The Triglyceride–Glucose Index, a Marker of Insulin Resistance, Is Associated with the Myocardial Performance Index in Asymptomatic Subjects
Abstract
1. Introduction
2. Materials and Method
2.1. Ethical Approval
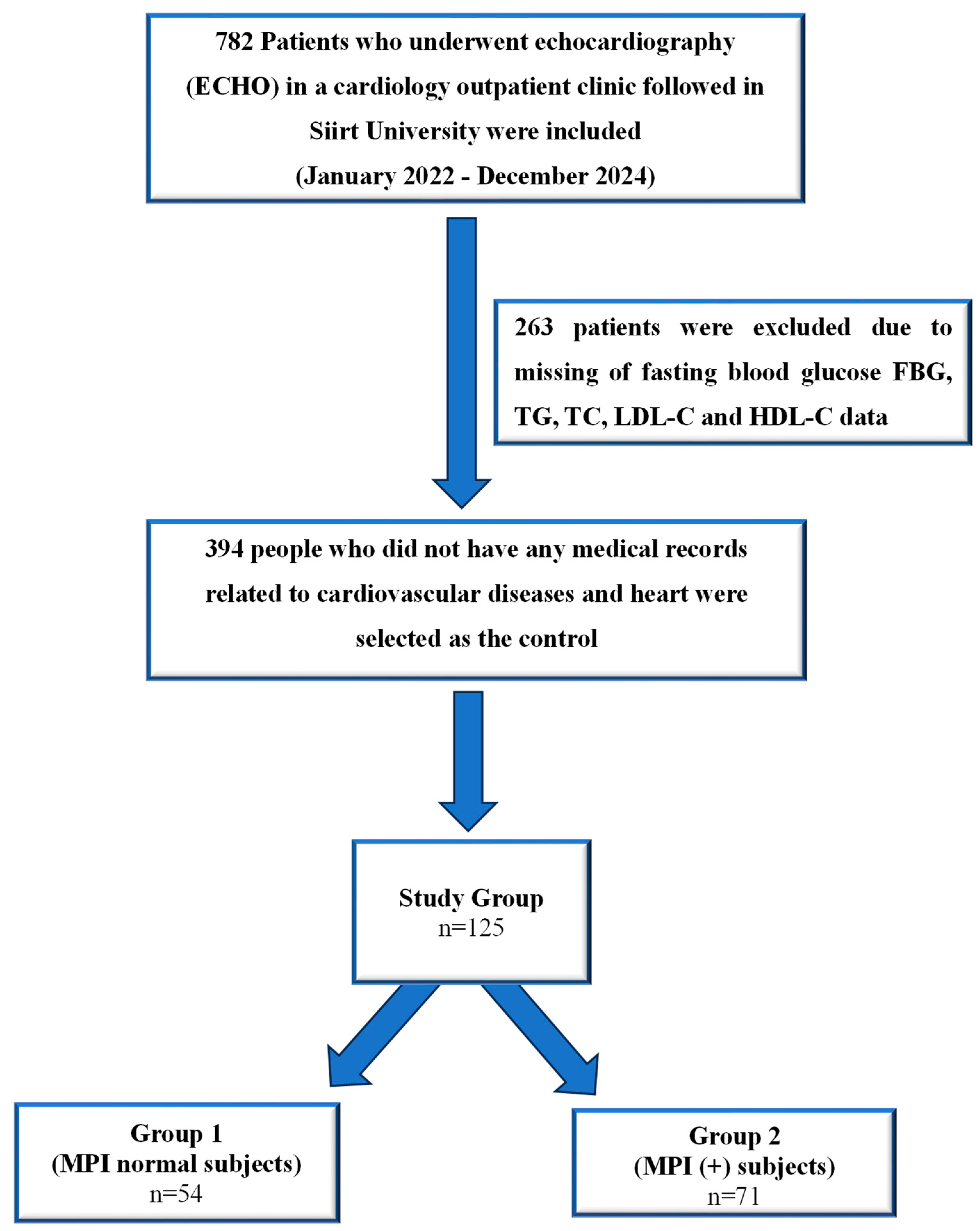
2.2. Study Populations
2.3. The Inclusion Criteria
2.4. The Exclusion Criteria
2.5. Definition of Metabolic Syndrome (MetS)
- Central obesity, defined as a waist circumference of ≥ 94 cm in men and ≥80 cm in women
- Elevated serum triglycerides, defined as TG levels of ≥150 mg/dL
- Reduced HDL, defined as HDL < 40 mg/dL in men and <50 mg/dL in women
- Hypertension, defined as either a systolic blood pressure of ≥130 mmHg and/or a diastolic blood pressure of ≥85 mmHg or a history of hypertension
- IR, defined as either a history of diabetes or the use of antidiabetic drug treatment
2.6. Blood Pressure Measurement
2.7. Electrocardiogram (ECG) Scan
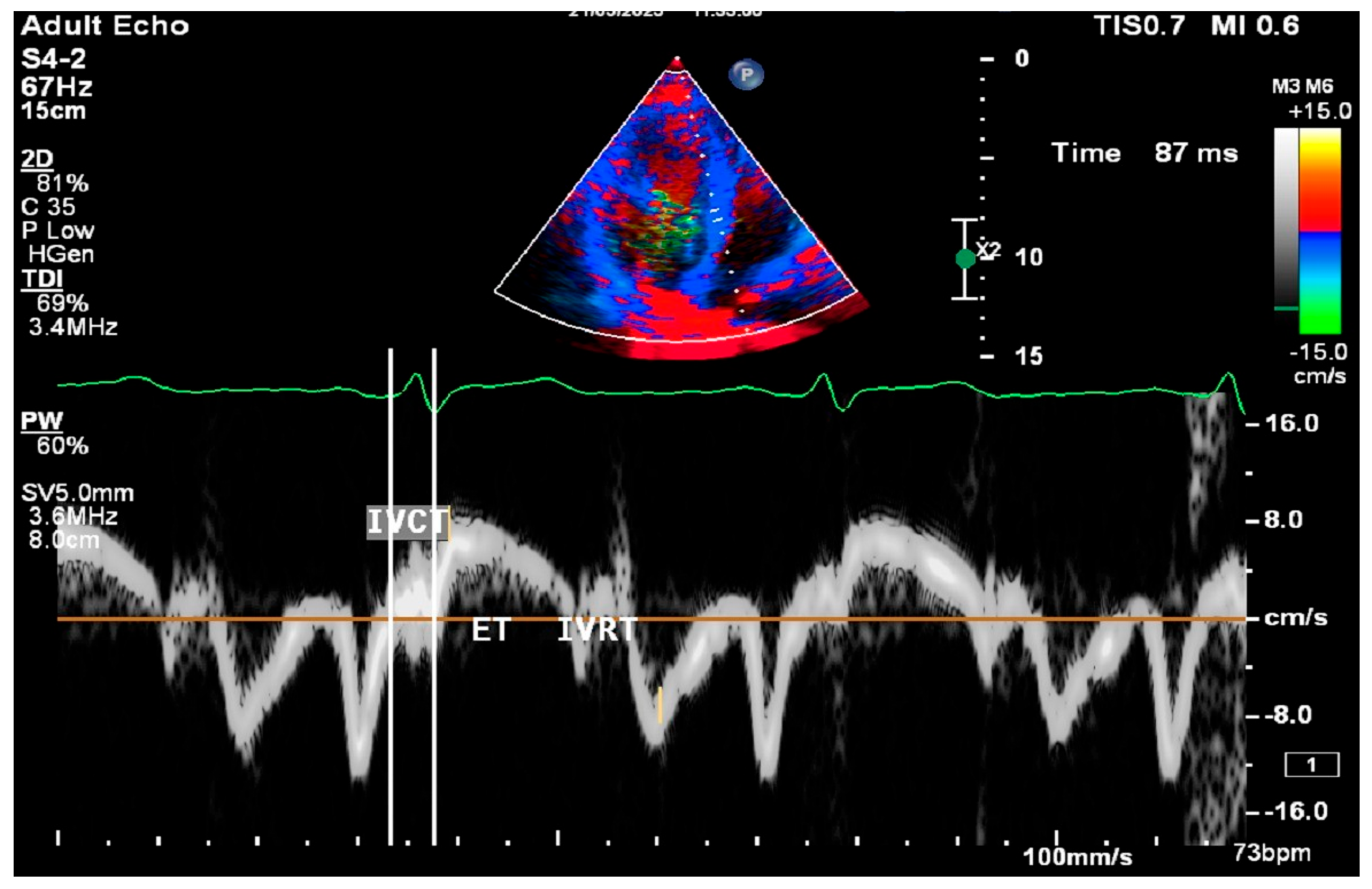
2.8. Cardiac Ultrasound Examinations
2.9. Statistical Analysis
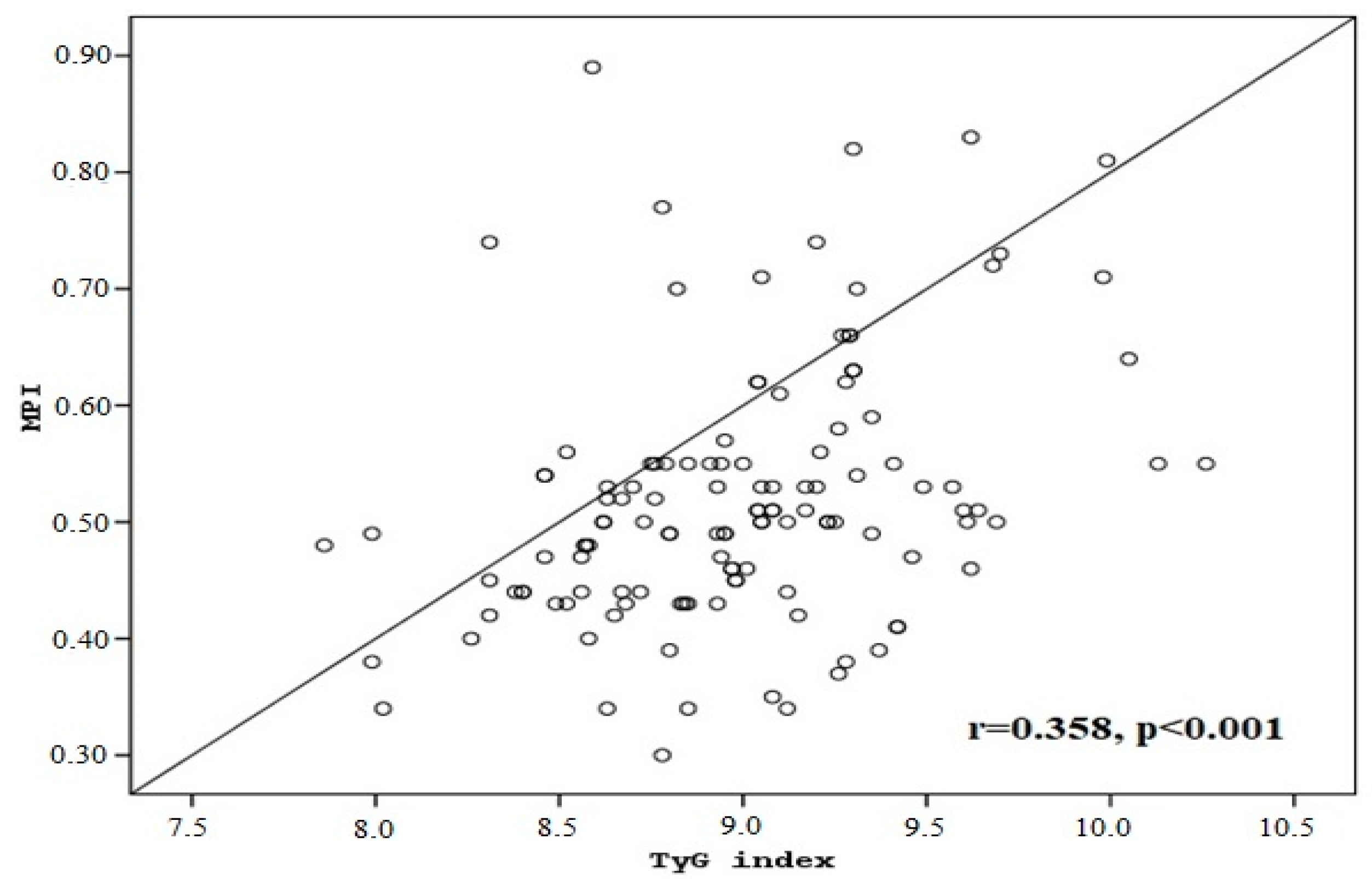
3. Results
4. Discussion
4.1. Novelty and Clinical Implications
4.2. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Artola Arita, V.; Beigrezaei, S.; Franco, O.H. Risk factors for cardiovascular disease: The known unknown. Eur. J. Prev. Cardiol. 2024, 31, e106–e107. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The global burden of cardiovascular diseases and risk: A compass for future health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Tei, C.; Ling, L.H.; Hodge, D.O.; Bailey, K.R.; Oh, J.K.; Rodeheffer, R.J.; Tajik, A.J.; Seward, J.B. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function—A study in normals and dilated cardiomyopathy. J. Cardiol. 1995, 26, 357–366. [Google Scholar] [PubMed]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271, Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 412. [Google Scholar] [CrossRef]
- Harjai, K.J.; Scott, L.; Vivekananthan, K.; Nunez, E.; Edupuganti, R. The Tei index: A new prognostic index for patients with symptomatic heart failure. J. Am. Soc. Echocardiogr. 2002, 15, 864–868. [Google Scholar] [CrossRef]
- Cheung, M.M.; Smallhorn, J.F.; Redington, A.N.; Vogel, M. The effects of changes in loading conditions and modulation of inotropic state on the myocardial performance index: Comparison with conductance catheter measurements. Eur. Heart J. 2004, 25, 2238–2242. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Moller, J.E.; Sondergaard, E.; Poulsen, S.H.; Egstrup, K. The Doppler echocardiographic myocardial performance index predicts left ventricular dilatation and cardiac death after myocardial infarction. Cardiyology 2001, 95, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, K.; Otsuji, Y.; Yoshifuku, S.; Kuwahara, E.; Yuasa, T.; El Rahim, A.E.R.A.; Matsukida, K.; Kumanohoso, T.; Toyonaga, K.; Kisanuki, A.; et al. Noninvasive estimation of impaired hemodynamics for patients with acute myocardial infarction by Tei index. J. Am. Soc. Echocardiogr. 2004, 17, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.T.; Kirkpatrick, J.N.; Mor-Avi, V.; Decara, J.M.; Lang, R.M. Age dependency of the Tei index of myocardial performance. J. Am. Soc. Echocardiogr. 2004, 17, 350–352. [Google Scholar] [CrossRef]
- Arnlöv, J.; Ingelsson, E.; Risérus, U.; Andrén, B.; Lind, L. Myocardial performance index, a Doppler-derived index of global left ventricular function, predicts congestive heart failure in elderly men. Eur. Heart J. 2004, 25, 2220–2225. [Google Scholar] [CrossRef]
- Karvounis, H.I.; Papadopoulos, C.E.; Zaglavara, T.A.; Nouskas, I.G.; Gemitzis, K.D.; Parharidis, G.E.; Louridas, G.E. Evidence of left ventricular dysfunction in asymptomatic elderly patients with non-insulin-dependent diabetes mellitus. Angiology 2004, 55, 549–555. [Google Scholar] [CrossRef]
- Orem, C.; Küçükosmanoğlu, M.; Hacihasanoğlu, A.; Yilmaz, R.; Kasap, H.; Erdoğan, T.; Kaplan, Ş.; Çelik, Ş. Association of Doppler-derived myocardial performance index with albuminuria in patients with diabetes. J. Am. Soc. Echocardiogr. 2004, 17, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, R.; Aslan, M.; Nas, N.; Güzel, T. Relationship between presystolic wave and subclinical left ventricular dysfunction as assessed by myocardial performance index in patients with metabolic syndrome. Int. J. Cardiovasc. Imaging 2023, 39, 2175–2182. [Google Scholar] [CrossRef]
- Voulgari, C.; Moyssakis, I.; Papazafiropoulou, A.; Perrea, D.; Kyriaki, D.; Katsilambros, N.; Tentolouris, N. The impact of metabolic syndrome on left ventricular myocardial performance. Diabetes Metab. Res. Rev. 2010, 26, 121–127. [Google Scholar] [CrossRef]
- Sreenivasa Kumar, M.L.; Rajasekhar, D.; Vanajakshamma, V.; Latheef, K. Impact of metabolic syndrome on global left ventricular function: As evaluated by the myocardial performance index. J. Saudi Heart Assoc. 2014, 26, 145–151. [Google Scholar] [CrossRef]
- Mao, Q.; Zhou, D.; Li, Y.; Wang, Y.; Xu, S.C.; Zhao, X.H. The Triglyceride-Glucose Index Predicts Coronary Artery Disease Severity and Cardiovascular Outcomes in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Dis. Markers 2019, 2019, 6891537. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, H.; Kan, F.; Ji, N. Association between the triglyceride glucose (TyG) index and the risk of acute kidney injury in critically ill patients with heart failure: Analysis of the MIMIC-IV database. Cardiovasc. Diabetol. 2023, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Behnoush, A.H.; Khanmohammadi, S.; Mardasi, K.G.; Sharifkashani, S.; Sahebkar, A.; Vinciguerra, C.; Cannavo, A. Triglyceride-glucose index and heart failure: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 244. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, X. Association between the triglyceride glucose index and heart failure: NHANES 2007–2018. Front. Endocrinol. 2024, 14, 1322445. [Google Scholar] [CrossRef]
- Li, X.; Chan, J.S.K.; Guan, B.; Peng, S.; Wu, X.; Lu, X.; Zhou, J.; Hui, J.M.H.; Lee, Y.H.A.; Satti, D.I.; et al. Triglyceride-glucose index and the risk of heart failure: Evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc. Diabetol. 2022, 21, 229. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, G.; Wu, K.; Wu, W.; Huang, Z.; Wang, X.; Chen, Z.; Cai, Z.; Cai, Z.; Lan, Y.; et al. Relationship between cumulative exposure to triglyceride-glucose index and heart failure: A prospective cohort study. Cardiovasc. Diabetol. 2023, 22, 239. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, J.; Tang, H.; Guo, Z.; Dong, W.; Wang, Y.; Meng, X.; Zhang, K.; Wang, W.; Shao, C.; et al. High triglyceride-glucose (TyG) index is associated with poor prognosis of heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 2023, 22, 263. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, Z.; Chen, J.; Bao, X.; Xu, N.; Guo, S.; Gu, R.; Wang, W.; Wei, Z.; Wang, L. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc. Diabetol. 2022, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shi, X.; Liu, W.; Wang, Z.; Li, R.; Xu, X.; Wang, C.; Li, L.; Wang, R.; Xu, T. Association between triglyceride glucose-body mass index and heart failure in subjects with diabetes mellitus or prediabetes mellitus: A cross-sectional study. Front. Endocrinol. 2023, 14, 1294909. [Google Scholar] [CrossRef]
- Fang, Y.; Shen, J.; Lyu, L. Value of the triglyceride-glucose index and related parameters in heart failure patients. Front. Cardiovasc. Med. 2024, 11, 1397907. [Google Scholar] [CrossRef]
- Huang, R.; Lin, Y.; Ye, X.; Zhong, X.; Xie, P.; Li, M.; Zhuang, X.; Liao, X. Triglyceride-glucose index in the development of heart failure and left ventricular dysfunction: Analysis of the ARIC study. Eur. J. Prev. Cardiol. 2022, 29, 1531–1541. [Google Scholar] [CrossRef]
- Oksen, D.; Aslan, M. Impact of oxidative stress on myocardial performance in patients with diabetes: A focus on subclinical left ventricular dysfunction. BMJ Open Diabetes Res. Care 2024, 12, e004153. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, A.R.; Turan, T.; Gürbak, İ.; Korkmaz, L.; Ağaç, M.T.; Çelik, Ş. The relationship between presystolic wave and subclinical left ventricular dysfunction in asymptomatic hypertensive patients. Blood Press. Monit. 2016, 21, 277–281. [Google Scholar] [CrossRef] [PubMed]

| MPI | ||||
|---|---|---|---|---|
| Parameters | Group 1 (n = 54) | Group 2 (n = 71) | Total (n = 125) | p Values |
| Age (years) | 45.8 ± 9.5 | 45.3 ± 10.4 | 45.5 ± 10 | 0.788 a |
| Female | 46 ± 10.1 | 47.8 ± 10.3 | 47 ± 10.1 | 0.550 a |
| Male | 45.7 ± 9.4 | 43.9 ± 10.3 | 44.7 ± 9.9 | 0.431 a |
| Gender n (%) | 0.795 c | |||
| Female | 21 (38.9%) | 26 (36.6%) | 47 (37.6%) | |
| Male | 33 (61.1%) | 45 (63.4%) | 78 (62.4%) | |
| BMI (kg/m2) | 27.8 ± 5.1 | 30.1 ± 5.6 | 29.1 ± 5.5 | 0.028 a |
| Obesity n (%) | 0.039 b | |||
| <30 kg/m2 | 40 (74.1%) | 39 (54.9%) | 79 (63.2%) | |
| ≥30 kg/m2 | 14 (25.9%) | 32 (45.1%) | 46 (36.8%) | |
| Smoking n (%) | 0.062 c | |||
| No | 39 (72.2%) | 39 (54.9%) | 78 (62.4%) | |
| Yes | 15 (27.8%) | 32 (45.1%) | 47 (37.6%) | |
| Metformin n (%) | 0.627 c | |||
| No | 39 (72.2%) | 54 (76.1%) | 93 (74.4%) | |
| Yes | 15 (27.8%) | 17 (23.9%) | 32 (25.6%) | |
| Metabolic Syndrome n (%) | 0.001 b | |||
| No | 36 (66.7%) | 26 (36.6%) | 62 (49.6%) | |
| Yes | 18 (33.3%) | 45 (63.4%) | 63 (50.4%) | |
| SBP (mmHg) | 131.3 ± 16.4 | 133.3 ± 14.7 | 132.4 ± 15.4 | 0.494 d |
| DBP (mmHg) | 84.4 ± 8.3 | 83.5 ± 9.5 | 83.9 ± 9.1 | 0.575 d |
| Glucose (mg/dL) | 101.9 ± 15.6 | 108 ± 20.1 | 105.3 ± 18.4 | 0.069 d |
| Triglyceride (mg/dL) | 148.1 ± 53.5 | 189 ± 72.7 | 171.3 ± 68 | 0.001 d |
| TyG index | 8.77 ± 0.39 | 9.14 ± 0.41 | 8.98 ± 0.44 | <0.001 d |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| p Values | OR (CI 95%) | p Values | OR (CI 95%) | |
| Obesity (kg/m2) | ||||
| ≥30, against <30 | 0.03 | 2.34 (1.08–5.05) | ns | |
| Metabolic Syndrome | ||||
| Yes, against no | 0.001 | 3.46 (1.64–7.28) | ns | |
| Triglyceride (mg/dL) | ||||
| per mg/dL increment | 0.002 | 1.01 (1.004–1.018) | ns | |
| TyG index | ||||
| per unit increment | <0.001 | 1.042 (1.035–1.051) | 0.006 | 1.038 (1.031–1.046) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nas, N.; Aslan, M.; Saglik, S.; Uzun, H. The Triglyceride–Glucose Index, a Marker of Insulin Resistance, Is Associated with the Myocardial Performance Index in Asymptomatic Subjects. Medicina 2025, 61, 987. https://doi.org/10.3390/medicina61060987
Nas N, Aslan M, Saglik S, Uzun H. The Triglyceride–Glucose Index, a Marker of Insulin Resistance, Is Associated with the Myocardial Performance Index in Asymptomatic Subjects. Medicina. 2025; 61(6):987. https://doi.org/10.3390/medicina61060987
Chicago/Turabian StyleNas, Necip, Muzaffer Aslan, Semih Saglik, and Hafize Uzun. 2025. "The Triglyceride–Glucose Index, a Marker of Insulin Resistance, Is Associated with the Myocardial Performance Index in Asymptomatic Subjects" Medicina 61, no. 6: 987. https://doi.org/10.3390/medicina61060987
APA StyleNas, N., Aslan, M., Saglik, S., & Uzun, H. (2025). The Triglyceride–Glucose Index, a Marker of Insulin Resistance, Is Associated with the Myocardial Performance Index in Asymptomatic Subjects. Medicina, 61(6), 987. https://doi.org/10.3390/medicina61060987






