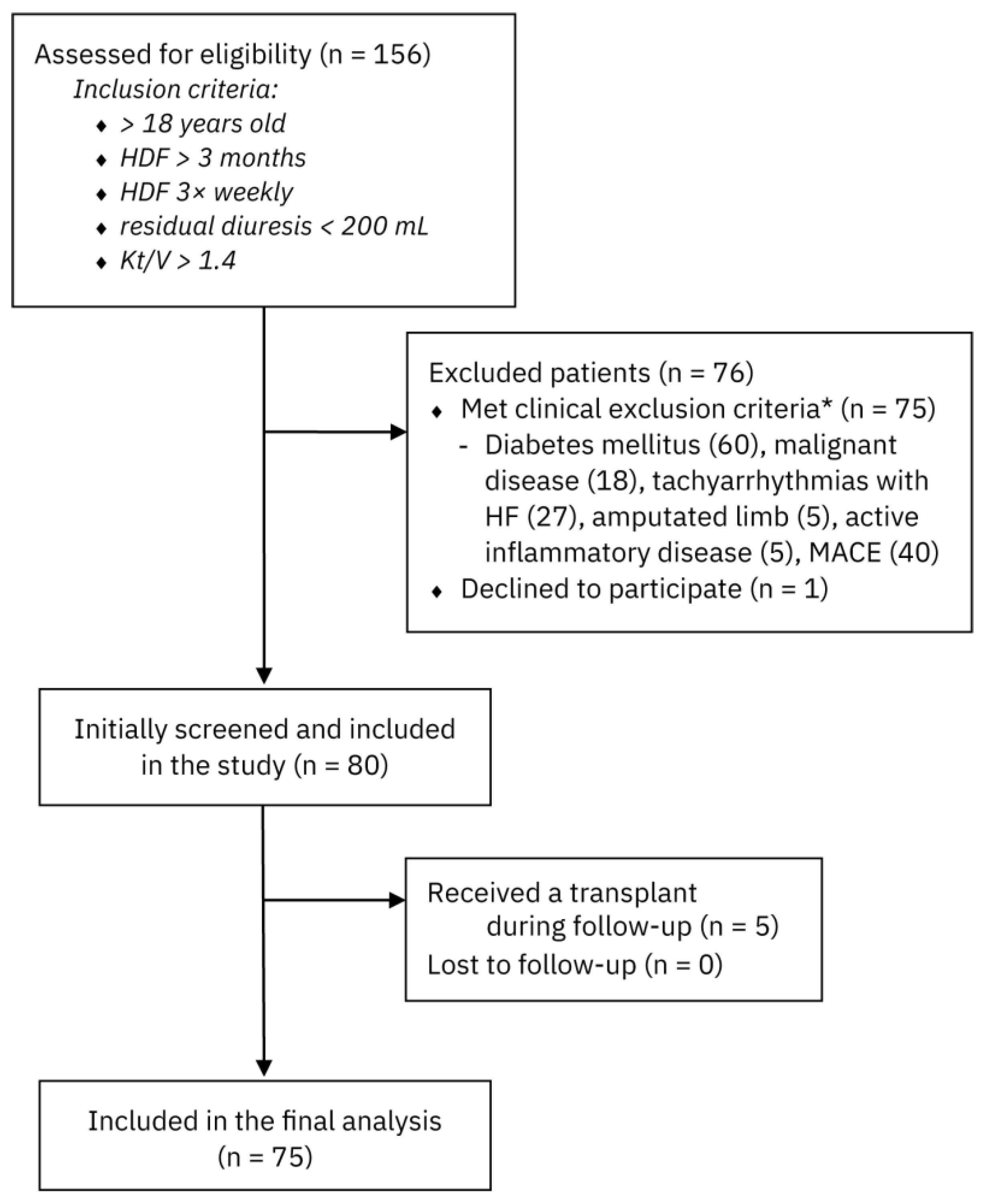
2.1. Study Population and Design
2.2. Demographic Data, Anthropometric Analyses, and Biochemical Investigations
2.3. Assessments of Peripheral and Central Haemodynamic Parameters
2.4. Statistical Analysis
3. Results
3.1. Demographic, Clinical, and Biochemical Characteristics
3.2. Parameters of Peripheral and Central Haemodynamics
3.3. Outcomes and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| HD | haemodialysis |
| DKD | diabetes mellitus |
| MACE | major adverse cardiovascular event |
| AVF | arteriovenous fistulas |
| TCVC | tunnelled central venous catheter |
| ESKD | end-stage kidney disease |
| FMD | flow-mediated dilation |
| IgA | immunoglobulin A |
| PD | peritoneal dialysis |
| sICAM-1 | intercellular adhesion molecule-1 |
| sVCAM-1 | vascular adhesion molecule-1 |
| hs-CRP | high-sensitivity CRP |
| CIMT | carotid intima–media thickness |
| PWV | pulse wave velocity |
| AI | augmentation index |
| MAP | mean arterial pressure |
| PP | pulse pressure |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| HR | heart rate |
| PTH | parathyroid hormone |
| BCM | bioimpedance method |
| ELISA | enzyme-linked immunosorbent assay |
| RAAS | renin angiotensin aldosterone system |
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.C. Cardiovascular disease in patients with chronic renal failure. Lancet 1996, 348, 1673–1674. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J. Cardiovascular Mortality in End-Stage Renal Disease. Am. J. Med. Sci. 2003, 325, 163–167. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health; US Department of Health and Human Services. United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health: Bethesda, MD, USA, 2020. [Google Scholar]
- Makar, M.S.; Pun, P.H. Sudden Cardiac Death Among Hemodialysis Patients. Am. J. Kidney Dis. 2017, 69, 684–695. [Google Scholar] [CrossRef]
- De Bie, M.K.; Van Dam, B.; Gaasbeek, A.; Van Buren, M.; Van Erven, L.; Bax, J.J.; Schalij, M.J.; Rabelink, T.J.; Jukema, J.W. The current status of interventions aiming at reducing sudden cardiac death in dialysis patients. Eur. Heart J. 2009, 30, 1559–1564. [Google Scholar] [CrossRef]
- Herzog, C.A. Cardiac arrest in dialysis patients: Approaches to alter an abysmal outcome. Kidney Int. 2003, 63, S197–S200. [Google Scholar] [CrossRef]
- Tri Tai Truyen, T.T.; Uy-Evanado, A.; Holmstrom, L.; Reinier, K.; Chugh, H.; Jui, J.; Herzog, C.A.; Chugh, S.S. Sudden Cardiac Arrest Associated with Hemodialysis: A Community-Based Study. Kidney360 [Internet]. 2025 January 17. Available online: https://journals.lww.com/10.34067/KID.0000000705 (accessed on 11 May 2025).
- Umanath, K.; Lewis, J.B. Update on Diabetic Nephropathy: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 884–895. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease: A Statement From the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar]
- Bessa, J.; Albino-Teixeira, A.; Reina-Couto, M.; Sousa, T. Endocan: A novel biomarker for risk stratification, prognosis and therapeutic monitoring in human cardiovascular and renal diseases. Clin. Chim. Acta 2020, 509, 310–335. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Adam, E.; Lyon, M.; Depontieu, F.; Motte, V.; Landolfi, C.; Lortat-Jacob, H.; Bechard, D.; Lassalle, P.; Delehedde, M. Endocan or endothelial cell specific molecule-1 (ESM-1): A potential novel endothelial cell marker and a new target for cancer therapy. Biochim. Biophys. Acta BBA—Rev. Cancer 2006, 1765, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Takir, M.; Kostek, O.; Covic, A.; Kanbay, M. Endocan: A New Molecule Playing a Role in the Development of Hypertension and Chronic Kidney Disease? J. Clin. Hypertens. 2014, 16, 914–916. [Google Scholar] [CrossRef]
- Samouilidou, E.; Bountou, E.; Papandroulaki, F.; Papamanolis, M.; Papakostas, D.; Grapsa, E. Serum Endocan Levels are Associated With Paraoxonase 1 Concentration in Patients With Chronic Kidney Disease. Ther. Apher. Dial. 2018, 22, 325–331. [Google Scholar] [CrossRef]
- Poon, P.Y.K.; Ng, J.K.C.; Fung, W.W.S.; Chow, K.M.; Kwan, B.C.H.; Li, P.K.T.; Szeto, C.-C. Relationship between Plasma Endocan Level and Clinical Outcome of Chinese Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2019, 44, 1259–1270. [Google Scholar] [CrossRef]
- Balta, S.; Mikhailidis, D.P.; Demirkol, S.; Ozturk, C.; Celik, T.; Iyisoy, A. Endocan: A novel inflammatory indicator in cardiovascular disease? Atherosclerosis 2015, 243, 339–343. [Google Scholar] [CrossRef]
- Malyszko, J.; Koc-Żórawska, E.; Malyszko, J.S. Endocan Concentration in Kidney Transplant Recipients. Transplant. Proc. 2018, 50, 1798–1801. [Google Scholar] [CrossRef]
- Khalaji, A.; Behnoush, A.H.; Mohtasham Kia, Y.; Alehossein, P.; Bahiraie, P. High circulating endocan in chronic kidney disease? A systematic review and meta-analysis. Battaglia Y, editor. PLoS ONE 2023, 18, e0289710. [Google Scholar] [CrossRef]
- Samouilidou, E.; Athanasiadou, V.; Grapsa, E. Prognostic and Diagnostic Value of Endocan in Kidney Diseases. Int. J. Nephrol. 2022, 2022, 3861092. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Unal, H.U.; Eyileten, T.; Gok, M.; Cetinkaya, H.; Oguz, Y.; Sari, S.; et al. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014, 86, 1213–1220. [Google Scholar] [CrossRef]
- Kim, J.S.; Ko, G.J.; Kim, Y.G.; Lee, S.Y.; Lee, D.Y.; Jeong, K.H.; Lee, S.H. Plasma Endocan as a Predictor of Cardiovascular Event in Patients with End-Stage Renal Disease on Hemodialysis. J. Clin. Med. 2020, 9, 4086. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Hsu, B.G.; Wang, C.H.; Tsai, J.P. Endocan as a Potential Marker for Predicting All-Cause Mortality in Hemodialysis Patients. J. Clin. Med. 2023, 12, 7427. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Lin, Y.L.; Hsu, B.G. Serum Endocan Is a Risk Factor for Aortic Stiffness in Patients Undergoing Maintenance Hemodialysis. Medicina 2024, 60, 984. [Google Scholar] [CrossRef]
- Malyszko, J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin. Chim. Acta 2010, 411, 1412–1420. [Google Scholar] [CrossRef]
- Qiu, C.R.; Fu, Q.; Sui, J.; Zhang, Q.; Wei, P.; Wu, Y.; Zhu, K.; Lu, Y.; Zong, B. Serum Endothelial Cell–Specific Molecule 1 (Endocan) Levels in Patients With Acute Myocardial Infarction and Its Clinical Significance: A Pilot Study. Angiology 2017, 68, 354–359. [Google Scholar] [CrossRef]
- Whayne, T.F. Endocan in Hypertension and Cardiovascular Disease. Angiology 2014, 65, 757–759. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Endocan—the new endothelial activation marker independently associated with soluble endothelial adhesion molecules in uraemic patients with cardiovascular disease. Clin. Biochem. 2015, 48, 425–430. [Google Scholar] [CrossRef]
- Blacher, J.; Safar, M.E.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003, 63, 1852–1860. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Lu, Q.; Cheng, L.T.; Wang, T.; Wan, J.; Liao, L.L.; Zeng, J.; Qin, C.; Li, K.-J. Visceral Fat, Arterial Stiffness, and Endothelial Function in Peritoneal Dialysis Patients. J. Ren. Nutr. 2008, 18, 495–502. [Google Scholar] [CrossRef]

| Parameters | 1st Tercile of Serum Endocan Levels (2.99–4.67 µg/L) | 2nd Tercile of Serum Endocan Levels (4.68–6.29 µg/L) | 3rd Tercile of Serum Endocan Levels (6.30–7.93 µg/L) | p-Value |
|---|---|---|---|---|
| Number of patients | 25 (33.3%) | 25 (33.3%) | 25 (33.3%) | |
| Sex | 0.214 | |||
| Male | 18 (72.0) | 14 (56.0) | 12 (48.0) | |
| Female | 7 (28.0) | 11 (44.0) | 13 (52.0) | |
| Age (years) | 50.00 [41.00, 64.00] | 71.00 [64.00, 75.00] | 77.00 [72.00, 81.00] | <0.001 |
| HD vintage (months) | 31.00 [6.00, 96.00] | 53.00 [21.00, 109.00] | 62.00 [48.00, 97.00] | 0.083 |
| Interdialytic weight gain (kg) | 2.56 (1.06) | 2.28 (0.99) | 2.44 (1.03) | 0.629 |
| Body mass index (kg/m2) | 27.39 [24.40, 30.44] | 25.91 [22.50, 28.22] | 26.40 [23.20, 29,70] | 0.448 |
| Diabetes mellitus | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Vascular access | 0.027 | |||
| Central venous catheter | 6 (24.0) | 15 (60.0) | 13 (52.0) | |
| AV fistula | 19 (76.0) | 10 (40.0) | 12 (48.0) | |
| Follow up (months) | 60.00 [60.00, 60.00] | 38.00 [20.00, 60.00] | 23.00 [14.00, 32.00] | <0.001 |
| Dialysis dose (Kt/V) | 1.61 (0.19) | 1.66 (0.18) | 1.67 (0.20) | 0.491 |
| Haematological counts | ||||
| Leucocytes (WBCs) (×109/L) | 7.00 (1.86) | 6.75 (2.08) | 7.34 (2.25) | 0.596 |
| Erythrocytes (RBCs) (×1012/L) | 3.78 (0.46) | 3.68 (0.43) | 3.60 (0.43) | 0.342 |
| Haemoglobin concentration (g/L) | 109.64 (10.24) | 105.88 (9.50) | 106.64 (8.26) | 0.330 |
| HbA1c (%) | 4.8 [4.6, 5.1] | 4.8 [4.5, 4.9] | 4.9 [4.4, 5.0] | 0.598 |
| Thrombocytes (×109/L) | 214.00 [178.00, 238.00] | 189.00 [177.00, 275.00] | 174.00 [135.00, 226.00] | 0.369 |
| Serum biochemical parameters | ||||
| CRP (mg/dL) | 5.50 [4.50, 8.00] | 7.80 [4.00, 13.42] | 17.60 [11.80, 24.40] | <0.001 |
| Albumin (g/L) | 39.00 [36.70, 40.30] | 36.60 [34.30, 37.90] | 37.10 [35.20, 39.00] | 0.026 |
| Glucose (mmol/L) | 4.80 [4.40, 5.10] | 4.50 [4.30, 4.90] | 4.70 [4.40, 5.00] | 0.475 |
| Iron (mmol/L) | 10.20 [9.30, 12.10] | 9.30 [7.60, 14.20] | 8.90 [6.50, 11.10] | 0.091 |
| Ferritin (ng/mL) | 289.00 [123.40, 437.50] | 437.60 [252.70, 687.60] | 434.90 [286.80, 546.00] | 0.070 |
| Total cholesterol (mmol/L) | 4.53 (1.13) | 4.43 (1.13) | 3.96 (1.26) | 0.196 |
| LDL cholesterol (mmol/L) | 2.12 [1.89, 2.67] | 2.12 [1.76, 2.35] | 1.87 [1.43, 2.23] | 0.236 |
| Calcium (mmol/L) | 2.23 [2.07, 2.34] | 2.33 [2.18, 2,41] | 2.21 [2.04, 2.30] | 0.139 |
| Phosphorus (mmol/L) | 1.77 (0.37) | 1.63 (0.45) | 1.58 (0.39) | 0.491 |
| PTH (pg/mL) | 222.00 [112.00, 307.00] | 348.00 [253.00, 423.00] | 283.00 [162.00, 403.00] | 0.085 |
| Endocan (µg/L) | 4.34 (0.47) | 5.73 (0.36) | 6.79 (0.40) | <0.001 |
| Therapy: | ||||
| RAAS inhibitor (%) | 15 (60.0) | 19 (76.0) | 15 (60.0) | 0.390 |
| Calcium channel blocker (%) | 16 (64.0) | 11 (44.0) | 15 (60.0) | 0.321 |
| Beta blocker (%) | 16 (64.0) | 15 (60.0) | 12 (48.0) | 0.492 |
| Statin (%) | 15 (60.0) | 17 (68.0) | 15 (60.0) | 0.796 |
| Cause of ESKD: | 0.447 | |||
| Glomerulonephritis (%) | 7 (28.0) | 6 (24.0) | 8 (32.0) | |
| Arterial hypertension (%) | 5 (20.0) | 7 (28.0) | 9 (36.0) | |
| Chronic pyelonephritis (%) | 3 (12.0) | 4 (16.0) | 3 (12.0) | |
| Polycystic kidney disease (%) | 4 (16.0) | 2 (8.0) | 0 (0.0) | |
| Tubulointerstitial nephritis (%) | 0 (0.0) | 3 (12.0) | 2 (8.0) |
| Parameters | 1st Tercile of Serum Endocan Levels (2.99–4.67 µg/L) | 2nd Tercile of Serum Endocan Levels (4.68–6.29 µg/L) | 3rd Tercile of Serum Endocan Levels (6.30–7.93 µg/L) | p-Value |
|---|---|---|---|---|
| PWV (m/s) | 8.17 (1.22) | 10.46 (1.64) | 12.62 (1.51) | <0.001 |
| PWV after HD (m/s) | 7.88 (1.57) | 10.36 (1.64) | 11.94 (1.45) | <0.001 |
| Peripheral SBP (mmHg) | 143.00 [137.00, 152.00] | 152.00 [125.00, 164.00] | 157.00 [149.00, 173.00] | 0.026 |
| Peripheral DBP (mmHg) | 88.20 (14.07) | 87.36 (14.98) | 93.28 (14.83) | 0.308 |
| MAP (mmHg) | 112.00 [98.00, 122.00] | 117.00 [100.00, 121.00] | 120.00 [114.00, 134.00] | 0.049 |
| PP (mmHg) | 55.40 (8.09) | 62.64 (19.62) | 69.88 (19.59) | 0.012 |
| HR (per minute) | 75.00 [72.00, 79.00] | 71.13 [64.00, 74.00] | 70.00 [60.00, 78.00] | 0.151 |
| AI | 23.48 (8.23) | 27.36 (15.05) | 38.56 (10.38) | <0.001 |
| Central SBP (mmHg) | 130.00 [126.00, 141.00] | 133.00 [118.00, 148.00] | 146.00 [131.00, 163.00] | 0.024 |
| Central DBP (mmHg) | 88.12 (15.57) | 87.64 (15.58) | 94.60 (15.24) | 0.213 |
| Central PP (mmHg) | 43.00 [39.00, 47.00] | 46.00 [38.00, 49.00] | 51.00 [44.00, 69.00] | 0.029 |
| Peripheral SBP after HD (mmHg) | 130.00 [120.00, 140.00] | 142.00 [125.00, 162.00] | 149.00 [131.00, 158.00] | 0.059 |
| Peripheral DBP after HD (mmHg) | 84.16 (14.82) | 84.60 (19.17) | 86.24 (14.97) | 0.895 |
| MAP after HD (mmHg) | 100.00 [92.00, 116.00] | 104.00 [96.00, 127.00] | 117.00 [104.00, 125.00] | 0.143 |
| PP after HD (mmHg) | 52.00 [41.00, 58.00] | 58.00 [47.00, 67.00] | 61.00 [48.00, 73.00] | 0.124 |
| HR after HD (per minute) | 83.52 (14.41) | 77.91 (11.52) | 75.47 (12.79) | 0.087 |
| AI after HD | 18.00 [13.00, 32.00] | 30.00 [15.00, 40.00] | 30.00 [19.00, 43.00] | 0.036 |
| Central SBP after HD (mmHg) | 124.64 (17.96) | 131.88 (22.78) | 133.60 (19.55) | 0.257 |
| Central DBP after HD (mmHg) | 84.64 (16.58) | 85.20 (20.22) | 88.20 (14.80) | 0.738 |
| Central PP after HD (mmHg) | 42.00 [30.00, 50.00] | 47.00 [34.00, 55.00] | 44.00 [33.00, 53.00] | 0.461 |
| Parameters | 1st Tercile of Serum Endocan Levels (2.99–4.67 µg/L) | 2nd Tercile of Serum Endocan Levels (4.68–6.29 µg/L) | 3rd Tercile of Serum Endocan Levels (6.30–7.93 µg/L) | p-Value |
|---|---|---|---|---|
| Number of patients | 25 (33.3) | 25 (33.3) | 25 (33.3) | |
| Number of patients who experienced a major adverse cardiovascular event (MACE) (%) | 3 (12.0) | 9 (36.0) | 20 (80.0) | <0.001 |
| Cause of death: | <0.001 | |||
| not applicable (surviving participants) (%) | 21 (84.0) | 8 (32.0) | 0 (0.0) | |
| MACE (%) | 1 (4.0) | 8 (32.0) | 20 (80.0) | |
| other causes (%) | 3 (12.0) | 9 (36.0) | 5 (20.0) |
| Outcome | MACE | MACE-Related Mortality | All-Cause Mortality | |||
|---|---|---|---|---|---|---|
| Variable | HR (95%-CI) | p-Value | HR (95%-CI) | p-Value | HR (95%-CI) | p-Value |
| Serum endocan concentration | 4.09 (1.72–9.74) | 0.00144 | 2.64 (1.23–5.65) | 0.0125 | 1.86 (1.07–3.23) | 0.0272 |
| Age | 1.01 (0.96–1.07) | 0.711 | 1.02 (0.97–1.08) | 0.386 | 1.03 (0.99–1.08) | 0.11 |
| Sex (male) | 1.96 (0.87–4.41) | 0.103 | 1.04 (0.46–2.37) | 0.921 | 1.30 (0.67–2.52) | 0.434 |
| Duration of haemodialysis | 1.01 (1.00–1.01) | 0.0688 | 1.00 (1.00–1.01) | 0.284 | 1.01 (1.00–1.01) | 0.00778 |
| Serum albumin concentration | 0.98 (0.85–1.12) | 0.736 | 1.05 (0.91–1.22) | 0.504 | 0.99 (0.89–1.10) | 0.824 |
| BMI | 1.00 (0.93–1.07) | 0.997 | 0.99 (0.92–1.06) | 0.751 | 0.99 (0.93–1.05) | 0.638 |
| Central systolic pressure | 0.99 (0.98–1.01) | 0.453 | 1.00 (0.99–1.02) | 0.731 | 1.00 (0.99–1.02) | 0.48 |
| Augmentation index | 1.01 (0.98–1.04) | 0.7 | 1.02 (0.99–1.05) | 0.253 | 1.00 (0.98–1.03) | 0.926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šafer, M.; Feldi, I.; Šahinović, I.; Tolj, I.; Pirić, M.; Šojat, D.; Oštarijaš, E.; Mihaljević, D. Serum Endocan as a Predictor of Survival and Cardiovascular Events in Patients Without Diabetic Kidney Disease on Chronic Haemodialysis: A Prospective, Observational Study. Medicina 2025, 61, 991. https://doi.org/10.3390/medicina61060991
Šafer M, Feldi I, Šahinović I, Tolj I, Pirić M, Šojat D, Oštarijaš E, Mihaljević D. Serum Endocan as a Predictor of Survival and Cardiovascular Events in Patients Without Diabetic Kidney Disease on Chronic Haemodialysis: A Prospective, Observational Study. Medicina. 2025; 61(6):991. https://doi.org/10.3390/medicina61060991
Chicago/Turabian StyleŠafer, Mario, Ivan Feldi, Ines Šahinović, Ivana Tolj, Marko Pirić, Dunja Šojat, Eduard Oštarijaš, and Dubravka Mihaljević. 2025. "Serum Endocan as a Predictor of Survival and Cardiovascular Events in Patients Without Diabetic Kidney Disease on Chronic Haemodialysis: A Prospective, Observational Study" Medicina 61, no. 6: 991. https://doi.org/10.3390/medicina61060991
APA StyleŠafer, M., Feldi, I., Šahinović, I., Tolj, I., Pirić, M., Šojat, D., Oštarijaš, E., & Mihaljević, D. (2025). Serum Endocan as a Predictor of Survival and Cardiovascular Events in Patients Without Diabetic Kidney Disease on Chronic Haemodialysis: A Prospective, Observational Study. Medicina, 61(6), 991. https://doi.org/10.3390/medicina61060991






