Survival Outcomes in Metastatic Germ Cell Tumors: A Multicenter Study from Turkey
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
4. Results
4.1. Patient Characteristics
4.2. First-Line Treatment and Response
4.3. Salvage Treatment
4.4. Survival Analysis
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Research Program NCI. SEER*Explorer: An Interactive Website for SEER Cancer Statistics 2024 [Updated 5 November 2024]. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 2 April 2025).
- Lauritsen, J.; Sauve, N.; Tryakin, A.; Jiang, D.M.; Huddart, R.; Heng, D.Y.C.; Terbuch, A.; Winquist, E.; Chovanec, M.; Hentrich, M.; et al. Outcomes of relapsed clinical stage I versus de novo metastatic testicular cancer patients: An analysis of the IGCCCG Update database. Br. J. Cancer 2023, 129, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J. Clin. Oncol. 1997, 15, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.D.; Birch, R.; Einhorn, L.H.; Irwin, L.; Greco, F.A.; Loehrer, P.J. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N. Engl. J. Med. 1987, 316, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.M.; Stenning, S.P. The International Germ Cell Consensus Classification: A new prognostic factor-based staging classification for metastatic germ cell tumours. Clin. Oncol. 1997, 9, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Sauve, N.; Collette, L.; Daugaard, G.; de Wit, R.; Albany, C.; Tryakin, A.; Fizazi, K.; Stahl, O.; Gietema, J.A.; et al. Predicting Outcomes in Men with Metastatic Nonseminomatous Germ Cell Tumors (NSGCT): Results From the IGCCCG Update Consortium. J. Clin. Oncol. 2021, 39, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Collette, L.; Sauve, N.; Daugaard, G.; Feldman, D.R.; Tandstad, T.; Tryakin, A.; Stahl, O.; Gonzalez-Billalabeitia, E.; De Giorgi, U.; et al. Survival and New Prognosticators in Metastatic Seminoma: Results From the IGCCCG-Update Consortium. J. Clin. Oncol. 2021, 39, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Albany, C.; Adra, N.; Snavely, A.C.; Cary, C.; Masterson, T.A.; Foster, R.S.; Kesler, K.; Ulbright, T.M.; Cheng, L.; Chovanec, M.; et al. Multidisciplinary clinic approach improves overall survival outcomes of patients with metastatic germ-cell tumors. Ann. Oncol. 2018, 29, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Tanuma, K.; Kawai, K.; Nitta, S.; Shiga, M.; Kawahara, T.; Negoro, H.; Onozawa, M.; Inoue, T.; Nishiyama, H.; Miyazaki, J. Improved survival of poor-risk non-seminomatous germ cell tumor patients: Real-world data from a single institute in Japan. Jpn. J. Clin. Oncol. 2022, 53, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.A.; Gongora, A.B.L.; Dzik, C.; Jardim, D.L.; Piva, M.; Carcano, F.M.; Bertollo, G.; Trindade, K.; Fontes, M.S.; Soares, A.; et al. Multicenter Database of Patients with Germ-Cell Tumors: A Latin American Cooperative Oncology Group Registry (LACOG 0515). Clin. Genitourin. Cancer 2023, 21, e104–e113. [Google Scholar] [CrossRef] [PubMed]
- Incesu, R.B.; Morra, S.; Scheipner, L.; Barletta, F.; Baudo, A.; Garcia, C.C.; Tappero, S.; Piccinelli, M.L.; Tian, Z.; Saad, F.; et al. A population-based validation of the IGCCCG Update Consortium for survival in metastatic non-seminoma testis cancer. Jpn. J. Clin. Oncol. 2024, 54, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Pagliaro, L.; Laplanche, A.; Flechon, A.; Mardiak, J.; Geoffrois, L.; Kerbrat, P.; Chevreau, C.; Delva, R.; Rolland, F.; et al. Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): A phase 3, multicentre, randomised trial. Lancet Oncol. 2014, 15, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Le Teuff, G.; Flechon, A.; Pagliaro, L.; Mardiak, J.; Geoffrois, L.; Laguerre, B.; Chevreau, C.; Delva, R.; Rolland, F.; et al. Personalized Chemotherapy on the Basis of Tumor Marker Decline in Poor-Prognosis Germ-Cell Tumors: Updated Analysis of the GETUG-13 Phase III Trial. J. Clin. Oncol. 2024, 42, 3270–3276. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Bagrodia, A.; Baraban, E.; Fankhauser, C.D.; Ged, Y.M.A. Testicular Germ Cell Tumors: A Review. JAMA 2025, 333, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, L.; Ardizzone, D.; Ravi, P.; Bagrodia, A.; Mego, M.; Daneshmand, S.; Nicolai, N.; Nazzani, S.; Giannatempo, P.; Franza, A.; et al. Risk of residual cancer after complete response following first-line chemotherapy in men with metastatic non-seminomatous germ cell tumour and International Germ Cell Cancer Cooperative Group intermediate/poor prognosis: A multi-institutional retrospective cohort study. Eur. J. Cancer 2023, 182, 144–154. [Google Scholar] [CrossRef]
- Fazekas, F.E.; Ujfaludi, Z.; Biro, K.; Pahi, Z.G.; Buzogany, I.; Sukosd, F.; Pankotai, T.; Beothe, T. Complex treatment of residual metastatic germ cell cancer: A single center experience. J. Biotechnol. 2024, 389, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Regouc, M.; Belge, G.; Lorch, A.; Dieckmann, K.P.; Pichler, M. Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer. Cancers 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Ditonno, F.; Franco, A.; Manfredi, C.; Fasanella, D.; Abate, M.; La Rocca, R.; Crocerossa, F.; Iossa, V.; Falagario, U.G.; Cirillo, L.; et al. The Role of miRNA in Testicular Cancer: Current Insights and Future Perspectives. Medicina 2023, 59, 2033. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | All Cohort (N = 316) N (%) | Seminoma (N = 75) N (%) | Non-Seminoma (N = 241) N (%) |
|---|---|---|---|
| Median age, years (IQR) | 28 (23–36) | ||
| Under 40 | 260 (82.3) | 51 (68) | 209 (86.4) |
| Over 40 | 56 (17.7) | 24 (32) | 32 (13.3) |
| Location of primary tumor | |||
| Gonadal | 293 (92.7) | 72 (96) | 221 (91.7) |
| Retroperitoneal | 13 (4.1) | 0 (0) | 13 (5.4) |
| Mediastinal | 10 (3.2) | 3 (4) | 7 (2.9) |
| Tumor histology | |||
| Pure seminoma | 75 (23.7) | 75 (100) | |
| Non-seminoma | 241 (76.3) | 241 (100) | |
| Mixed germ cell tumors | 201 (63.6) | 201(83.4) | |
| Embrional carcinoma | 26 (8.2) | 26 (10.8) | |
| Yolksac | 7 (2.2) | 7 (2.9) | |
| Teratoma | 5 (1.6) | 5 (2.1) | |
| Choriocarcinoma | 2 (0.6) | 2 (0.8) | |
| IGCCCG risk at diagnosis | |||
| Good | 168 (53.2) | 67 (89.3) | 101 (41.9) |
| Intermediate | 80 (25.3) | 8 (10.7) | 72 (29.9) |
| Poor | 68 (21.5) | 68 (28.2) | |
| Stage at metastatic diagnosis | |||
| IIA | 42 (13.3) | 14 (18.7) | 28 (11.6) |
| IIB | 52 (16.5) | 18 (24) | 34 (14.1) |
| IIC | 56 (17.7) | 22 (29.3) | 34 (14.1) |
| IIIA | 57 (18.0) | 8 (10.7) | 49 (20.3) |
| IIIB | 42 (13.3) | 8 (10.7) | 34 (14.1) |
| IIIC | 67 (21.2) | 5 (6.7) | 62 (25.7) |
| Metastatic sites | |||
| Lymph nodes/retroperitoneal | 304 (96.2) | 74 (98.7) | 230 (95.4) |
| Pulmonary | 119 (37.7) | 7 (9.3) | 112 (46.5) |
| NPVM | 56 (17.7) | 7 (9.3) | 49 (20.3) |
| Liver | 37 (11.7) | 3 (4) | 34 (14.1) |
| Brain | 8 (2.5) | 1 (1.3) | 7 (2.9) |
| Bone | 19 (6.0) | 3 (4) | 16 (6.6) |
| Postorchiectomy median AFP ng/mL (IQR) | 15 (3.3–344) | - | |
| AFP level < 1000 | 238 (79.1) | 167 (72.6) | |
| AFP level 1000–10,000 | 42 (14) | 42 (18.3) | |
| AFP level ≥ 10,000 | 21 (7) | 21 (9.1) | |
| Postorchiectomy median hCG mlU/mL (IQR) | 16 (1–505) | ||
| hCG level < 5000 | 257 (87.7) | 67 (98.5) | 190 (84.4) |
| hCG level 5000–50,000 | 17 (5.8) | 1 (1.5) | 16 (7.1) |
| hCG level ≥ 50,000 | 19 (6.5) | 19 (8.4) | |
| Postorchiectomy median LDH | 306 (200–543) | ||
| <2.5 × ULN | 217 (72.1) | 55 (77.5) | 162 (70.4) |
| >2.5 × ULN | 84 (27.9) | 16 (22.5) | 68 (29.6) |
| Relapse from Stage I | 33 (10.4) | 16 (21.3) | 17 (7.1) |
| Platinum resistance (PFI < 3 months) | 57 (18) | 6 (8) | 51 (21.1) |
| Progression after first-line treatment | 110 (34.8) | 15 (20) | 95 (39.4) |
| IPFSG Risk score for salvage treatment | 125 (39.6) | 15 (20) | 110 (45.6) |
| Very low | 7 (2.2) | 7 (9.3) | 0 (0) |
| Low | 22 (7) | 4 (5.3) | 18 (7.5) |
| Intermediate | 23 (7.3) | 2 (2.7) | 21(8.7) |
| High | 30 (9.5) | 1 (1.3) | 29 (12) |
| Very high | 42 (13.3) | 1 (1.3) | 41 (17) |
| Unknown | 1 (0.3) | 1 (0.4) | |
| Progression after second-line treatment | 44 (13.9) | 1 (1.3) | 43 (17.8) |
| Exitus | 34 (10.8) | 2 (2.7) | 32 (13.3) |
| Treatment Profile | No of Patients (%) |
|---|---|
| Metastatic first-line chemotherapy | 316 (100) |
| 3 × BEP | 133 (42.1) |
| 4 × BEP | 120 (38) |
| 4 × BEP + 2 × EP | 22 (7) |
| 4 × EP | 14 (4.4) |
| 4 × VIP | 10 (3.2) |
| 4 × BEP + 2 × VIP | 5 (1.6) |
| 3 × BEP + 1 × EP | 4 (1.3) |
| 3 × EP | 4 (1.3) |
| 6 × EP | 2 (0.6) |
| 3 × TIP | 1 (0.3) |
| 4 × VIP + 2 × EP | 1 (0.3) |
| Responses after first-line treatment | |
| Complete response | 123 (38.1) |
| Partial response | |
| STM (−) | 129 (39.9) |
| STM (+) | 35 (10.8) |
| Stable disease | 12 (3.7) |
| Progressive disease | 27 (7.4) |
| Additional treatment after first-line | |
| RPLND | 64 (20.3) |
| Radiotherapy | 13 (4.1) |
| Metastasectomy | 9 (2.8) |
| Chemotherapy | 28 (8.9) |
| Pathology after additional surgery | |
| Viable Tumor | 28 (8.9) |
| Necrosis/Fibrosis | 30 (9.5) |
| Teratoma | 15 (4.7) |
| Metastatic second-line (salvage) treatment | 125 (39.6) |
| CDCT | 46 (14.6) |
| 4 × TIP | 33 (10.4) |
| 4 × VIP | 7 (2.2) |
| GemPOX | 4 (1.3) |
| VeIP | 2 (0.6) |
| HDCT | 79 (25) |
| Responses after salvage treatment | |
| Complete response | 32 (10.1) |
| Partial response | |
| STM (−) | 72 (22.8) |
| STM (+) | 7 (2.2) |
| Stable disease | 5 (1.6) |
| Progressive disease | 9 (2.8) |
| Additional treatment after second-line | |
| RPLND | 9 (2.8) |
| Radiotherapy | 10 (3.2) |
| Metastasectomy | 7 (2.2) |
| Maintanence oral etoposide | 28 (8.9) |
| Third-line and beyond treatment | |
| GemPOX/GemOX | 24 (7.6) |
| HDCT | 6 (1.9) |
| TIP | 2 (0.6) |
| Brentuximab | 2 (0.6) |
| IGCCCG Prognostic Group | |||||
|---|---|---|---|---|---|
| Good | Intermediate | Poor | p | ||
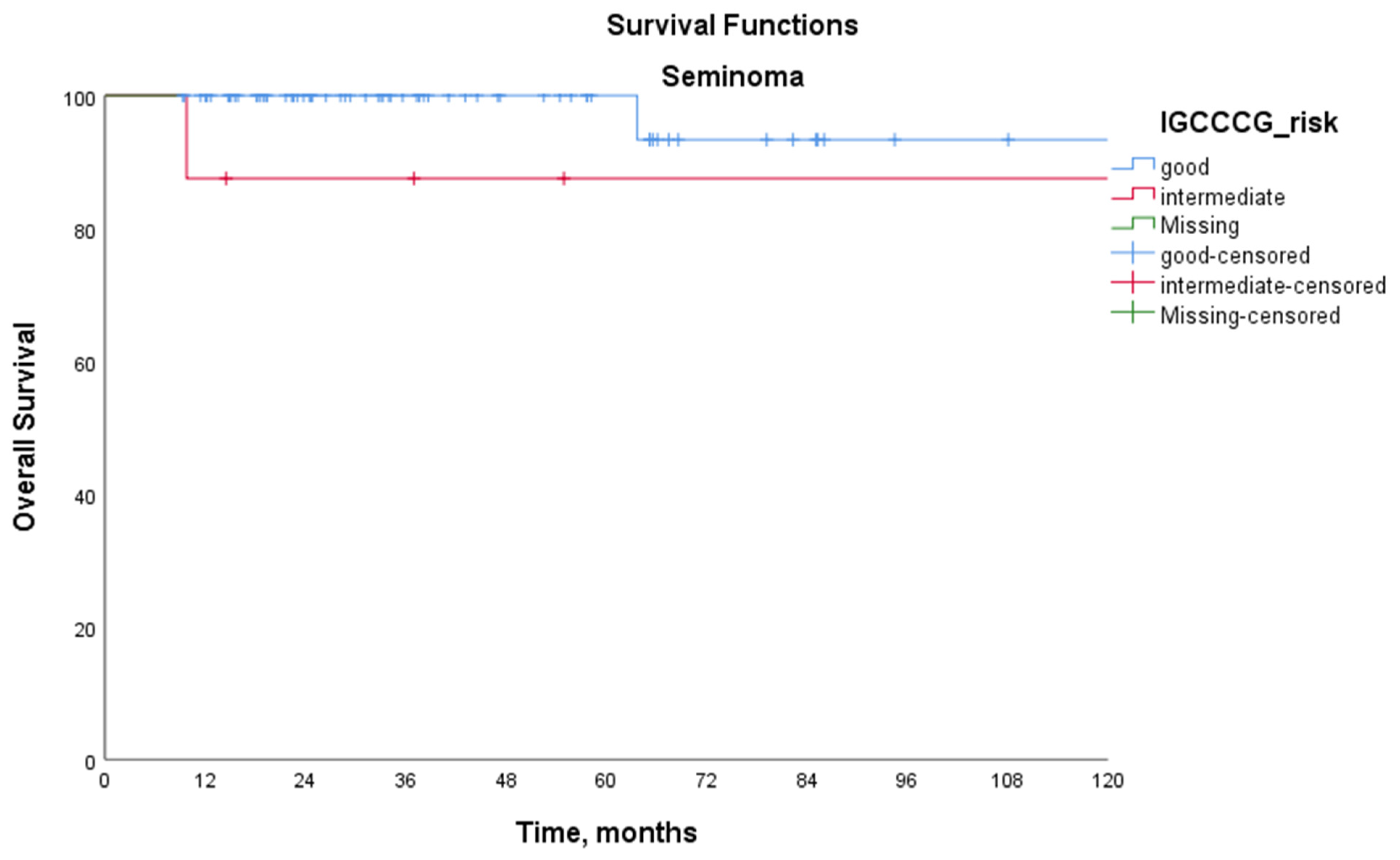
| Seminoma | 5-year PFS (%) | 82.8 | 46.9 | - | 0.030 |
| 5-year OS (%) | 100 | 87.5 | - | 0.186 | |
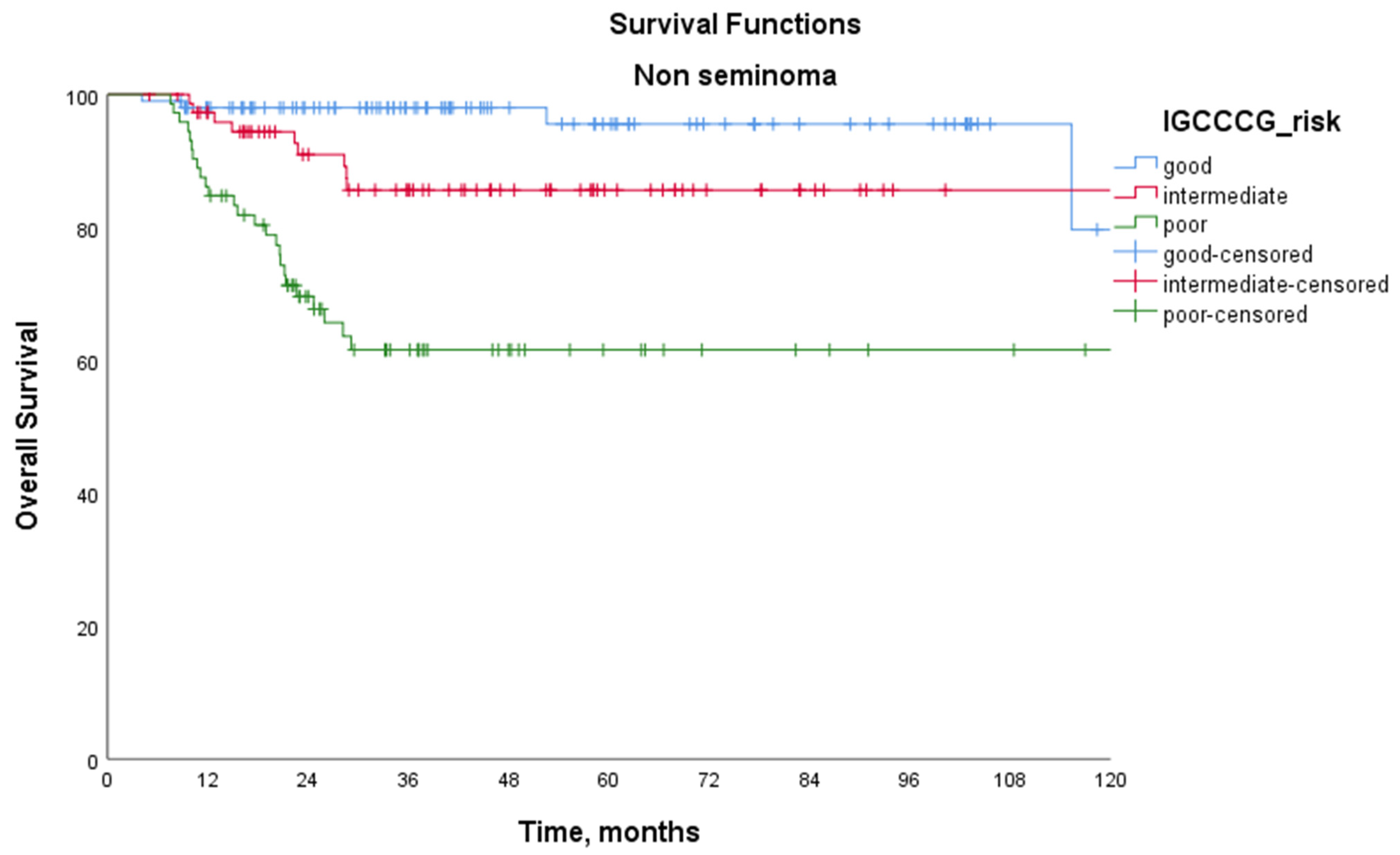
| Non-seminoma | 5-year PFS (%) | 80.9 | 51.9 | 30.9 | <0.001 |
| <0.001 | |||||
| 0.001 | |||||
| 5-year OS (%) | 96.6 | 86.9 | 65.1 | 0.046 | |
| <0.001 | |||||
| 0.001 | |||||
| All cohort | 5-year PFS (%) | 81.4 | 51.3 | 30.9 | <0.001 |
| <0.001 | |||||
| 0.001 | |||||
| 5-year OS (%) | 97.7 | 87.1 | 65.1 | 0.017 | |
| <0.001 | |||||
| 0.001 | |||||
| IPFSG Score | Salvage | N. of Patients | N. of Events | 3 Year OS % | p |
|---|---|---|---|---|---|
| Very low | CDCT | 5 | 0 | - | - |
| HDCT | 2 | 0 | - | ||
| Low | CDCT | 8 | 0 | - | - |
| HDCT | 14 | 0 | - | ||
| Intermediate | CDCT | 8 | 2 | 87.5 | 0.570 |
| HDCT | 15 | 3 | 84.8 | ||
| High | CDCT | 10 | 3 | 61.7 | 0.371 |
| HDCT | 20 | 5 | 83.4 | ||
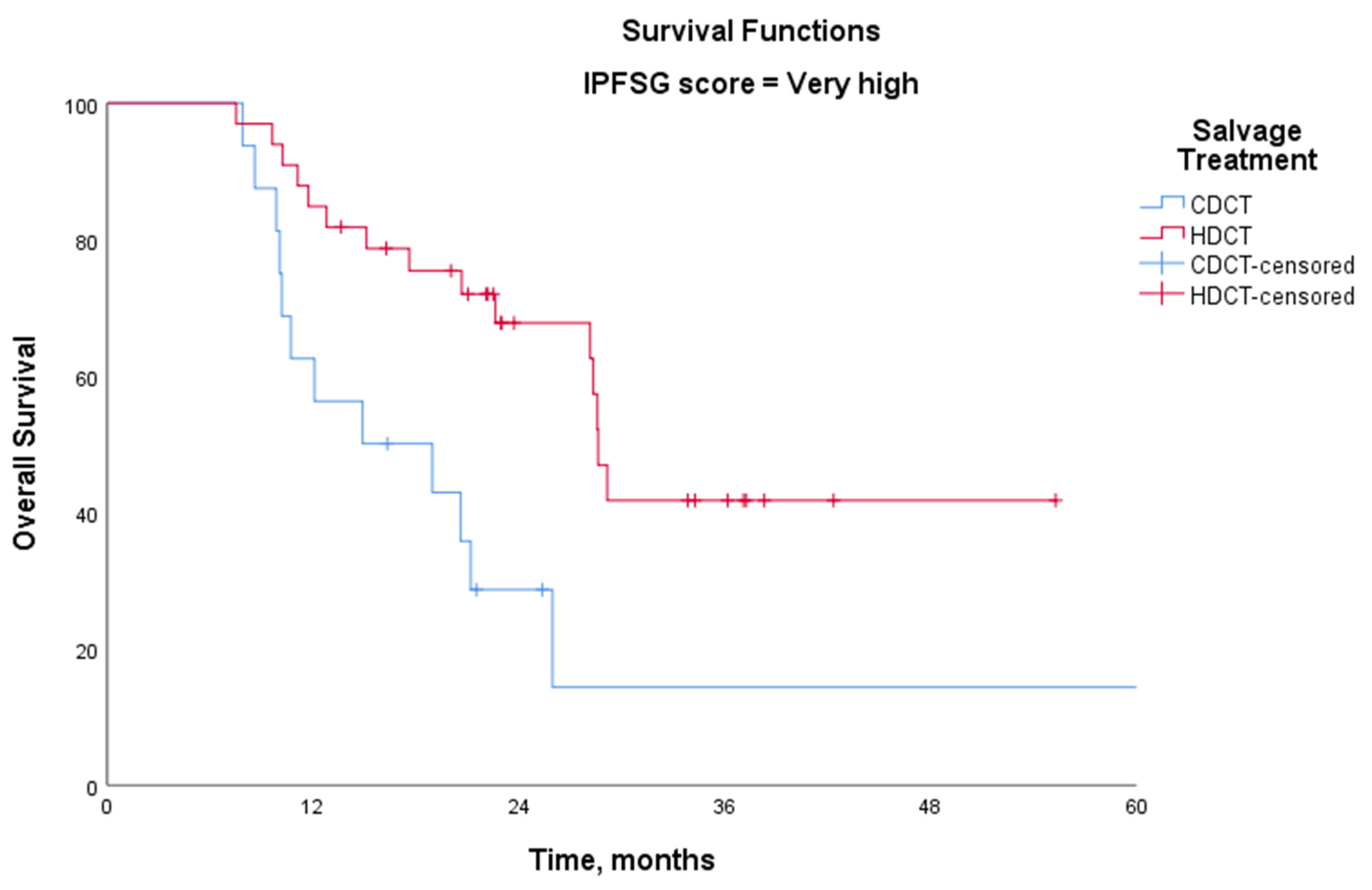
| Very high | CDCT | 14 | 10 | 16.3 | 0.007 |
| HDCT | 28 | 10 | 55.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiran Keskin, G.S.; Yetginoglu, O.; Vurgun, S.; Guzel, E.Z.; Ozkan, F.E.; Yilmaz, M.; Soylemez, C.M.; Agyol, Y.; Akbas, S.; Sagiroglu, M.F.; et al. Survival Outcomes in Metastatic Germ Cell Tumors: A Multicenter Study from Turkey. Medicina 2025, 61, 951. https://doi.org/10.3390/medicina61060951
Yildiran Keskin GS, Yetginoglu O, Vurgun S, Guzel EZ, Ozkan FE, Yilmaz M, Soylemez CM, Agyol Y, Akbas S, Sagiroglu MF, et al. Survival Outcomes in Metastatic Germ Cell Tumors: A Multicenter Study from Turkey. Medicina. 2025; 61(6):951. https://doi.org/10.3390/medicina61060951
Chicago/Turabian StyleYildiran Keskin, Gul Sema, Ozge Yetginoglu, Sertac Vurgun, Evrican Zin Guzel, Fariz Emrah Ozkan, Mesut Yilmaz, Cem Murat Soylemez, Yesim Agyol, Sinem Akbas, Muhammed Fatih Sagiroglu, and et al. 2025. "Survival Outcomes in Metastatic Germ Cell Tumors: A Multicenter Study from Turkey" Medicina 61, no. 6: 951. https://doi.org/10.3390/medicina61060951
APA StyleYildiran Keskin, G. S., Yetginoglu, O., Vurgun, S., Guzel, E. Z., Ozkan, F. E., Yilmaz, M., Soylemez, C. M., Agyol, Y., Akbas, S., Sagiroglu, M. F., Yildirim, G., Semiz, H. S., Tatli, A. M., Ekinci, F., Cosar, R., Acar, R., Aykan, M. B., Erturk, I., & Karadurmus, N. (2025). Survival Outcomes in Metastatic Germ Cell Tumors: A Multicenter Study from Turkey. Medicina, 61(6), 951. https://doi.org/10.3390/medicina61060951






