Systematic Review and Meta-Analysis of Acute Mortality and Complication Rates Following Leadless Pacemaker Placement Using National-Level Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
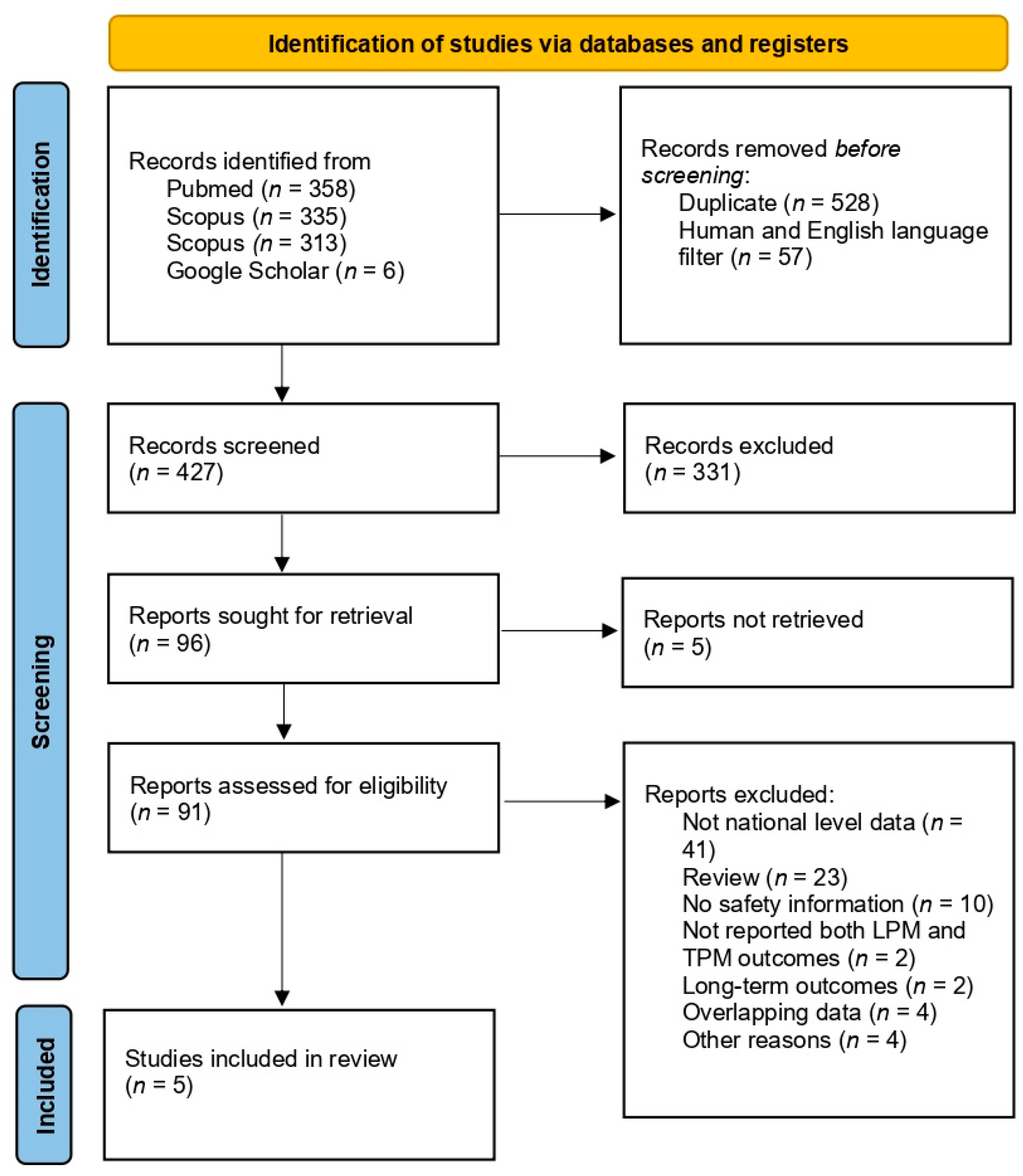
2.3. Selection of Studies and Data Extraction
2.4. Meta-Analysis Strategy
2.5. Risk of Bias and Certainty of Evidence Assessment
3. Results
3.1. Included Study Characteristics
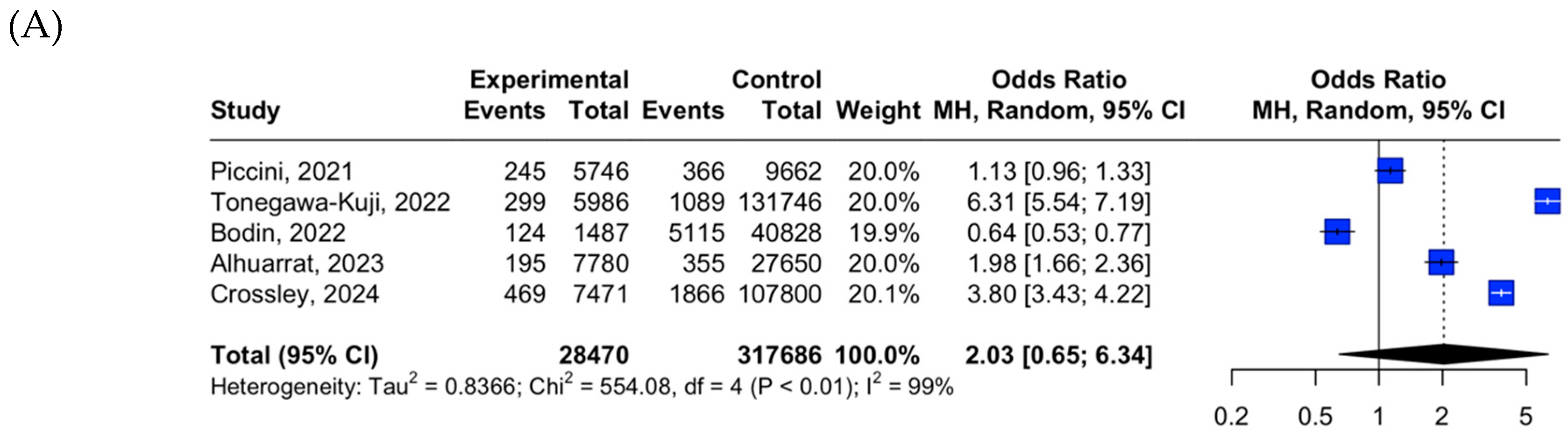
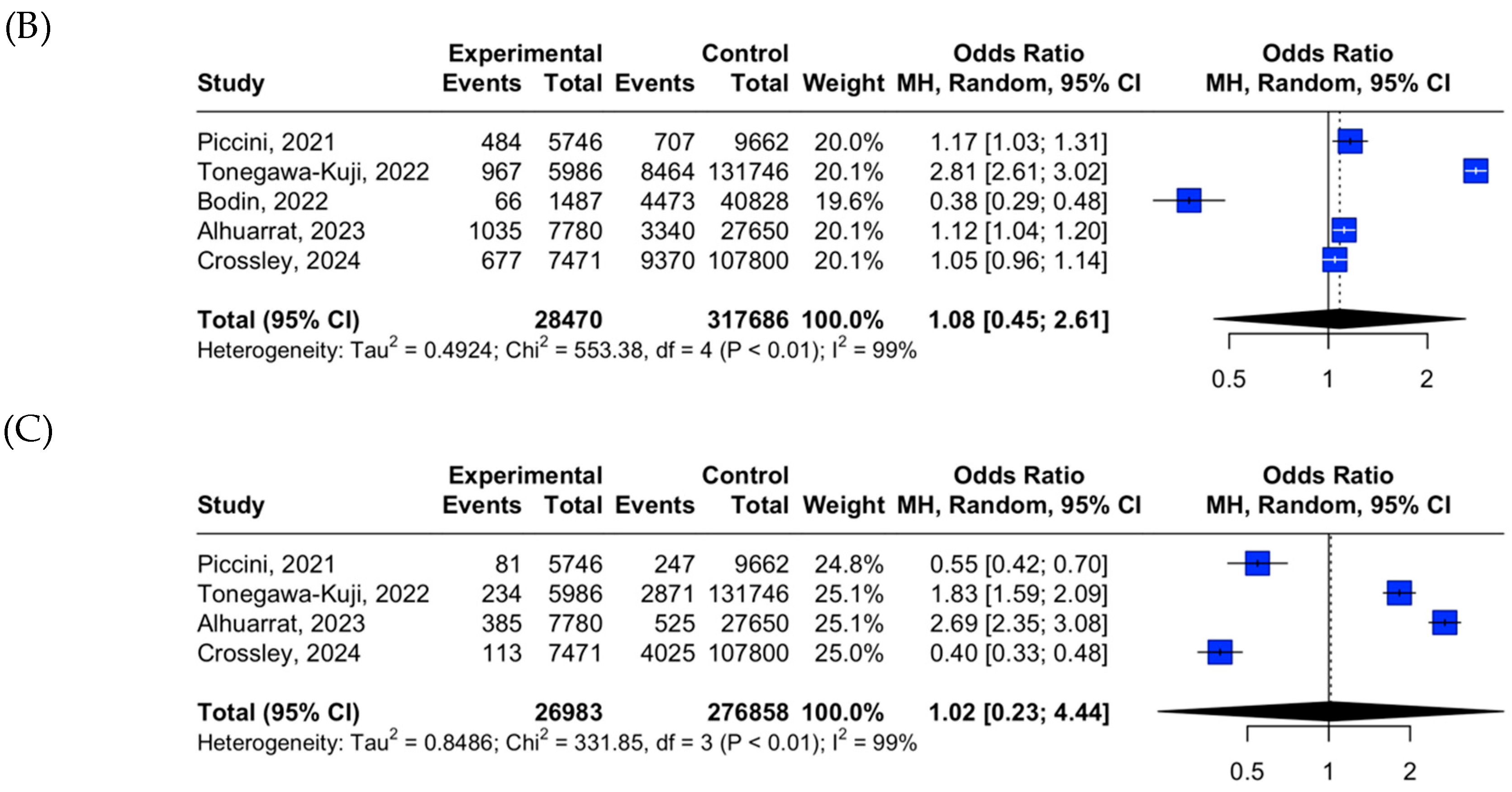
3.2. LPM vs. TPM Placement Outcome Data
3.3. Risk of Bias and Certainty of Evidence Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| IQR | Interquartile range |
| LPM | Leadless pacemaker |
| OR | Odds ratio |
| PMSI | Programme de Médicalisation des Systèmes d’Information |
| SD | Standard deviation |
| TPMs | Transvenous pacemakers |
| USA | United States of America |
References
- Mulpuru, S.K.; Madhavan, M.; McLeod, C.J.; Cha, Y.M.; Friedman, P.A. Cardiac Pacemakers: Function, Troubleshooting, and Management: Part 1 of a 2-Part Series. J. Am. Coll. Cardiol. 2017, 69, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Egas, D.; Rodriguez, F.; Jaswal, A.; Jeilan, M.; Milasinovic, G.; Fagih, A. Al Burden of Bradycardia and Barriers to Accessing Bradycardia Therapy in Underserved Countries. Eur. Heart J. Suppl. 2023, 25, H1–H7. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS Guideline on Cardiac Physiologic Pacing for the Avoidance and Mitigation of Heart Failure. Heart Rhythm 2023, 20, e17–e91. [Google Scholar] [CrossRef] [PubMed]
- Aquilina, O. A Brief History of Cardiac Pacing. Images Paediatr. Cardiol. 2006, 8, 17–81. [Google Scholar] [PubMed]
- Spickler, J.W.; Rasor, N.S.; Kezdi, P.; Misra, S.N.; Robins, K.E.; LeBoeuf, C. Totally Self-Contained Intracardiac Pacemaker. J. Electrocardiol. 1970, 3, 325–331. [Google Scholar] [CrossRef]
- Shen, E.N.; Ishihara, C.H.; Uehara, D.R. Leadless Pacemaker: Report of the First Experience in Hawai‘i. Hawai’i J. Med. Public Health 2018, 77, 79–82. [Google Scholar]
- National Institute for Health and Care Excellence. Leadless Cardiac Pacemaker Implantation for Bradyarrhythmias Interventional Procedures Guidance Your Responsibility. Available online: www.nice.org.uk/guidance/ipg626 (accessed on 24 September 2024).
- La Fazia, V.M.; Lepone, A.; Pierucci, N.; Gianni, C.; Barletta, V.; Mohanty, S.; Della Rocca, D.G.; La Valle, C.; Torlapati, P.G.; Al-Ahmad, M.; et al. Low Prevalence of New-Onset Severe Tricuspid Regurgitation Following Leadless Pacemaker Implantation in a Large Series of Consecutive Patients. Heart Rhythm 2024, 21, 2603–2604. [Google Scholar] [CrossRef]
- Meredith, A.; Markovic, N.; Kakar, P.; Kim, H.; Aziz, E.F. Leadless Intracardiac Pacemaker Implantation in Patients with Bradyarrhythmias after Spinal Cord Injury. Heart Rhythm Case Rep. 2021, 7, 669–673. [Google Scholar] [CrossRef]
- National Institute for Health and Care Research PROSPERO. International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 27 March 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R. Available online: https://docs.posit.co/previous-versions/ (accessed on 22 January 2024).
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide; Chapman & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021; Chapter 4; ISBN 978-0-367-61007-4. [Google Scholar]
- Migliavaca, C.B.; Stein, C.; Colpani, V.; Barker, T.H.; Ziegelmann, P.K.; Munn, Z.; Falavigna, M. Meta-Analysis of Prevalence: I2 Statistic and How to Deal with Heterogeneity. Res. Synth. Methods 2022, 13, 363–367. [Google Scholar] [CrossRef]
- CASP. CASP Checklist for Cross-Sectional Studies, 2017. Critical Appraisal Skills Programme, UK. 2017. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 1 October 2024).
- Schünemann, H.J.; Higgins, J.P.T.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H. Cochrane GRADEing Methods Group Assessing Certainty in the Evidence in the Context of a Systematic Review. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2022; Version 6.3. [Google Scholar]
- Prasad, M. Introduction to the GRADE Tool for Rating Certainty in Evidence and Recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- Brennan, S.E.; Johnston, R.V. Research Note: Interpreting Findings of a Systematic Review Using GRADE Methods. J. Physiother. 2023, 69, 198–202. [Google Scholar] [CrossRef]
- Clémenty, N.; Fernandes, J.; Carion, P.L.; de Léotoing, L.; Lamarsalle, L.; Wilquin-Bequet, F.; Wolff, C.; Verhees, K.J.P.; Nicolle, E.; Deharo, J.C. Pacemaker Complications and Costs: A Nationwide Economic Study. J. Med. Econ. 2019, 22, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Nassar, S.; Nguyen, A.; Khan, M.U.; Sattar, Y.; Alruwaili, W.; Gonuguntla, K.; Mazek, H.; Asad, Z.U.A.; Agarwal, S.; et al. Contemporary Trends of Leadless Pacemaker Implantation in the United States. J. Cardiovasc. Electrophysiol. 2024, 35, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Bockstedt, L.; Longacre, C.; Higuera, L.; Stromberg, K.; Crossley, G.; Kowal, R.C.; Piccini, J.P. Leadless vs. Transvenous Single-Chamber Ventricular Pacing in the Micra CED Study: 2-Year Follow-Up. Eur. Heart J. 2022, 43, 1207–1215. [Google Scholar] [CrossRef]
- Crossley, G.H.; Piccini, J.P.; Longacre, C.; Higuera, L.; Stromberg, K.; El-Chami, M.F. Leadless versus Transvenous Single-Chamber Ventricular Pacemakers: 3 Year Follow-up of the Micra CED Study. J. Cardiovasc. Electrophysiol. 2023, 34, 1015–1023. [Google Scholar] [CrossRef]
- Vincent, L.; Grant, J.; Peñalver, J.; Ebner, B.; Maning, J.; Olorunfemi, O.; Goldberger, J.J.; Mitrani, R.D. Early Trends in Leadless Pacemaker Implantation: Evaluating Nationwide in-Hospital Outcomes. Heart Rhythm 2022, 19, 1334–1342. [Google Scholar] [CrossRef]
- Haddadin, F.; Majmundar, M.; Jabri, A.; Pecha, L.; Scott, C.; Daher, M.; Kumar, A.; Kalra, A.; Fram, R.; Haddadin, F.; et al. Clinical Outcomes and Predictors of Complications in Patients Undergoing Leadless Pacemaker Implantation. Heart Rhythm 2022, 19, 1289–1296. [Google Scholar] [CrossRef]
- Khan, M.Z.; Nguyen, A.; Khan, M.U.; Sattar, Y.; Alruwaili, W.; Gonuguntla, K.; Sohaib Hayat, H.M.; Mendez, M.; Nassar, S.; Abideen Asad, Z.U.; et al. Association of Chronic Kidney Disease and End-Stage Renal Disease with Procedural Complications and Inpatient Outcomes of Leadless Pacemaker Implantations across the United States. Heart Rhythm 2024, 21, 1695–1702. [Google Scholar] [CrossRef]
- Ueyama, H.A.; Miyamoto, Y.; Hashimoto, K.; Watanabe, A.; Kolte, D.; Latib, A.; Kuno, T.; Tsugawa, Y. Comparison of Patient Outcomes Between Leadless vs Transvenous Pacemakers Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2024, 17, 1779–1791. [Google Scholar] [CrossRef]
- Kiblboeck, D.; Blessberger, H.; Ebner, J.; Boetscher, J.; Maier, J.; Reiter, C.; Kellermair, J.; Steinwender, C.; Saleh, K. Feasibility, Timing and Outcome of Leadless Cardiac Pacemaker Implantation in Patients Undergoing Cardiac Implantable Electronic Device Extraction. Clin. Res. Cardiol. 2024, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, T.; Nagashima, M.; Kono, H.; Sadohara, Y.; Hirokami, J.; Kuji, R.; Korai, K.; Fukunaga, M.; Hiroshima, K.; Ando, K. Clinical Outcome for Heart Failure Hospitalizations in Patients with Leadless Pacemaker. J. Arrhythmia 2022, 38, 730–735. [Google Scholar] [CrossRef]
- Tsz-Kin Tam, M.; Kin-Yin Chan, A.; Chi-Kin Au, A.; Cheung, L.; Chin-Pang Chan, G.; Yat-Sun Chan, J. Effect of Low Body Mass Index in Outcome of Micra Leadless Pacemaker Implantation. J. Hong Kong Coll. Cardiol. 2022, 29, 43–52. [Google Scholar] [CrossRef]
- Nagashima, M.; Hiroshima, K.; Katsuki, T.; Ando, K. Clinical Outcome for Heart Failure Hospitalizations in Patients with Leadless Pacemaker. Eur. Heart J. 2023, 44, ehad655.400. [Google Scholar] [CrossRef]
- Piccini, J.P.; El-Chami, M.; Wherry, K.; Crossley, G.H.; Kowal, R.C.; Stromberg, K.; Longacre, C.; Hinnenthal, J.; Bockstedt, L. Contemporaneous Comparison of Outcomes Among Patients Implanted with a Leadless vs Transvenous Single-Chamber Ventricular Pacemaker. JAMA Cardiol. 2021, 6, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Clementy, N.; Bisson, A.; Pierre, B.; Herbert, J.; Babuty, D.; Fauchier, L. Leadless or Conventional Transvenous Ventricular Permanent Pacemakers: A Nationwide Matched Control Study. J. Am. Heart Assoc. 2022, 11, e025339. [Google Scholar] [CrossRef]
- Tonegawa-Kuji, R.; Kanaoka, K.; Mori, M.; Nakai, M.; Iwanaga, Y. Mortality and 30-Day Readmission Rates After Inpatient Leadless Pacemaker Implantation: Insights From a Nationwide Readmissions Database. Can. J. Cardiol. 2022, 38, 1697–1705. [Google Scholar] [CrossRef]
- Al Alhuarrat, M.D.; Kharawala, A.; Renjithlal, S.; Eid, M.M.; Varrias, D.; Mohammed, M.; Grushko, M.; Biase, L. Di Comparison of In-Hospital Outcomes and Complications of Leadless Pacemaker and Traditional Transvenous Pacemaker Implantation. Europace 2023, 25, euad269. [Google Scholar] [CrossRef]
- Crossley, G.H.; Longacre, C.; Higuera, L.; Stromberg, K.; Cheng, A.; Piccini, J.P.; El-Chami, M.F. Outcomes of Patients Implanted with an Atrioventricular Synchronous Leadless Ventricular Pacemaker in the Medicare Population. Heart Rhythm 2024, 21, 66–73. [Google Scholar] [CrossRef]
- Boveda, S.; Lenarczyk, R.; Haugaa, K.H.; Iliodromitis, K.; Finlay, M.; Lane, D.; Prinzen, F.W.; Dagres, N. Use of Leadless Pacemakers in Europe: Results of the European Heart Rhythm Association Survey. EP Eur. 2018, 20, 555–559. [Google Scholar] [CrossRef]
- Ngo, L.; Nour, D.; Denman, R.A.; Walters, T.E.; Haqqani, H.M.; Woodman, R.J.; Ranasinghe, I. Safety and Efficacy of Leadless Pacemakers: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, 19212–19232. [Google Scholar] [CrossRef] [PubMed]
- Darlington, D.; Brown, P.; Carvalho, V.; Bourne, H.; Mayer, J.; Jones, N.; Walker, V.; Siddiqui, S.; Patwala, A.; Kwok, C.S. Efficacy and Safety of Leadless Pacemaker: A Systematic Review, Pooled Analysis and Meta-Analysis. Indian Pacing Electrophysiol. J. 2022, 22, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.R.; ElRefai, M.; Foley, P.; Rao, A.; Sharman, D.; Somani, R.; Sporton, S.; Wright, G.; Zaidi, A.; Pepper, C. UK Expert Consensus Statement for the Optimal Use and Clinical Utility of Leadless Pacing Systems on Behalf of the British Heart Rhythm Society. Arrhythm. Electrophysiol. Rev. 2022, 11, e19. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, A.; Campos, B.; Alonso-Martín, C.; Guerra-Ramos, J.M.; Rodríguez-Font, E.; Moreno-Weidmann, Z.; Alcalde-Rodríguez, Ó.; Méndez-Zurita, F.J.; Santaló, M.; Espinosa-Viamonte, H.; et al. Performance of the Micra Cardiac Pacemaker in Nonagenarians. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 307–312. [Google Scholar] [CrossRef]
- Knops, R.E.; Reddy, V.Y.; Ip, J.E.; Doshi, R.; Exner, D.V.; Defaye, P.; Canby, R.; Bongiorni, M.G.; Shoda, M.; Hindricks, G.; et al. A Dual-Chamber Leadless Pacemaker. N. Engl. J. Med. 2023, 388, 2360–2370. [Google Scholar] [CrossRef]

| Study | Country | Database Name | Period | Sample Size | Age Mean ± SD or Median (IQR) | Objective | ||
|---|---|---|---|---|---|---|---|---|
| LPM | TPM | LPM | TPM | |||||
| Piccini, 2021 [31] | USA | Medicare Claims Data | 9 March 2017–1 December 2018 | 3726 | 7265 | 79.4 ± 9.5 | 82.0 ± 8.1 | 30-day and 6-month complication rates |
| Bodin, 2022 [32] | France | PMSI | 1 January 2017–1 September 2020 | 1487 | 40,828 | 70.7 ± 18.4 | 83.4 ± 9.1 | 30-day complication rate, including all-cause death and cardiovascular death |
| Tonegawa-Kuji, 2022 [33] | USA | Nationwide Readmissions Database | April 2017–December 2019 | 5986 | 131,746 | 79 (70–86) | 78 (70–85) | In-hospital complications and 30-day readmission |
| Alhuarrat, 2023 [34] | USA | National Inpatient Sample Database | 2016–2019 | 7780 | 27,650 | 77.1 ± 12.1 | 81.3 ± 9.4 | In-hospital outcome and procedural complications |
| Crossley, 2024 [35] | USA | Medicare Claims Data | 5 February 2020–1 December 2021 | 7471 | 107,800 | 79.0 ± 10.2 | 78.7 ± 8.0 | 30 days and 6 months overall and device-related complications, and all-cause mortality |
| Study | Complication Assessment Period | Acute Complications |
|---|---|---|
| Piccini, 2021 [31] | 30-day | Embolism and thrombosis, puncture site events, cardiac effusion and perforation, device-related, and other complications. |
| Bodin, 2022 [32] | In-hospital | Embolism and thrombosis, puncture site events, cardiac effusion and perforation, device-related, and other complications. |
| Tonegawa-Kuji, 2022 [33] | 30-day | Cardiac tamponade, pneumothorax, hemothorax, major bleeding, transfusion, and device-related complications. |
| Alhuarrat, 2023 [34] | In-hospital | Bleeding, vascular events, venous thromboembolism, device-related, cardiac, pulmonary, infection, and neurologic complications. |
| Crossley, 2024 [35] | 30-day | Embolism and thrombosis, puncture site events, cardiac effusion and perforation, device-related, and other complications. |
| Authors | Question | Recruitment | Exposure | Outcome | Confounding | Follow up | Results | Applicability | Fit | Implications | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Piccini, 2021 [31] | Yes | Yes | Yes | Yes | Cannot t tell | Yes | Yes | Yes | Cannot tell | Yes | 9.0 |
| Bodin, 2022 [32] | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | Cannot tell | Yes | 9.0 |
| Tonegawa-Kuji, 2022 [33] | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Cannot tell | Cannot tell | Yes | 8.5 |
| Alhuarrat, 2023 [34] | Yes | Yes | Yes | Yes | Cannot tell | Can’t tell | Yes | Yes | Cannot tell | Yes | 8.5 |
| Crossley, 2024 [35] | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | Cannot tell | Yes | 9.0 |
| Outcome | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty of Evidence |
|---|---|---|---|---|---|---|
| Acute mortality | Low | Serious | Not serious | Serious | Not assessed | Low |
| Acute overall complications | Low | Serious | Not serious | Serious | Not assessed | Low |
| Acute device-related complications | Low | Serious | Not serious | Serious | Not assessed | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarsenbayeva, A.; Baimbetov, A.; Puodziukynas, A.; Baimakhanov, B.; Sapunov, A.; Bizhanov, K. Systematic Review and Meta-Analysis of Acute Mortality and Complication Rates Following Leadless Pacemaker Placement Using National-Level Data. Medicina 2025, 61, 974. https://doi.org/10.3390/medicina61060974
Sarsenbayeva A, Baimbetov A, Puodziukynas A, Baimakhanov B, Sapunov A, Bizhanov K. Systematic Review and Meta-Analysis of Acute Mortality and Complication Rates Following Leadless Pacemaker Placement Using National-Level Data. Medicina. 2025; 61(6):974. https://doi.org/10.3390/medicina61060974
Chicago/Turabian StyleSarsenbayeva, Akmoldir, Adil Baimbetov, Aras Puodziukynas, Bolatbek Baimakhanov, Alexander Sapunov, and Kenzhebek Bizhanov. 2025. "Systematic Review and Meta-Analysis of Acute Mortality and Complication Rates Following Leadless Pacemaker Placement Using National-Level Data" Medicina 61, no. 6: 974. https://doi.org/10.3390/medicina61060974
APA StyleSarsenbayeva, A., Baimbetov, A., Puodziukynas, A., Baimakhanov, B., Sapunov, A., & Bizhanov, K. (2025). Systematic Review and Meta-Analysis of Acute Mortality and Complication Rates Following Leadless Pacemaker Placement Using National-Level Data. Medicina, 61(6), 974. https://doi.org/10.3390/medicina61060974







